Childhood is a key period in life for the development of healthy eating habits that can persist for a long time(Reference Totland, Gebremariam and Lien1–Reference Madruga, Araujo and Bertoldi5). The transition from pre-school to school can influence changes in children’s food consumption(Reference Duyff6). Previous literature has shown that eating habits are established early in life and can be maintained throughout childhood; suggesting that the task of modifying behaviours at later stages will be challenging(Reference Northstone and Emmett2,Reference Rauber, Hoffman and Vitolo3) . However, tracking of eating habits has mainly been assessed in adults or between adolescence and adulthood, while few studies have evaluated tracking of diet during childhood(Reference Madruga, Araujo and Bertoldi5,Reference Northstone and Emmett7–Reference Ambrosini, Emmett and Northstone10) . Understanding how children’s eating patterns are acquired is essential in public health planning, particularly for supporting strategies to improve the quality of children’s diet.
Maternal behaviours during and after pregnancy might modulate the child’s nutrition status. In fact, children whose mothers smoked during pregnancy are more likely to be overweight throughout their lives(Reference Oken, Levitan and Gillman11,Reference Rayfield and Plugge12) . Breastfeeding is also one of the factors that play a significant role in the establishment of long-term eating habits among children(Reference de Lauzon-Guillain, Jones and Oliveira13–Reference Issanchou15) and its duration is inversely associated with the child’s risk of being overweight later on(Reference Harder, Bergmann and Kallischnigg16).
Childhood is also an important period for the development of healthy eating behaviours(Reference Duyff6). During this period, food choices can be influenced by several environmental factors(Reference Birch, Savage and Ventura17), such as parental behaviours (e.g. parental food habits) and family characteristics(Reference Vilela, Oliveira and Pinto18–Reference Kral and Rauh21). In particular, parental sociodemographic status seems to have a strong influence in children’s food intake, since low levels of maternal education have been associated with lower compliance with dietary recommendations(Reference Northstone and Emmett2,Reference Kyttala, Erkkola and Lehtinen-Jacks20,Reference Aranceta, Perez-Rodrigo and Ribas22) , and with a higher consumption of energy-dense foods (EDF)(Reference Vilela, Oliveira and Pinto18), compared with children of mothers with higher education levels. This negative effect of lower education levels on children’s food intake might be, in part, the result of parents’ poorer health literacy (e.g. ability to obtain, process and apply basic health information)(Reference Rauber, da Costa Louzada and Feldens23,Reference Zoellner, You and Connell24) .
Nevertheless, children with older and well-educated mothers tend to have a healthier diet(Reference Vereecken and Maes25,Reference Vereecken, Keukelier and Maes26) . Maternal age also appears to be an important determinant, as children of younger mothers seem to have a diet similar to that of children of less-educated mothers, presenting, for instance, lower consumption of whole-wheat bread and fruit(Reference Vereecken and Maes25,Reference Rogers and Emmett27) .
Understanding whether children’s food consumption changes during childhood, when food consumption remains stable and what factors influence diet is important since it allows to formulate interventions that promote a healthy diet at an early childhood stage(Reference Rauber, Hoffman and Vitolo3,Reference Lake, Rugg-Gunn and Hyland28,Reference Smithers, Brazionis and Golley29) . Thus, the present study aimed to evaluate the tracking of food consumption, including the evaluation of a score of a healthy eating index in pre-school and school age and its association with early life factors and maternal sociodemographic characteristics.
Methods
Participants
The present study was based on the population-based birth cohort Generation XXI(Reference Larsen, Kamper-Jorgensen and Adamson30). For this cohort, all newborns in the five public maternity hospitals in the metropolitan area of Porto were invited to participate, between 2005 and 2006. Of invited mothers, 91⋅4 % agreed to participate, with a total of 8647 children enrolled at baseline. At 4 years of age, the first follow-up of the entire cohort was performed, with 86 % of the children being evaluated. The second follow-up was performed at 7 years of age, and 80 % of the children were re-evaluated. During the follow-up periods, participants were invited to participate in a face-to-face interview. For those families that were not able to be present during the face-to-face interview, data were collected by telephone with a shorter version of the questionnaire. For the final sample, we considered all children with complete information on dietary habits consumption in the FFQ at both ages (n 5046). After exclusion of children with diseases that might influence the analysis of food intake (celiac disease, allergy and food intolerance, n 15) and children with congenital malformations that might affect feeding (n 18), 5013 children were included in the final sample.
