Despite hope of identifying a specific cognitive deficit characteristic of schizophrenia, generalised neuropsychological impairment is the most common finding in individuals with an established schizophrenic illness (Reference Heinrichs and ZakzanisHeinrichs & Zakzanis, 1998; Reference Joyce and HuddyJoyce & Huddy, 2004). Two key issues remain to be resolved. What are the earliest emerging neuropsychological impairments in schizophrenia? Are such impairments specific to schizophrenia-related disorders? We report data from a longitudinal birth cohort study on neuropsychological functioning at the age of 13 years in relation to adult psychiatric outcomes at 26 years.
METHOD
Participants were members of the Dunedin Multidisciplinary Health and Development Study – a prospective general-population birth cohort of 1037 individuals born in Dunedin, New Zealand, between April 1972 and March 1973. Study members were assessed on ten occasions between the ages of 3 and 26 years (Reference Poulton, Caspi and MoffittPoulton et al, 2000; Reference Cannon, Caspi and MoffittCannon et al, 2002). The protocol was approved by the ethics review boards of the three participating universities.
Psychiatric interviews using the Diagnostic Interview Schedule (Reference Robins, Cottler and BucholzRobins et al, 1995) were available at the age of 26 years for 979 of the 1019 cohort members still living (96%). Research diagnoses of past-year Axis 1 disorders were grouped for this analysis into the following: schizophreniform disorder (3.7%), manic episode (2.0%) and depressive or anxiety disorder (28.5%). The remainder of the Dunedin Study members comprised the control group. Because the youth of the cohort made the ultimate diagnostic outcome uncertain for some, we grouped study members meeting criteria for schizophrenia (1% of the cohort) and schizophreniform disorder (2.7% of the cohort) under the term schizophreniform disorder. Diagnostic procedures are described elsewhere (Reference Poulton, Caspi and MoffittPoulton et al, 2000; Reference Cannon, Caspi and MoffittCannon et al, 2002). Briefly, data from interviews and collateral reports were used to make research diagnoses. All those receiving this diagnosis reported both hallucinations and delusions, and 70% had received treatment. Interviewers were masked to previous neuropsychological data.
In 1985–1986, two clinical psychologists administered a 50-min neuropsychological test battery to the Dunedin Study Members. The battery comprised: Rey–Osterreith Complex Figure Test; Rey Auditory–Verbal Learning Test (four trials); Wisconsin Card Sort Test (three categories); Mazes; Trail Making Test; Grooved Pegboard and Verbal Fluency (Reference LezakLezak, 1983). Only Dunedin Study members with both diagnostic data at the age of 26 years and neuropsychological data at the age of 13 years (69% of the cohort) could be included in this analysis. Participants who were missing either did not take part in the assessment at 26 years of age (4%) or the assessment at 13 years of age (14%), lived too far away to come to the unit for neuropsychological testing (11%), or were unable to undergo testing for varied reasons (2%). The children who underwent testing did not differ significantly from the remainder of the cohort on measures of family socio-economic status, IQ, gender, or behaviour problems (Reference Frost, Moffitt and McGeeFrost et al, 1989). A similar proportion of the children who were tested developed an adult schizophreniform disorder outcome, as compared with the whole cohort (3.5% v. 3.7%).
To examine specific cognitive functions within the context of broadly normal IQ, we excluded Dunedin Study members with IQ scores of >2 s.d. below the mean (n=16). One participant who had suffered a severe head injury was also excluded. Ultimately, 699 individuals were included in this analysis, comprising four groups: schizophreniform disorder (n=23); mania (n=10); depression/anxiety disorder (n=196); and controls (n=470). Test scores were standardised so that mean=0 and s.d.=1. Regression equations were performed using three dummy variables (one for each diagnostic status) and using the control group as the reference category. All regression coefficients were adjusted for gender and average socio-economic status of the family throughout childhood and adolescence (Reference Wright, Caspi and MoffittWright et al, 1999).
RESULTS
At age 13, the schizophreniform disorder group differed significantly from the control group on the following test scores: Trail Making Test, part B score, time to completion: β=–0.76, 95% CI –0.35 to –1.2, P<0.001; Trail Making Test, part B score minus part A score: β=–0.74, 95% CI –0.33 to –1.16, P<0.001; Grooved Pegboard, right hand: β=–0.68, 95% CI –0.22 to –1.1, P=0.002; Grooved Pegboard, left hand: β=–0.41, 95% CI –0.008 to –0.81, P=0.045 (Pegboard effects persisted following adjustment for hand preference); and Verbal Fluency: β=–0.46, 95% CI –0.91 to –0.02, P=0.043. All significant differences between schizophreniform v. control groups were in the moderate range, s.d.=0.4–0.8. The mania group did not differ significantly from the control group on any test score at age 13 years. Although this study had poor power to detect differences between mania v. control groups, Fig. 1 shows that the effects were generally not as large as the differences between schizophreniform v. control groups, and for some tests (i.e. Trail-Making Test and Grooved Pegboard) differences were in the opposite direction. The depression and anxiety group differed significantly from the control group only on the Trail Making Test, part A (β=–0.23, 95% CI –0.06 to –0.4, P=0.008) and part B (β=–0.19, 95% CI –0.03 to –0.36, P=0.02). Effect sizes for the differences between depression and anxiety group and control group were not as large as the differences between the schizophreniform group and control group (Fig. 1). The study had ample power to detect the small differences between the depression and anxiety group and control group.
