Pregnancy during adolescence in Jamaica and elsewhere is associated with a high prevalence of low birth weight( Reference Fraser, Brockert and Ward 1 , Reference Jackson, Eggleston and Lee 2 ). It has been proposed that this is due to an inability of the adolescent mother to provide the nutrient needs for her own growth and the growth of her fetus. In particular, the requirement for amino acids increases as pregnancy progresses in order to sustain increased rates of protein deposition( Reference Duggleby and Jackson 3 ) and to support increased availability of glucose through gluconeogenesis( Reference Kalhan, Rossi and Gruca 4 ).
Dispensable amino acids comprise the bulk of maternal amino acids transferred to the fetus in pregnancy( Reference Lemons, Adcock and Jones 5 ). A good example is glycine, which is a provider of methyl groups needed for the synthesis of DNA necessary for cell division to support maternal and fetal tissue deposition( Reference Lamers, Williamson and Gilbert 6 , Reference Lamers, Williamson and Theriaque 7 ). In addition, most of fetal cartilage is synthesised during late pregnancy and glycine constitutes 25 % of the amino acids in cartilage( Reference Meier, Teng and Battaglia 8 ). Therefore, as pregnancy progresses, there is a higher fetal demand for glycine. From our earlier study, glycine flux decreased by 39 % from the first trimester to the third trimester in pregnant adolescent girls( Reference Thame, Fletcher and Baker 9 ). Plasma glycine concentration in pregnant adolescent girls also decreased significantly from trimester 1 to 3, indicating a reduced availability. These findings suggested that after an overnight fast pregnant adolescent girls had a shortage in glycine supply in late pregnancy because they could not maintain production similar to their adult counterparts. However, de novo synthesis of glycine during pregnancy is not known. On the basis of evidence that most of de novo glycine synthesis is from serine in humans( Reference Gregory, Cuskelly and Shane 10 ), we hypothesised that unlike adult counterparts adolescent girls cannot maintain glycine flux in late pregnancy because of decreased synthesis from serine. The objective of the present study was to measure and compare glycine and serine kinetics of pregnant adolescent girls and adult women in the first and third trimesters. The present study is part of the phase 2 study of a larger study of amino acid metabolism in pregnancy( Reference Thame, Hsu and Gibson 11 ).
Methods
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving human subjects were approved by the Ethics Committee of the University of the West Indies and by the Institutional Review Board for Human Subject Research of Baylor College of Medicine & Affiliated Hospitals. Written informed consent was obtained from each study participant at recruitment.
In total, eleven pregnant adolescent girls, aged 16–17 years, and twelve pregnant adult women attending the antenatal clinic at the University Hospital of the West Indies were invited to participate in the study, and were enrolled consecutively. Women with chronic illnesses such as diabetes mellitus, hypertension, heart disease or a genetic abnormality such as sickle cell disease or women with multiple gestations were excluded. All the adolescents were studied at 13 and 29 weeks of gestation. The twelve adult women were studied at 13 weeks of gestation; however, only ten adults were studied at 29 weeks of gestation because one had a spontaneous abortion and the other had a premature delivery at 24 weeks and the baby did not survive. Data from these two adults were excluded from the analysis.
Maternal weight was measured at the time of each study to the nearest 0·01 kg using a Tanita digital scale (CMS Weighing Equipment Ltd). Maternal weight gain from the first to the third trimester was calculated. Maternal height was measured at the first study to the nearest 0·1 cm using a stadiometer (CMS Weighing Equipment Ltd). Gestational age was determined by the last menstrual period and confirmed by an ultrasound measurement performed at the time of the first experimental study. Birth weight was measured to the nearest 0·01 kg using a Tanita model 1583 digital baby scale (CMS Weighing Equipment Ltd); crown–heel length was measured to the nearest 0·1 cm using a Harpenden infantometer (CMS Weighing Equipment Ltd); and head circumference was measured using a fibre-glass tape measure.
Tracer infusion protocol
All the participants were studied after an 8-h overnight fast on two occasions: 13·1 (se 0·4) weeks of gestation (trimester 1) and 28·8 (se 0·4) weeks of gestation (trimester 3). Participants were admitted to the obstetric ward in the evening and were allowed to have their last meal of the day at 22.00 hours. After 8 h, an intravenous catheter (Sensecure, 18G; Morningside Pharmaceuticals Ltd) was inserted into the antecubital vein of one arm for the infusion of isotopes, whereas a second catheter was inserted in an anti-flow direction into the dorsal vein of the contralateral hand for drawing blood samples. The cannula was kept patent with intermittent infusions of heparinised saline.
Sterile solutions of 2,2-2H2-glycine and 15N-serine (Cambridge Isotope Laboratories) were prepared in isotonic saline. Baseline blood samples were collected before the start of the infusion protocol. Simultaneous primed constant infusions of 2H2-glycine and 15N-serine (prime=4 µmol/kg, infusion=4 µmol/kg per h, respectively) were initiated and maintained for 4 h. Further blood samples were collected at 10-min intervals during the last 30 min of the infusions. At the end of each of the tracer infusion periods, the catheters were removed, the subjects were provided lunch and discharged.
