Twin-to-twin transfusion syndrome (TTTS) is a serious complication in monochorionic diamniotic (MD) twin pregnancies, accounting for approximately 10% of cases (Lewi et al., Reference Lewi, Jani, Blickstein, Huber, Gucciardo, Van Mieghem and Deprest2008; Lutfi et al., Reference Lutfi, Allen, Fahey, O’Connell and Vincer2004; Murgano et al., Reference Murgano, Khalil, Prefumo, Mieghem, Rizzo, Heyborne and D’Antonio2020). In the past decades, fetoscopic laser photocoagulation of the placental communicating vessels (FLP) has dramatically improved the perinatal outcomes of TTTS (Akkermans et al., Reference Akkermans, Peeters, Klumper, Lopriore, Middeldorp and Oepkes2015; Hecher et al., Reference Hecher, Gardiner, Diemert and Bartmann2018; Mascio et al., Reference Mascio, Khalil, D’Amico, Buca, Panici, Flacco and D’Antonio2020; Murata et al., Reference Murata, Takano, Kagawa, Sumie and Nakata2018; Murgano et al., Reference Murgano, Khalil, Prefumo, Mieghem, Rizzo, Heyborne and D’Antonio2020; Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). Although the prediction of TTTS is essential for the management of MD twin pregnancies and ultrasonographic assessment at every 2 weeks after 16 weeks of gestation is recommended (Khalil et al., Reference Khalil, Rodgers, Baschat, Bhide, Gratacos, Hecher and Ville2016), there is limited available information regarding the ultrasonographic parameter for predicting evolving TTTS in the second trimester (Yamamoto et al., Reference Yamamoto, Ishii, Muto, Kawaguchi, Murata, Hayashi and Mitsuda2013). In the first trimester, the discordance in fetal nuchal translucency thickness and fetal crown-rump length has been reported as a risk factor of severe TTTS (El Kateb et al., Reference El Kateb, Nasr, Nassar, Bernard and Ville2007; Kagan et al., Reference Kagan, Gazzoni, Sepulveda-Gonzalez, Sotiriadis and Nicolaides2007; Sebire et al., Reference Sebire, Souka, Skentou, Geerts and Nicolaides2000), but these parameters have unsatisfying detection rates (approximately 50%) and are unavailable in the second trimester.
The ductus venosus (DV) is one of the unique vessels in the fetal circulation, which connects the umbilical vein to the right atrium. The blood flow in DV is directly influenced by atrial pressure–volume changes throughout the cardiac cycle, showing S-wave, D-wave and a-wave. In the pathophysiology of TTTS, the donor-recipient exchange of vasoactive mediators, such as angiotensin, contributes to systemic hypertension and hypervolemia in recipient twins (Kilby et al., Reference Kilby, Platt, Whittle, Oxley and Lindop2001; Mahieu-Caputo et al., Reference Mahieu-Caputo, Meulemans, Martinovic, Gubler, Delezoide, Muller and Dommergues2005). Moreover, it has been reported that these recipients’ hemodynamic changes influence ventricular relaxation and ventricular filling pressures (Divanovic et al., Reference Divanovic, Cnota, Ittenbach, Tan, Border, Crombleholme and Michelfelder2011; Takano et al., Reference Takano, Nakata, Nagasaki and Morita2019) and alter the DV Doppler waveform. Recently, some studies have shown an increase in the time interval and velocity–time integral (VTI) of the decelerating S-wave phase, and a decrease in those of the D-wave phase in recipient twins (Tachibana et al., Reference Tachibana, Glosemeyer, Diehl, Nakagawa, Wada, Kurihara and Hecher2015; Wohlmuth et al., Reference Wohlmuth, Osei, Moise, Wieser, Johnson, Papanna and Gardiner2016). However, the data about the time intervals and VTIs of the recipients’ DV Doppler flow are still limited. Furthermore, the predictive parameter of DV Doppler flow for evolving TTTS remains unclear.