Data collection
Data were collected by trained interviewers using structured questionnaires. The following variables collected at baseline were included in the present study: maternal sociodemographic characteristics at delivery (maternal working status, age and complete years of education), maternal and child characteristics during pregnancy and delivery (gestational diabetes, hypertension status, smoking status during pregnancy and weight gain during pregnancy; child’s sex, birth weight and birth weight for gestational age), being that the latter represent more the weight of metabolic and/or genetic factors. From the 4-year follow-up evaluation, we have included maternal and child’s BMI and both dietary intake, child’s physical activity and if the child was breastfeeding and the respective duration. From the 7-year follow-up, the children’s dietary intake and BMI were used.
Child’s dietary intake
Data on children’s food consumption were collected through an FFQ, applied by trained interviewers to the main caregiver of the child (usually the mother), referring to the 6 months prior to the interview, at both ages(Reference Vilela, Severo and Moreira31,Reference Vilela, Hetherington and Oliveira32) . At 4 years, the FFQ asked the frequency of intake of thirty-five food items, while at 7 years, the FFQ included thirty-eight food items. The nine possible frequency options were converted into daily consumption frequencies, ranging from ‘never’ to ‘≥4 times per d’. Similar food groups were created at both ages, based on the food recommendations of the Portuguese food wheel guide(Reference Rodrigues, Franchini and Graca33): ‘Dairy products’ (semi-skimmed milk, skimmed milk, cheese and yogurts); ‘Meat and meat products’ (pork, beef, rabbit, poultry and processed meat); ‘Fish and eggs’; ‘Cereal products and potatoes’ (rice, pasta, potatoes, bread and semi-sweet type biscuits); ‘Fruit and vegetables’ (vegetable soup, raw and cooked vegetables, fruit and fresh fruit juice); ‘Sweet foods’ (cakes, sweet pastry, chocolate, chocolate powder, chocolate milk and candies); ‘Salty snacks’ (crisps, pizzas and burgers) and ‘Soft drinks’ (sweetened carbonated drinks and other sweetened drinks, including diet drinks).
In a subsample of children, dietary intake was also collected using 3-d food diaries, two weekdays and one weekend day, at 4 and 7 years of age (n 2993), completed by the main caregiver(Reference Vilela, Severo and Moreira31,Reference Moreira, Severo and Oliveira34) . Parents were asked to record all the foods and drinks that children had consumed during those days, describing the amount, brand, recipe and place of consumption, through food diaries. The codification process was conducted a posteriori by a team of trained nutritionists, and nutrient intake was estimated using the software Food Processor SQL(35).
Healthy eating index
A healthy eating index, based on the WHO’s dietary recommendations, previously developed(Reference Vilela, Oliveira and Ramos36) for children at the age of 4 years, was adapted in this study. This index was developed to evaluate the quality of diet at 4 and 7 years of age and includes the eight previously defined food groups. For each food group, at 4 years, quartiles of the frequency of consumption were obtained and a score was assigned ranging from 1 to 4 points. The same cut-off points obtained to define the quartiles at 4 years of age were also used at 7 years. For foods that are considered healthier, such as fruit, vegetables, dairy products, fish and eggs, the lowest consumption quartile was scored with 1 point, intermediate quartiles with 2–3 points and the highest consumption quartile was scored with 4 points. Food groups with less healthy foods, such as meat, meat products, salty snacks, sweet foods and soft drinks, the score was assigned inversely, that is, the highest consumption quartile was assigned the lowest score. Scores assigned were summed up, resulting in an index with a total score ranging from 8 to 32 points at both ages, with higher scores reflecting a better adherence to dietary recommendations.
To validate the healthy eating index, we used dietary information collected through 3-d food diaries. We compared the healthy eating index score with nutrient intake evaluated by food diaries. The information used in the validation was children’s total energy intake (kJ/d), the percentage of energy from carbohydrates, proteins and fat and the fibre intake (g/d), at both ages. We predict that children with higher scores will have a higher intake of fibre and a lower intake of total fat and energy.
Other child’s characteristics
A detailed description of newborns’ anthropometrics is described elsewhere(Reference Fonseca, Severo and Santos37). Briefly, birth weight was abstracted from medical records by trained interviewers at the baseline evaluation. During the hospital stay, trained examiners weighed babies to the nearest 1 g using infant scales. Birth weight adjusted for gestational age was computed as a continuous variable according to the Canadian reference(Reference Kramer, Platt and Wen38). Accordingly, children were categorised as small for gestational age (children with birth weight below the 10th percentile for their gestational age and sex), appropriate for gestational age or large for gestational age (children weighing above the 90th percentile for their gestational age and sex).