Laboratory analyses
Blood samples were drawn into pre-chilled tubes containing heparin, centrifuged at 4°C and the plasma was separated and stored at −70°C for later analysis.
The plasma glycine and serine isotopic enrichments were measured by liquid chromatography-tandem MS. In brief, plasma glycine and serine were converted into their 5-(dimethylamino)-1-napthalenesulphonamide derivatives and analysed using a Kinetex C18 2·6 µ 100×2·1 mm column (Phenomenex) on a triple quadrupole mass spectrometer (TSQ Vantage; Thermo Scientific), equipped with an heated-electrospray ionisation source, a Accela pump (Thermo Scientific) and a Thermal PAL autosampler (Thermo Scientific). The ions were then analysed in the selected reaction monitoring mode. The transitions observed were precursor ions m/z 309, 310 and 311 to product ion m/z 170 at 19 eV for glycine and precursor ion m/z 339, 340 to product ion m/z 170 at 20 eV for serine. Instrumental control, data acquisition and analysis were performed by Xcalibur (version 2.1) software package (Thermo Scientific).
The 2H enrichment of plasma serine derived from the conversion of 2H2-glycine was measured after the separation and subsequent pyrolysis utilising GC-thermal conversion-isotope ratio MS technique (GC-TC-IRMS; Thermo Scientific Delta + XL). In brief, the amino acids in the plasma samples were first separated by Dowex AG-50W resin columns (Bio-Rad Life Sciences Research) and esterified using propanol–HCl (3:1) by incubating at 70°C for 1 h. Subsequently, the esterified samples were acetylated by acetone–triethylamine–acetic anhydride (5:2:2) and incubated at 70°C for 20 min. The samples were then injected into the GC-TC-IRMS for separation followed by pyrolysis to H in order to determine 2H enrichment of plasma serine.
Calculations
Total glycine or serine flux (Q) was calculated as follows:
where IE inf is the isotopic enrichment of the infusate, IE pl is the plateau isotopic enrichment of either glycine or serine in plasma and I is the tracer infusion rate. Enrichment of 15N-serine was obtained as the difference between the measured m+1 serine enrichment and the enrichment of 2H-serine derived from the 2H2-glycine tracer.
Endogenous glycine or serine flux was obtained by subtracting the tracer infusion rate.
The rate of conversion of serine to glycine (Q Ser→Gly) was calculated as follows:
where Q Gly is the total glycine flux, IE Gly is the plateau 15N-glycine enrichment in plasma and IE Ser is the plateau 15N-serine enrichment in plasma.
The rate of conversion of glycine to serine (Q Gly→Ser) was calculated as follows:
where Q Ser is the total serine flux, IE Ser is the plateau 2H-serine enrichment in plasma and IE Gly is the plateau 2H2-glycine enrichment in plasma.
Statistical analysis
Data are expressed as mean values with their standard errors. Maternal characteristics and pregnancy outcomes of the two groups were assessed using the non-paired t test. The D’Agostino–Pearson test was used to check for normalcy of distribution of the study data. The Grubb’s method was used to remove outliers. Differences in amino acid variables between the groups were analysed by mixed-model (repeated-measure two-factor) ANOVA. This model included the two age groups (adult and adolescent) and time of pregnancy (first and third trimesters). Post hoc comparisons were performed using Sidak’s test. As each group had different body weights, total body glycine and serine fluxes were not compared among groups. Only within-group comparisons were made for trimester 1–3 using the paired t test. Tests were considered statistically significant if P<0·05. Pearson’s correlation (one-tailed) was performed on newborn length and maternal third trimester glycine flux. Data analyses were performed using GraphPad Prism version 6 software (GraphPad Software).
Results
The maternal characteristics and pregnancy outcomes of the subjects have been published previously( Reference Thame, Hsu and Gibson 11 ). However, for convenience, some of the relevant data are presented in Table 1. In brief, at 13 weeks of gestation, there were no significant differences in body weight or BMI between the groups, although the adolescents tended to be lighter with lower BMI compared with the adults. Similarly, there were no significant differences in body weight at 29 weeks of gestation and in weekly weight gain from week 13 to 29 of gestation. Gestational age at birth was significantly longer in the adolescent group (39·5 (se 0·3) weeks) compared with the adult group (38 (se 0·4) weeks; P<0·05), but the average length of their newborn babies was significantly shorter, with the crown–heel length being 47 cm compared with 49·5 cm (P<0·05).
Table 1 Maternal characteristics and pregnancy outcomes of study subjects (Mean values with their standard errors)
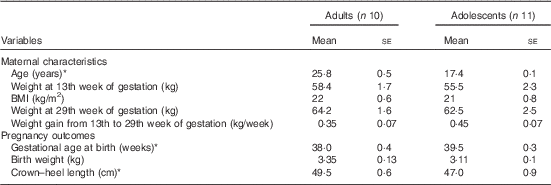
* Significantly different: P<0·05 (unpaired t test).