This study aimed to assess the alterations of the time intervals and VTIs of recipients’ DV Doppler flow, and to investigate whether these parameters could predict TTTS in MD twin pregnancies in the second trimester.
Materials and Methods
The available ultrasonographic data of women with MD twin pregnancies who were referred to Toho University Omori Medical Center (Tokyo, Japan) between January 2016 and August 2019 were retrospectively analyzed. The chorionicity and zygosity were confirmed by ultrasonography in the first trimester or placental pathology after birth in all cases. Triplets or higher-order pregnancies and fetuses with chromosomal and structural abnormalities were excluded. Clinical records of all cases were reviewed to investigate perinatal complications and outcomes. The study protocol was approved by the ethics committee of Toho University Omori Medical Center (27-198, No. M16103, M1722417283), and written informed consent was obtained from all patients.
First, we examined the difference in DV parameters between the larger twins in uncomplicated MD twins and recipient twins in TTTS cases. The uncomplicated MD twins were defined as MD twins without complications, such as TTTS, twin anemia-polycythemia sequence and selective intrauterine growth restriction (sIUGR; defined as intertwin estimated fetal weight [EFW] discordance of >25%) type 2 or type 3 (Gratacos et al., Reference Gratacos, Lewi, Munoz, Acosta-Rojas, Hernandez-Andrade, Martinez and Deprest2007) until their birth. In this study, sIUGR type 1 was included, and sIUGR type 2 and type 3 were excluded because sIUGR type 2 and type 3 cases are subject for FLP in Japan, and it is unclear whether they develop TTTS or not. TTTS was diagnosed based on the following standard criteria: oligohydramnios (maximum deepest vertical pocket [MVP] of amniotic fluid of <2 cm) in one twin and polyhydramnios (MVP of >8 cm) in the other, together with markedly discordant bladder sizes. TTTS severity was classified using the Quintero staging system (Quintero et al., Reference Quintero, Morales, Allen, Bornick, Johnson and Kruger1999).
Second, we extracted the data at the pre-TTTS condition in the TTTS cases retrospectively. The pre-TTTS condition was defined as the condition within 2 weeks before when a TTTS case fulfilled the criteria. Then, we compared the DV parameters between the larger twins in uncomplicated MD twins and recipient twins at the pre-TTTS condition (prerecipient twins).
All ultrasonographic examinations were performed using the ARIETTA 60, 70 or 850 ultrasound machines with a C35 convex transducer and 2–8 MHz of the frequency range (Hitachi Ltd., Tokyo, Japan). EFW was calculated using the formula previously reported by the local population (Shinozuka, Reference Shinozuka2000). The intertwin EFW discordance rate was calculated in all cases using the following formula: [(A−B)/A] × 100, where A is the EFW of the larger or recipient twin, and B is the EFW of the smaller or donor twin. The Doppler flow of DV and the umbilical artery (UA) were assessed (Acharya et al., Reference Acharya, Wilsgaard, Berntsen, Maltau and Kiserud2005; Turan et al., Reference Turan, Turan, Sanapo, Willruth, Berg, Gembruch and Baschat2014). An abnormal Doppler flow was defined as an absent or reversed end-diastolic flow. In the uncomplicated MD twins, the ultrasonographic data with adequate images from both fetuses in the second trimester were analyzed. In TTTS cases, the ultrasonographic data at the diagnosis of TTTS were included. In pre-TTTS cases, the ultrasonographic data within 2 weeks before the diagnosis of TTTS were used.