Objective measurements of children’s height and weight at 4 and 7 years were performed by a team of trained examiners, according to standard procedures. Children’s BMI was defined by weight in kilograms divided by height in metres squared. This variable was then categorised using specific cut-offs for sex and age as defined by the WHO(39) (underweight/normal weight (≤1 sd); overweight (>1 sd to ≤2 sd); obesity (>2 sd)). A combined variable was created to evaluate the stability of BMI categories from 4 to 7 years of age: ‘maintain: normal weight’ (children with normal weight at both ages), ‘maintain: overweight/obesity’ (children with overweight or obesity at both ages), ‘increase’ (children that change from normal weight at 4 years to overweight or obesity at 7 years) and ‘decrease’ (children that change from overweight or obesity at 4 years to normal weight at 7 years).
Regular practice of structured physical exercise was used as qualitative variable and children were classified as non-practitioners or practitioners. The variable breastfeeding, collected as any breastfeeding duration in weeks, was categorised as never or less than 16 weeks, between 16 and 20 weeks and more than 20 weeks.
Maternal characteristics
Maternal working status at baseline was evaluated as a nominal variable, converted into working (part or full-time and working student) and not working (unemployed, student, seeking the first job, incapacitated and retired). As described earlier(Reference Santos, Severo and Gaillard40), mothers were considered as having gestational diabetes or hypertension during pregnancy when recorded on obstetrical records as a diagnosis during the current pregnancy. Maternal smoking status was asked at baseline for each of the pregnancy trimesters and categorised as not smoking or smoking during that period (less than one cigarette/d or at least one cigarette/d, respectively). Weight gained during pregnancy was obtained by subtracting the self-reported final pre-delivery weight by the preconceptional weight, and the results were categorised as ≤10, >10 and ≤20, >20 kg.
Objective measures of maternal height and weight were performed when the child was 4 years of age by a team of trained examiners, according to standard procedures. Maternal BMI was defined by weight in kilograms divided by height in metres squared. This variable was then categorised as normal or underweight (<25 kg/m2) and overweight/obese (≥25 kg/m2), according to the WHO cut-offs(41).
Information on maternal dietary intake at the 4 years evaluation was collected through an FFQ adapted from a previous questionnaire validated for the general adult population(Reference Lopes, Aro and Azevedo42) and for pregnant women in a subsample of this cohort(Reference Pinto, Severo and Correia43). This FFQ collected information on food intake during the previous year and included eighteen food items with nine response options ranging from ‘never’ to ‘four or more times per d’. Frequencies of consumption were converted into daily frequencies. A maternal food index previously developed was used in this study to evaluate the influence of maternal food intake on the child’s food intake(Reference Durao, Severo and Oliveira44). Briefly, a score was obtained considering eight food items/groups: milk, fish, red meat, bread, fruit, vegetables, EDF and sugar-sweetened beverages (SSB). For each item/food group, quartiles of the frequency of consumption were obtained and a score was assigned with a range from 1 to 4 points, according to increasing quartiles of food consumption (milk, bread, fruit and vegetables) or decreasing quartiles of food consumption (red meat, EDF and SSB). The food score ranged from 8 to 32 points, where higher scores represent better dietary quality.
Ethical considerations
The Generation XXI project was conducted in accordance with the guidelines defined in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the Hospital de São João/University of Porto Medical School. The legal representatives of each participant were informed about benefits and potential discomforts, through written informed consent, with the information of all the examinations to be carried out during the evaluation, at baseline and in the subsequent follow-up evaluations.
Statistical analyses
Categorical variables were described as frequencies and compared using the McNemar test. Continuous variables were summarised by means and standard deviations or medians and interquartile ranges. Comparison between groups was performed using Student’s t test for paired samples or the Wilcoxon test, respectively. The change in score on the healthy eating index at both ages was compared using Student’s t test for paired samples. The correlation between nutrient intake and the score of the healthy eating index, at both ages, was obtained using the Pearson correlation coefficient.