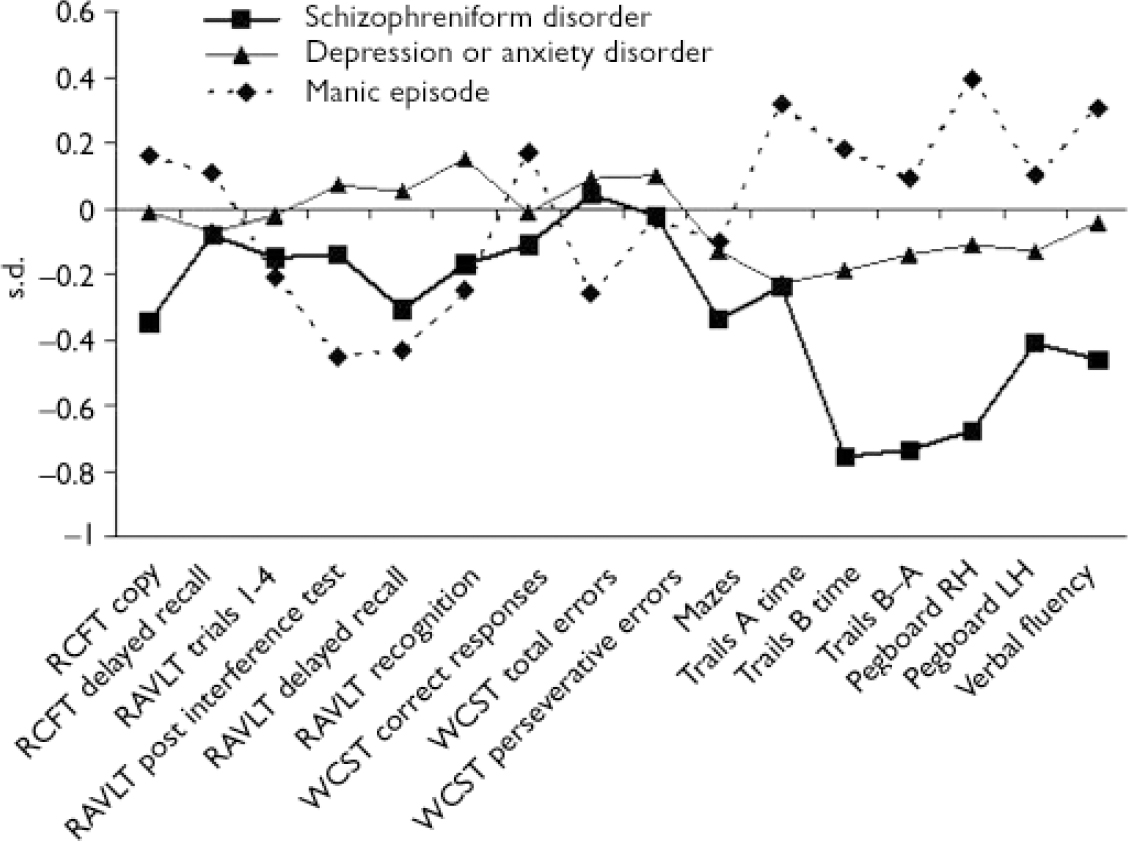
Fig. 1 Standardised scores on a neuropsychological test battery for 13-year-olds who later developed schizophreniform disorder (n=23), manic episode (n=10) or depression or anxiety disorder (n=196). The regression coefficients are interpretable as s.d. unit differences between each psychiatric group and the control group, adjusted for gender and family socio-economic status. RH, right hand; LH, left hand; RCFT, Rey–Osterreith Complex Figure Test; RVLT, Rey Auditory–Verbal Learning Test; WCST, Wisconsin Card Sort Test.
DISCUSSION
This study is limited by the small number of Dunedin Study members having schizophrenia-related disorders or mania, and by the rather old-fashioned nature of the neuropsychological battery. However, these limitations are compensated for by the prospective nature of the data. Our results expand on previous work showing that impairments in motor performance and attentional or executive performance are evident many years before onset of schizophrenia (Reference Erlenmeyer-Kimling, Rock and RobertsErlenmeyer-Kimling et al, 2000; Reference Niendam, Bearden and RossoNiendam et al, 2003), and suggest the hypothesis that integrated higher-level cortical activity is already affected. Our findings correspond to the speed-of-processing dimension identified as one of seven separable cognitive factors in schizophrenia (Reference Neuchterlein, Barch and GoldNeuchterlein et al, 2004).
There are two noteworthy aspects to our results. First, memory and learning impairments were not found in the current analysis but are evident in studies of first-episode patients, indicating that these impairments may emerge later in the developmental course of the disorder (e.g. Reference Joyce, Hutton and MutsatsaJoyce et al, 2002). Second, our results suggest some specificity of early motor and attentional or executive impairment to future schizophrenia-related outcomes rather than affective disorder outcomes (though power was limited by the size of the mania group).
This study further emphasises the importance of studying cognitive impairment in schizophrenia within the context of brain development (Reference Thompson, Vidal and GieddThompson et al, 2001).
Acknowledgements
We thank the Dunedin Study members, their collateral informants, unit research staff, and study founder, Phil Silva. This research was supported by the Wellcome Trust, NARSAD, UK, Medical Research Council grant G0100527, US-NIMH grants MH45070 and MH49414, and the William T. Grant Foundation. T.M. is a Royal Society–Wolfson Merit Award holder.




eLetters
No eLetters have been published for this article.