The DV Doppler waveform was assessed in the midsagittal plane or cross-sectional plane. The sampling volume was adjusted to 3 mm and covered the whole DV vessels. The ultrasound Doppler settings were as follows: pulse repetition frequency of 3.6−4.2 kHz, wall filter of 57.7−71.4 Hz and sweep speed of 133−200 cm/s. Using the acquired DV Doppler waveform, time intervals and VTIs were measured in each phase (Figure 1). All measurements of the VTIs and time intervals were performed by one operator (M.T.). The measuring operator was blinded to fetal size discordance, amniotic fluid volume and other parameters except for information about larger or smaller twin and recipient or donor twin to identify fetuses. The measurements of time intervals included the acceleration times of S-wave (S1), deceleration times of S-wave (S2) and times of D-wave (D), and their values were normalized to the following cardiac cycle lengths: S1 (%), S2 (%) and D (%), respectively. The measurements of VTIs included the accelerating phase of S-wave (VTI-1), decelerating phase of S-wave (VTI-2) and VTI of D-wave (VTI-diast), and their values were normalized to the following total VTIs: VTI-1 (%), VTI-2 (%) and VTI-diast (%), respectively.
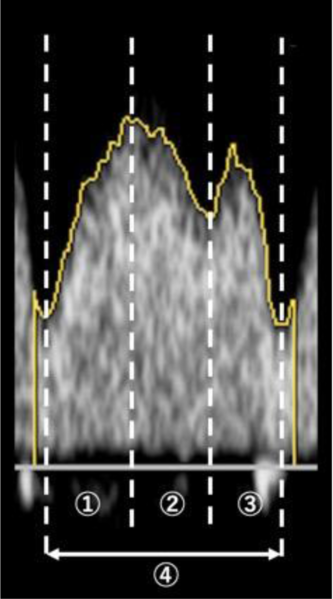
Fig. 1. Ultrasonogram for the measurement of time intervals and velocity–time integrals (VTI) in ductus venosus Doppler waveform. Measurements of time intervals were performed; acceleration times of S-wave (![]() , S1), deceleration times of S-wave (
, S1), deceleration times of S-wave (![]() , S2) and times of D-wave (
, S2) and times of D-wave (![]() , D), and their values were normalized to cardiac cycle length (
, D), and their values were normalized to cardiac cycle length (![]() ). S1 (%) = S1 (ms)/cardiac cycle length (ms), S2 (%) = S2 (ms)/cardiac cycle length (ms) and D (%) = D (ms)/cardiac cycle length (ms). Measurements of VTI were performed; accelerating phase of S-wave (
). S1 (%) = S1 (ms)/cardiac cycle length (ms), S2 (%) = S2 (ms)/cardiac cycle length (ms) and D (%) = D (ms)/cardiac cycle length (ms). Measurements of VTI were performed; accelerating phase of S-wave (![]() , VTI-1), decelerating phase of S-wave (
, VTI-1), decelerating phase of S-wave (![]() , VTI-2) and VTI of D-wave (
, VTI-2) and VTI of D-wave (![]() , VTI-diast), and their values were normalized to total VTI (
, VTI-diast), and their values were normalized to total VTI (![]() ). VTI-1 (%) = VTI-1/total VTI, VTI-2 (%) = VTI-2/total VTI and VTI-diast (%) = VTI-diast/total VTI.
). VTI-1 (%) = VTI-1/total VTI, VTI-2 (%) = VTI-2/total VTI and VTI-diast (%) = VTI-diast/total VTI.
Statistical Analysis
The normality of the data was assessed using the Shapiro–Wilk test. Welch’s t-test and Mann–Whitney U test were performed to compare independent data between two groups. The receiver-operating characteristic (ROC) curve analysis was performed to assess how well the DV parameters could detect the evolution of TTTS, comparing the DV parameters between the larger twins in uncomplicated MD twins and prerecipient twins in pre-TTTS cases. To validate the results of the ROC analysis, the area under the curve (AUC) was also assessed. Using the threshold values, sensitivity, specificity, positive predictive value and negative predictive value were calculated. Two-sided p values <.05 were considered statistically significant in all tests. All statistical analyses were performed using the SPSS software version 20.0 (IBM Corp., Armonk, NY, USA).