The associations between the score of the healthy eating index at 4 years, early life factors and sociodemographic characteristics, and the score of the healthy eating index at 7 years were evaluated through linear regression models – regression coefficients (β) and respective 95 % CI. In order to analyse the direct and indirect associations between exposures (family and maternal characteristics) and the outcome, a step-by-step approach was used: predefined blocks of variables were fitted separately, with each block mutually adjusted, and introduced cumulatively into the analysis, with a fixed order. Model 1 was adjusted for maternal sociodemographic characteristics (maternal age, education and work status at delivery); model 2 was further adjusted for maternal and child’s characteristics during pregnancy and at delivery (gestational diabetes, hypertension, weight gain during pregnancy, smoking status, child’s weight for the gestational age and breastfeeding); model 3 included those variables in model 2 plus maternal and child’s characteristics at the 4 years evaluation (maternal BMI, maternal dietary score and child’s physical exercise); model 4 included all the previous variables plus the healthy eating index score at 4 years; finally, model 5 included all the previous variables plus children BMI’s stability from 4 to 7 years. An interaction effect between child’s BMI stability and maternal BMI was tested in model 5 by including an interaction term in the final models. All models were adjusted for child’s sex. Models’ goodness-of-fit was evaluated through adjusted R 2.
The software used was the Statistical Package for the Social Sciences (released 2011. IBM SPSS Statistics for Windows, version 22.0.; IBM Corp.). We considered a significance level lower than 0⋅05.
Results
Table 1 shows the sociodemographic and early life characteristics. At baseline, mothers presented a mean age of 30 (sd 5⋅2) years, had an average of 11 (sd 4⋅3) years of education and 77 % were working. Among the children included in the analysis (49 % girls), the majority were normal weight at both ages; only 0⋅5 % were underweight. However, the percentage of obese children increased from 4 to 7 years old (9⋅8 v. 15⋅0 %).
Table 1. Sociodemographic characteristics, pregnancy and early-life characteristics
(Mean values and standard deviations; numbers of participants and percentages)
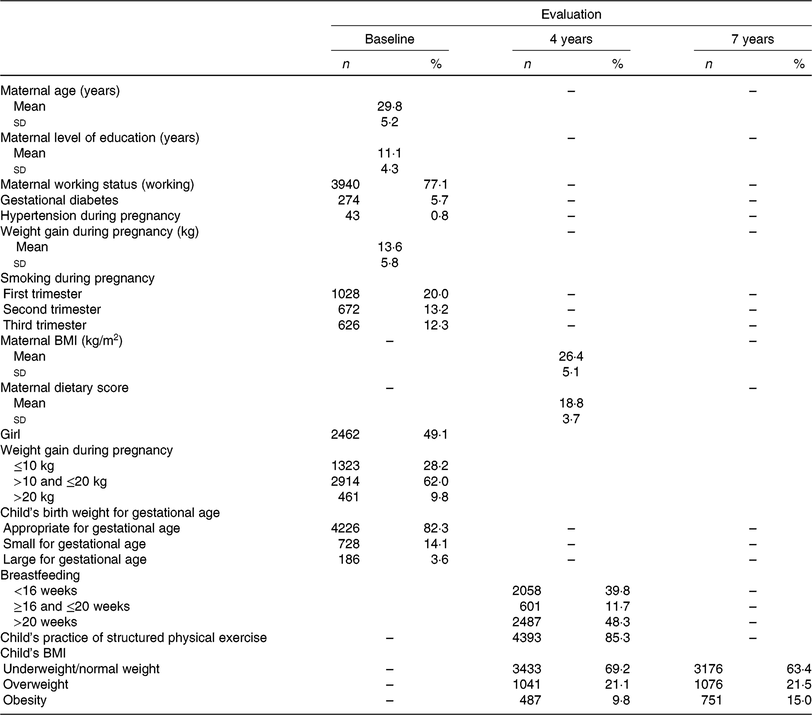
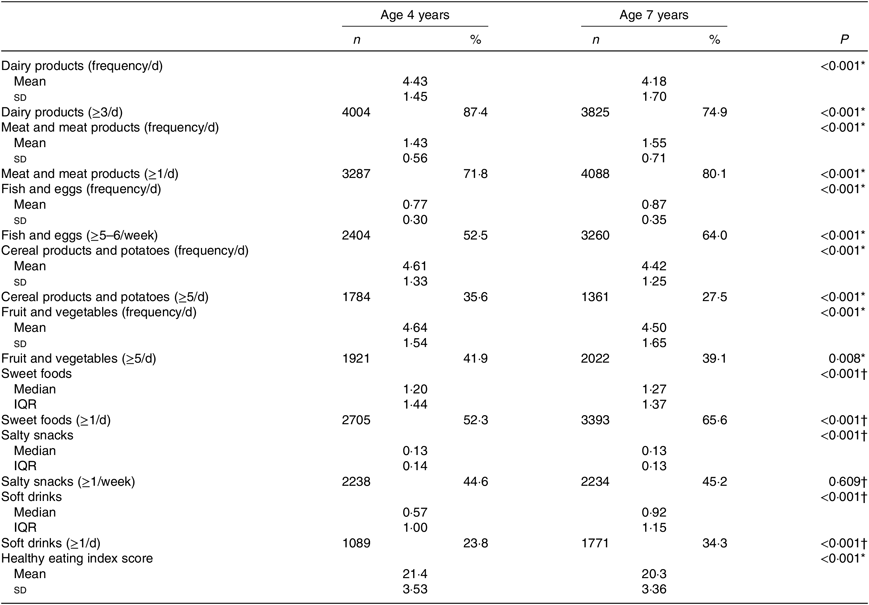
Table 2 presents the comparison of daily dietary intake and the scores in the healthy eating index, between 4 and 7 years of age. The daily frequency of consumption of ‘Meat and meat products’, ‘Fish and eggs’, ‘Sweet foods’ and ‘Soft drinks’ increased significantly from 4 to 7 years. However, the frequency of consumption of ‘Cereal products and potatoes’ and ‘Dairy products’ has decreased in the same period. The proportion of children who followed the Portuguese dietary recommendations for healthy foods and the proportion of children who consumed daily EDF are also described in Table 2. At the age of 4 years, almost 90 % of the children consumed ‘Dairy products’ at least three times per d, while at the age of 7 years, there was a significant decrease in the daily frequency. From 4 to 7 years, there was an increase in the proportion of children with daily consumption of ‘Meat and meat products’ (72 v. 80 %, P < 0·001) and weekly consumption of ‘Fish and eggs’ (≥5–6/week: 53 v. 64 %, P < 0·001). At both ages, less than half of children were consuming five daily servings of ‘Fruit and vegetables’. The proportion of children consuming ‘Cereal products and potatoes’ daily decreased from 4 to 7 years (≥5/d: 35·6 v. 27·5 %). Around 45 % of children consumed ‘Salty snacks’ weekly, at both ages. The daily consumption frequency of ‘Sweets foods’ and ‘Soft drinks’ increased significantly from 4 to 7 years (52 v. 66 %, P < 0·001 and 23 v. 34 %, P < 0·001, respectively). The healthy eating index score decreased slightly from 4 to 7 years (21·4 (sd 3·53) v. 20·3 (sd 3·63), respectively, P < 0·001). The scores ranged from 12 to 32 points at 4 years and from 11 to 31 points at 7 years (Table 2).
Table 2. Children’s dietary intake at 4 and 7 years of age
(Mean values and standard deviations; numbers of participants and percentages; medians and interquartile ranges (IQR))
* Student’s t test for paired samples.
† Wilcoxon test.
The correlation between the healthy eating index score at 4 and 7 years with nutrient intake was obtained through 3-d food diaries (Supplementary Table S1). At both ages, the healthy eating index was positively and significantly correlated with protein (r 0·111, P < 0·001 and r 0·157, P < 0·001) and fibre intake (r 0·205, P < 0·001 and r 0·253, P < 0·001), but inversely correlated with energy intake (r −0·060, P = 0·006 and r −0·063, P < 0·001), total fat intake (r −0·106, P < 0·001 at 4 years only) and saturated fat (r −0·141, P < 0·001 and r −0·073, P < 0·001). The intake of carbohydrates, monounsaturated and polyunsaturated fat did not present a statistically significant correlation (P > 0·01) with the healthy eating index scores, at both ages (Supplementary Table S1).
The relationship between a children’s healthy eating index at 4 and 7 years, by tertiles, is presented in Fig. 1. Around 40 % of the children who were in the lowest tertile of the score at 4 years of age continued in the lowest tertile at the age of 7 years, while the remaining moved to a higher tertile. Of those children within the second tertile of the healthy eating index score at 4 years of age, one-third remained in the same tertile at 7 years, 20 % moved to the first tertile, while the remaining moved to a higher tertile. Of those children within the highest tertile of the score at 4 years, most remained in the highest tertile at 7 years (71 %), while one-fifth moved to the second tertile and less than 10 % moved to the first tertile.

Fig. 1. Relationship between children’s healthy eating index (tertile) at 4 years and their healthy eating index (tertile) at 7 years. ![]() , First tertile;
, First tertile; ![]() , second tertile;
, second tertile; ![]() , third tertile.
, third tertile.