Results
The data of DV parameters were obtained from 123 MD twin pregnancies, including 44 uncomplicated MD twins, 20 pre-TTTS cases and 79 TTTS cases. The clinical characteristics of each group are summarized in Table 1. There were no statistically significant differences in gestational age at ultrasonographic examinations among the groups. The intertwin EFW discordance rate of uncomplicated MD twins was significantly lower than those of pre-TTTS (p < .001) and TTTS cases (p < .001). In the TTTS cases, 53 (67.1%) were in stage I or II, and 26 (32.9%) were in stage III or IV. Eighteen of the recipient twins (22.8%) showed an abnormal DV Doppler flow. There were no cases of abnormal DV and UA Doppler flow in the larger twins and prerecipient twins. In the pre-TTTS cases, the median interval from ultrasonography to the diagnosis of TTTS was 8 days (range 4−13 days). Fifteen of the 20 pre-TTTS cases (75.0%) progressed to TTTS stage I or II, and the other 5 cases (25.0%) progressed to TTTS stage III or IV.
Table 1. Clinical characteristics of each group

Note: EFW, estimated fetal weight; MD, monochorionic diamniotic; TTTS: twin-to-twin transfusion syndrome. All data are given as mean and ±SD, median and range or number.
a p < .05 compared with uncomplicated MD twins.
Comparison between Uncomplicated MD Twins and TTTS Cases
The recipient twins in TTTS cases showed significantly shorter S1 (%), longer S2 (%), shorter D (%), smaller VTI-1 (%), larger VTI-2 (%) and smaller VTI-diast (%) than did the larger twins in uncomplicated MD twins (Table 2).
Table 2. Comparison of the parameters of ductus venosus Doppler flow

Note: MD, monochorionic diamniotic; TTTS, twin-to-twin transfusion syndrome; S1, acceleration times of S-wave; S2, deceleration times of S-wave; D, times of D-wave; VTI, velocity–time integrals. All data are given as mean and ±SD or median and range. P values show comparison with larger twins in uncomplicated MD twins.
a p < .05 compared with prerecipient twins in pre-TTTS.
Comparison between Uncomplicated MD Twins and Pre-TTTS Cases
Prerecipient twins in the pre-TTTS cases showed significantly shorter S1 (%), longer S2 (%), shorter D (%), smaller VTI-1 (%), larger VTI-2 (%) and smaller VTI-diast (%) than did the larger twins in uncomplicated MD twins (Table 2). As shown in Figure 2, the prerecipient twins in the pre-TTTS cases showed almost similar values to those of the recipient twins with TTTS stage I or II, showing no statistical significance (p > .05).
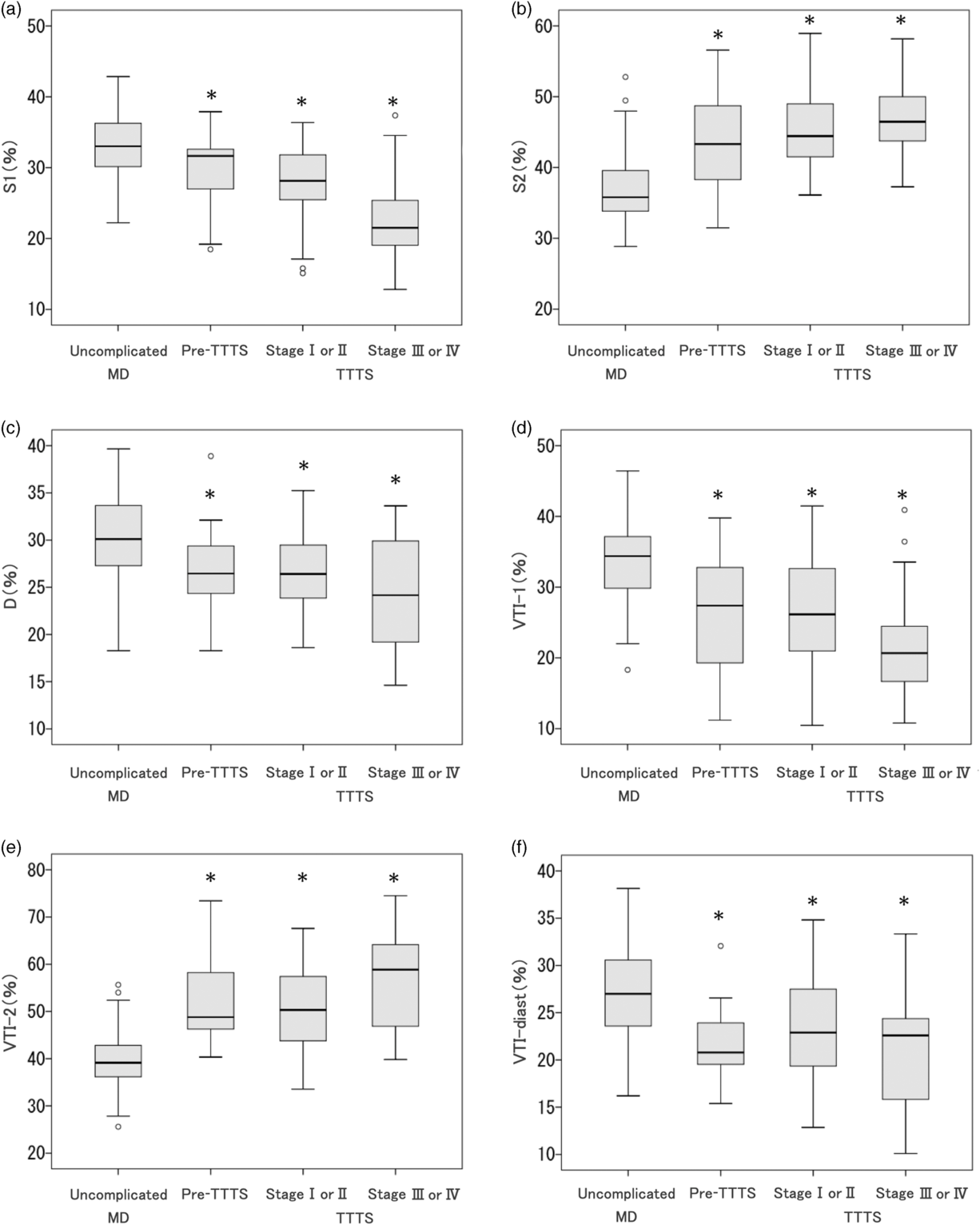
Fig. 2. Box plots showing the relationships of time intervals and velocity–time integrals (VTI) of ductus venosus Doppler flow between the larger twins in uncomplicated monochorionic diamniotic (MD) twins, prerecipient twins in pre-twin-to-twin transfusion syndrome (TTTS) cases and recipient twins in TTTS stage I or II and stage III or IV cases. (a) S1 (%); (b): S2 (%); (c) D (%); (d) VTI-1 (%); (e) VTI-2 (%); (f) VTI-diast (%). Note: *p < .05 compared with uncomplicated MD twins.
The ROC curve analysis was performed to differentiate uncomplicated MD twins from pre-TTTS cases using the DV parameters of the larger and prerecipient twins respectively (Table 3). VTI-2 (%) had better AUC than did the other DV parameters (Figure 3), and we identified a VTI-2 (%) cut-off value of 45.5, which had 87.0% sensitivity, 90.9% specificity, 81.0% positive predictive value and 93.0% negative predictive value for detecting prerecipient twins.
Table 3. Results of the receiver operating characteristic (ROC) curve analyses comparing the parameters between larger twins in uncomplicated monochorionic diamniotic twins and prerecipient twins in pre-twin-to-twin transfusion syndrome cases

Note: AUC, area under the curve; EFW, estimated fetal weight; S1, acceleration times of S-wave; S2, deceleration times of S-wave; D, times of D-wave; VTI, velocity–time integrals. VTI, velocity–time integrals.

Fig. 3. The receiver operating characteristic (ROC) curve for assessing how well the VTI-2 (%) detects prerecipient twins in pre-twin-to-twin transfusion syndrome (TTTS) cases.