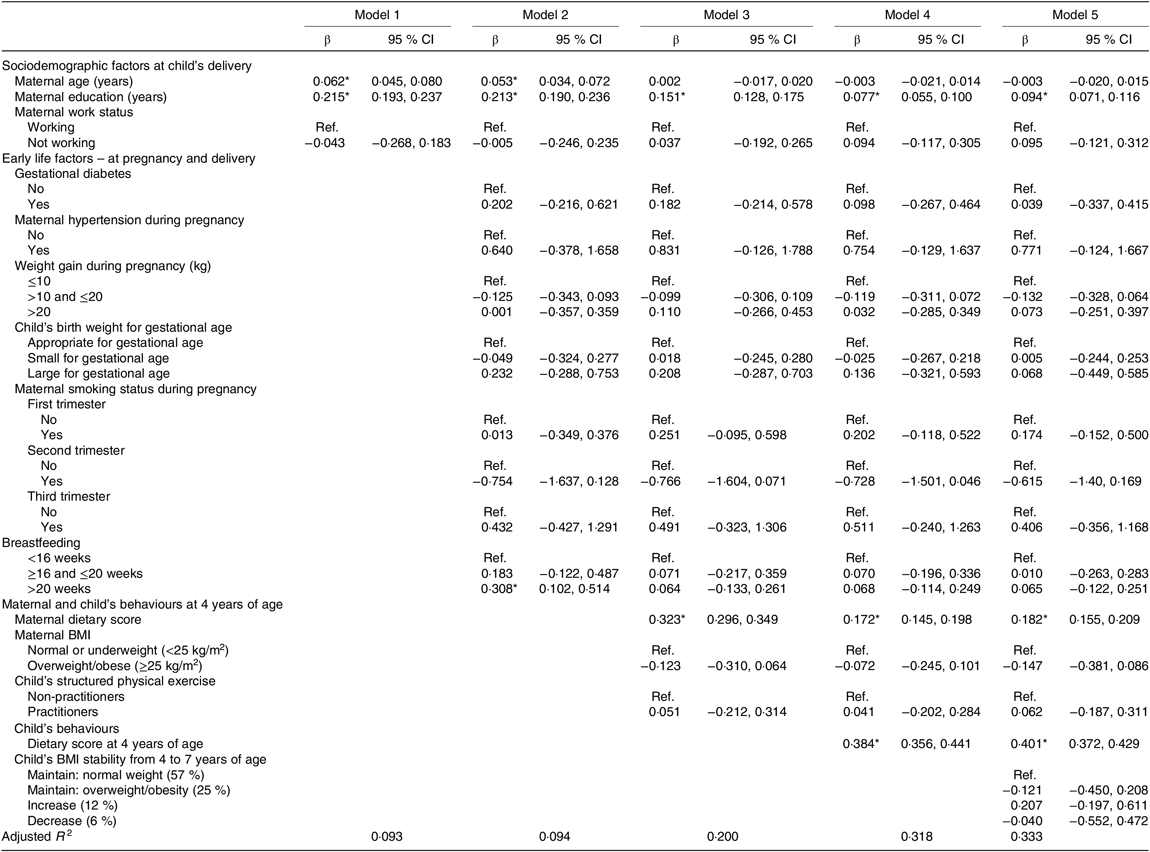
In the analysis presented in Table 3, the associations were considered by consecutive addition of blocks into the model. An increase of 1 year in maternal age and maternal education was associated with a 0·06- and 0·22-unit increase in the healthy eating index at 7 years, respectively (model 1). When maternal behaviours at the 4 years evaluation were added (model 3), maternal age ceased to be significantly associated with the healthy eating index score at 7 years. An increase of one unit in the maternal dietary score was associated with an increase of 0·32 units in the healthy eating index score at 7 years (model 3). A one-unit increase in healthy eating index at age 4 years was associated with a 0·38-unit increase in the healthy eating index at 7 years (model 3). Also, in this model, maternal education and maternal dietary score remained significantly associated with an increased score of the healthy eating index from 4 to 7 years of age. The addition of the child’s BMI stability in model 5 did not change significantly the previous associations. No association was found between early life characteristics (intra-uterine and infancy environment) and diet quality at 7 years of age.
Table 3. Associations between maternal and child characteristics and adherence to a healthy eating index at 7 years of age
(β-Coefficients and 95 % confidence intervals)
Ref., reference.
* Statistically significant associations.
† Blocks of variables (maternal sociodemographic characteristics; maternal and children characteristics during pregnancy and at delivery; maternal characteristics at 4-year evaluation; and children characteristics) were added sequentially into the analysis; models are adjusted for children’s sex. Model 5 also includes an interaction effect between child’s BMI stability and maternal BMI.
Comparison between models was performed through the adjusted R 2, which allows us to know the proportion of variation in the healthy eating index at 7 years that is explained by each model. Model 5 best explains this variation.
Discussion
The present study supports the hypothesis that food intake changes with the transition from pre-school to school years. These dietary modifications consisted mainly of an increase in the consumption of animal protein food sources (meat, fish and eggs), soft drinks and sweets, as well as a decrease in dairy products and cereals and potatoes consumption, which contributed to a lower score on the healthy eating index at 7 years.
The increased consumption of animal protein from 4 to 7 years is in accordance with previous studies that have shown that European adolescents greatly exceed the recommendations, mainly due to meat consumption(Reference Diethelm, Jankovic and Moreno45). The decrease in daily consumption of dairy products may be related to the substitution of these foods by SSB, since in the same period, and as reported in other studies, there has been an increase in SSB consumption(Reference Marshall, Eichenberger Gilmore and Broffitt46,Reference Vartanian, Schwartz and Brownell47) . Indeed, a previous systematic review and meta-analysis showed that increased SSB intake is associated with a lower consumption of milk and Ca, in both adults and children, although average effect sizes were small(Reference Vartanian, Schwartz and Brownell47). Even though the consumption of fruit and vegetables remained constant between 4 and 7 years of age, less than half of the children have an intake of at least five times per d of this food group. Consumption of foods high in sugar and energy (SSB and sweet foods) increased significantly between the two ages. Attention should be given to this trend in childhood, as previously reported from 2 to 4 years of age(35), since these food groups are associated with adverse health outcomes, namely a higher risk of obesity in children(Reference Gibson48).
Although the score on the healthy eating index decreased from preschool to school age, it was verified that the better the healthy eating index score at age 4 years, the better the healthy eating index score at age 7 years. This association might be sustained by the fact that the dietary pattern formed in the first years of life continues to strongly influence food intake, even if the child spends more time away from home. Another possibility is that although children are eating out-of-home more often, most of the food they consume is still consumed or prepared at home(Reference Singer, Moore and Garrahie49). The comparison between the healthy eating index score and child’s nutrients intake obtained through food diaries showed that the index was indeed evaluating a better diet, at both ages. Moreover, the comparison of tertiles of the healthy eating index score at 4 and 7 years of age showed that children in the highest tertile at age 4 years were most likely to remain in the highest tertile at age 7 years. This was true to a lesser extent for the children in the lowest tertile.
Maternal education and diet were independently associated with a better diet quality at 7 years. These results are in concordance with previous studies that showed that healthier eating habits among pre-school and school children have been associated with higher maternal education(Reference Northstone and Emmett2,Reference Aranceta, Perez-Rodrigo and Ribas22,Reference Rogers and Emmett27,Reference Bjelland, Brantsaeter and Haugen50–Reference North and Emmett52) . Lower maternal education increases the probability of a poorer food quality diet in children, as this sociodemographic condition probably limits the purchasing power and the ability to obtain adequate nutritional information(Reference Northstone and Emmett2). Plus, this association might be partly explained by the importance of parents as role models for children’s dietary intake. There is considerable evidence that children’s eating behaviours, including food preferences, are very similar to that of their parents(Reference Patrick and Nicklas53,Reference McLeod, Campbell and Hesketh54) . In concordance with previous reports, this study shows that maternal diet is one of the most important factors associated with the healthy eating index score at 7, with a better maternal dietary intake influencing the children’s diet positively(Reference Durao, Severo and Oliveira44,Reference Fisk, Crozier and Inskip55) . This suggests that particular efforts should be made towards nutritional education among less-educated mothers, encouraging them to adopt healthy eating habits(Reference Vereecken and Maes25,Reference Vereecken, Keukelier and Maes26,Reference McLeod, Campbell and Hesketh54) . Maternal work status may also influence dietary patterns in children, as was shown in a study in the UK(Reference Northstone and Emmett2). The same was not observed in our study, as future analysis will be required to fully understand the matter.