Discussion
This study demonstrates that the characteristic alterations of DV waveform have already occurred in recipient twins prior to fulfilling the criteria of TTTS. Furthermore, we report VTI-2 >45.5% as a possible predictive parameter for TTTS with high detection rates (87% sensitivity and 91% specificity) in the second trimester.
Recipient twins complicated with TTTS showed the characteristic DV Doppler waveforms, including the decrease of the accelerating component of S-wave (S1 and VTI-1), the increase of the decelerating component of S-wave (S2 and VTI-2) and the decrease of the D-wave component (D and VTI-diast; Figure 4), which are consistent with the findings of other reports (Tachibana et al., Reference Tachibana, Glosemeyer, Diehl, Nakagawa, Wada, Kurihara and Hecher2015; Wohlmuth et al., Reference Wohlmuth, Osei, Moise, Wieser, Johnson, Papanna and Gardiner2016). We considered that these alterations reflect the pathophysiological hemodynamic changes of TTTS. The deterioration of the diastolic function is considered to be induced by systemic hypertensive and hypervolemic changes among recipient twins caused by the paradoxically activated renin-angiotensin-aldosterone system (RAAS; Divanovic et al., Reference Divanovic, Cnota, Ittenbach, Tan, Border, Crombleholme and Michelfelder2011; Kilby et al., Reference Kilby, Platt, Whittle, Oxley and Lindop2001; Mahieu-Caputo et al., Reference Mahieu-Caputo, Meulemans, Martinovic, Gubler, Delezoide, Muller and Dommergues2005; Votava-Smith et al., Reference Votava-Smith, Habli, Cnota, Divanovic, Polzin, Lim and Michelfelder2015). In fact, we previously reported that recipient twins have an impaired ventricular relaxation function and increased ventricular filling pressure (Takano et al., Reference Takano, Nakata, Nagasaki and Morita2019). The impaired ventricular relaxation seems to prolong the duration before the opening of the atrioventricular valves in the diastolic phase (isovolumic relaxation time [IRT]), which results in higher values of S2 and VTI-2 because these components contain IRT. On the other hand, the increased ventricular filling pressure seemed to contribute to the decrease in D and VTI-diast, which contain the diastolic ventricular filling time. Divanovic et al. (Reference Divanovic, Cnota, Ittenbach, Tan, Border, Crombleholme and Michelfelder2011) reported that recipient twins show shortened diastolic ventricular filling time owing to their diastolic dysfunction, which supports our results.
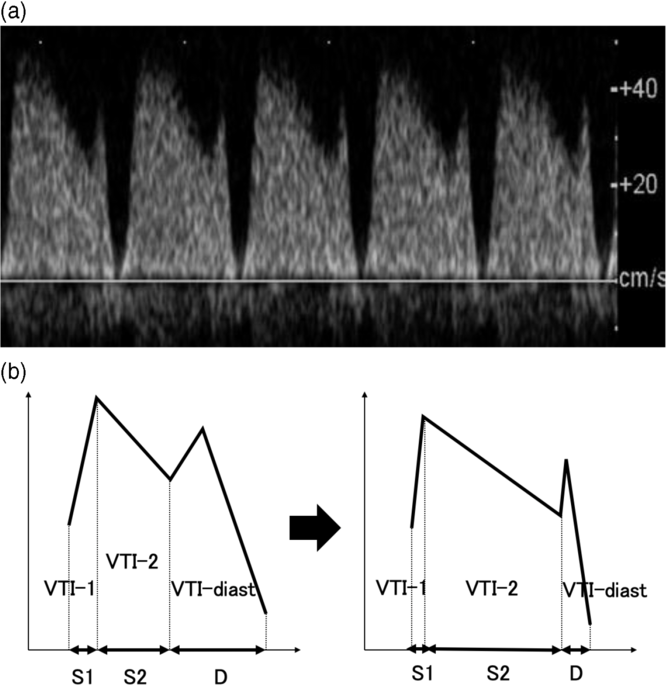
Fig. 4. Schema of the alterations of ductus venosus (DV) Doppler waveform in recipient twins with twin-to-twin transfusion syndrome (TTTS) (a) and ultrasonogram of recipient twin’s DV Doppler waveform with characteristic alterations (b). In this study, shorter S1, longer S2, shorter D, smaller VTI-1, larger VTI-2 and smaller VTI-diast were seen in prerecipient twins in pre-TTTS cases and recipient twins in TTTS cases than in larger twins in uncomplicated monochorionic diamniotic twins.