Excessive gestational weight gain during pregnancy and gestational diabetes were previously recognised as prenatal determinants of childhood obesity(Reference Olson, Strawderman and Dennison56,Reference Zhao, Liu and Qiao57) , which might be explained, in part, by the adoption of less healthy eating habits. However, we did not find any statistically significant association between these factors and the diet quality at 7 years. Moreover, maternal smoking status has been associated with children’s dietary intake(Reference Smithers, Brazionis and Golley29). Our results, however, are not in line with previous studies, which show an association between low scores in a healthy pattern and smoking during pregnancy(Reference Smithers, Brazionis and Golley29). A recent study combining information from four European cohorts (the Habeat Project) concluded that never or short duration of breastfeeding (but not the timing of complementary feeding) were associated with a less-healthy diet in early childhood, including a lower consumption of fruit and vegetables(Reference de Lauzon-Guillain, Jones and Oliveira13). However, in the present analysis, we did not find a significant association between breastfeeding and higher scores in the healthy eating index. This might be due to the reported longer breastfeeding duration time in Portugal, in comparison with other countries(Reference de Lauzon-Guillain, Jones and Oliveira13).
These results should be viewed in light of some limitations. The FFQ was answered by parents, in a face-to-face interview, who might not be aware of every food offered to children when they are with other caregivers or in school. However, in a subsample of children from the Generation XXI cohort, dietary information collected from 3-d food diaries was compared with dietary data from the FFQ, supporting the validity of the results, particularly for those foods that are more frequently consumed(Reference Vilela, Severo and Moreira31). Information about the duration of breastfeeding was collected only at the 4-year follow-up, which may represent a potential memory bias. In addition, for groups with minimum and maximum consumption, the proposed index is limited to assessing compliance with the WHO quantitative recommendations. Some parents might have also reported what, for them, the child is supposed to consume instead of what was actually consumed or report more healthy food and less unhealthy food. If this has occurred, our associations could be underestimated. The low correlation between the healthy eating index score and the nutrients’ intake might be a threat to the external validation of the index. Nevertheless, this might be partly explained by the nature of the collection method and the usually obtained low to medium correlations obtained between FFQ and food diaries(Reference Vilela, Severo and Moreira31).
The use of two methods to collect dietary intake information, food diaries and FFQ, can be considered a strength of this study, as it allows the reduction of report errors. The main strengths of the study are its longitudinal design, based on a relatively large sample size from participants of a population-based birth cohort regularly evaluated, with a high participation rate. This allows us to ensure a temporal sequence between several exposures and the tracking of children’s dietary intake. Only children with complete FFQ data were considered in our sample. The comparison of baseline characteristics between participants and non-participants showed that mothers included in our sample were slightly older and more educated. However, these differences do not seem to be very relevant (Cohen’s effect size values are not high: <0·40), and probably these differences are more likely to be due to large sample size rather than to substantial differences between participants. Finally, by performing the statistical analysis by models, we were able to easily identify the possible confounders of these associations.
Conclusion
This study suggests that promoting healthy eating habits at early ages is essential in order to prevent unfavourable eating habits later on. The quality of dietary intake decreases from preschool to school age and healthy eating habits established at 4 years influence healthy eating habits at 7 years. We could also verify that maternal sociodemographic and behavioural factors have a higher influence on the maintenance of healthy eating habits from 4 to 7 years, compared with metabolic or genetic factors. A higher maternal education and a healthier maternal diet increased the likelihood of children maintaining a healthy eating index score at school age. Interventions to promote healthy eating habits among preschool children should involve mothers as well. Greater focus should be given to those with lower education, with a particular emphasis on their diet and its consequences on children’s healthy eating habits.
Acknowledgements
The authors gratefully acknowledge the families enrolled in Generation XXI for their kindness, all members of the research team for their enthusiasm and perseverance and the participating hospitals and their staff for their help and support. The authors acknowledge the support from the Epidemiology Research Unit (EPI-Unit: UID-DTP/04750/2013).
Generation XXI was funded by the Health Operational Programme – Saúde XXI, Community Support Framework III and the Regional Department of Ministry of Health. It was supported by the Calouste Gulbenkian Foundation, by FEDER from the Operational Programme Factors of Competitiveness – COMPETE and through national funding from the Foundation for Science and Technology – FCT (Portuguese Ministry of Education and Science) under the project PTDC/SAU-EPI/121532/2010 (FEDER-Operational Programme Factors of Competitiveness – COMPETE – FCOMP-01-0124-FEDER-021177), and the PhD grant SFRH/BD/92389/2013 (S. V.). FCT had no role in the design, analysis or writing of this article.
The authors’ contributions are as follows: M. P. C. was responsible for the analysis and interpretation of the data and wrote the first draft of the paper; C. D., C. L. and S. V. were also responsible for the analysis and interpretation of the data. All authors contributed to the concept and design of the study and paper review.
There were no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519001028