It is interesting to note that even in the condition wherein the cases did not fulfill the criteria of TTTS, prerecipient twins showed alterations of the DV waveform, including an increase in S2 and VTI-2 and decreases in S1, VTI-1, D and VTI-diast, similar to those of recipient twins. We considered that the hemodynamic influence of paradoxically activated RAAS in the recipient twins precedes the development of polyhydramnios and oligohydramnios. In this study, VTI-2 (%) showed a better performance in predicting TTTS than did the other parameters. In the perspective of the pathophysiology of TTTS, it seemed reasonable to conclude that VTI-2 is a suitable parameter because higher VTI-2 reflects the deterioration of the ventricular relaxation function. Contrarily, Wohlmuth et al. (Reference Wohlmuth, Osei, Moise, Wieser, Johnson, Papanna and Gardiner2016) reported that VTI-diast (%) was more effective in predicting evolving TTTS than were the other DV parameters. A possible reason for this difference is that our study was only based on the prerecipient twin’s DV Doppler flow, whereas Wohlmuth et al.’s study assessed the intertwin differences between recipient and donor twins. Additionally, the difference in the number of cases might have influenced the results; our study included 20 pre-TTTS cases that developed TTTS, whereas Wohlmuth et al.’s study only analyzed seven cases. Furthermore, the sensitivity and specificity of VTI-2 >45.5% in the present study were as high as 87% and 91%, respectively, which are higher compared to the data of other reports (El Kateb et al., Reference El Kateb, Nasr, Nassar, Bernard and Ville2007; Kagan et al., Reference Kagan, Gazzoni, Sepulveda-Gonzalez, Sotiriadis and Nicolaides2007; Sebire et al., Reference Sebire, Souka, Skentou, Geerts and Nicolaides2000; Wohlmuth et al., Reference Wohlmuth, Osei, Moise, Wieser, Johnson, Papanna and Gardiner2016). Although the reason why VTI-2, as a parameter of VTI, is superior to S2, as a parameter of the time interval, is unclear; the advantage of VTI is that it is an integral parameter of time intervals and velocities and can assess not only the duration of temporal cardiac cycle changes but also the blood flow volume passing the DV.
A limitation of this study is that our analyses are retrospective and based on a small number of cases. In addition, only the data of 20 cases in the pre-TTTS condition out of the 79 TTTS cases could be obtained, because many cases have already been examined after fulfilling the criteria of TTTS, as our institution is a referral center. Thus, further large prospective studies are required to validate our findings. Last, this study focused on the prediction of TTTS, so this parameter is not for the prediction of perinatal outcomes.
In conclusion, the recipient twins complicated with TTTS have characteristic alterations of time intervals and VTIs of the DV Doppler flow, which is present even before fulfilling the criteria of TTTS. There is a possibility that VTI-2 is a useful parameter for predicting TTTS, and we recommend the measurement of VTI-2 in the management of MD twin pregnancies in the second trimester.
Clinical Trial Registration
This study was registered with the Japanese Clinical Trial Registry ‘UMIN-CTR’ (http://www.umin.ac.jp/ctr/index-j.htm) and was given trial ID numbers of UMIN000024486 and UMIN000037702.
Financial support
This work was partially supported by JSPS KAKENHI grant numbers JP16K1114 and 19K09788.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