When glycine kinetic parameters were expressed per kilogram of body weight, there was a significant effect of trimester on endogenous glycine flux (Table 2). Endogenous glycine flux and glycine flux derived from serine were significantly slower in adolescent girls in trimester 3 than in trimester 1 (P<0·05). There was a significant interaction between age group and time of pregnancy (P<0·05) on glycine flux derived from serine (Fig. 1), as glycine flux derived from serine decreased significantly from trimester 1 to 3 in the adolescent group but not in the adult group. When the kinetic parameters were expressed per whole body, endogenous glycine flux and glycine flux derived from serine were not different from trimester 1 to 3 in both groups.

Fig. 1 Weight-specific glycine flux derived from serine in pregnant adult women (n 10, ![]() ) and adolescent girls (n 11,
) and adolescent girls (n 11, ![]() ) at 13 and 29 weeks of gestation. * Significant interaction between age group and time of pregnancy (P<0·05), as flux decreased significantly from trimester 1 to 3 in the adolescent group (repeated-measures two-factor ANOVA).
) at 13 and 29 weeks of gestation. * Significant interaction between age group and time of pregnancy (P<0·05), as flux decreased significantly from trimester 1 to 3 in the adolescent group (repeated-measures two-factor ANOVA).
Table 2 Glycine and serine kinetics in pregnant adolescent girls and adult women at 13 and 29 weeks of gestation (Mean values with their standard errors)

* Trimester 1=13·1 (se 0·4) weeks of gestation; trimester 3=28·8 (se 0·4) weeks of gestation.
† Significant effect of trimester: P<0·05 (repeated-measures two-factor ANOVA).
‡ Significantly different from corresponding trimester 1 value: P<0·05 (post hoc Sidak’s multiple comparison).
§ Significant interaction between age group and trimester: P<0·05 (repeated-measures two-factor ANOVA).
There was no significant effect of trimester or age group on endogenous serine flux and serine flux derived from glycine, when the kinetic parameters were expressed either per kilogram of body weight or per whole body (Table 2).
There was a positive association between newborn crown–heel length and endogenous glycine flux in trimester 3 in adolescent girls (P=0·05) but not in adult women (Fig. 2).

Fig. 2 Association between newborn length and third trimester (29 weeks of pregnancy) glycine flux in pregnant adult women (a) and adolescent girls (b). (a) Pearson’s r −0·26, P=0·24; (b) Pearson’s r 0·52, P=0·05.
Discussion
To test the hypothesis that adolescent girls cannot maintain glycine flux in late pregnancy because of decreased synthesis from serine, endogenous glycine and serine fluxes, glycine flux derived from serine and serine flux derived from glycine were measured in adult women and adolescent girls after an overnight fast at the end of the first trimester and at the beginning of the third trimester of pregnancy. The results show that in pregnant adolescents glycine and serine fluxes and conversion of serine to glycine were significantly slower at trimester 3 compared with trimester 1. These findings corroborate our earlier finding that glycine flux decreased in the adolescent group in trimester 3, suggesting that the pregnant adolescent cannot maintain glycine production in late pregnancy because of decreased de novo synthesis from serine( Reference Thame, Fletcher and Baker 9 ).
The findings of the present study have several important implications for fetal development. Glycine has several critical functions with respect to the development of the fetus, which includes being a primary source of one-carbon necessary for both synthesis and methylation of DNA and new proteins( Reference Lamers, Williamson and Theriaque 7 ). It is also in high demand to synthesise glycine-rich collagen in late gestation( Reference Meier, Teng and Battaglia 8 ). Therefore, its availability is critical for optimal fetal growth. Our results showed that in adolescent mothers glycine flux in trimester 3 had a positive association with the baby’s length. It is therefore possible that inability of the adolescent girl to maintain glycine synthesis makes her fetus vulnerable to impaired cartilage synthesis, and thus linear growth.
In addition, some studies in pregnant ewes have reported that there is an absence of serine transport from maternal circulation to the fetus( Reference Chung, Teng and Timmerman 12 – Reference Moores, Rietberg and Battaglia 14 ). Maternal serine is used within the utero-placental tissues to synthesise glycine, some of which is delivered into the fetal circulation and used by the fetus to resynthesise serine( Reference Moores, Rietberg and Battaglia 14 ). In human studies, a higher concentration of serine and glycine was observed in the fetal umbilical vein than in a simultaneously obtained maternal arterial sample at term gestation, suggesting increased de novo synthesis by utero-placental tissues( Reference Cetin, Hirst and Corbetta 15 , Reference Hayashi, Sanada and Sagawa 16 ). In our present study, the percentage of serine flux derived from glycine (3–5 %) was much less compared with the percentage of glycine flux derived from serine (37–42 %), suggesting that maternal glycine supply was paramount and was being sustained to a large extent by de novo synthesis from serine. Therefore, a contributing factor to the slower glycine flux in adolescent girls in late pregnancy is decreased synthesis from serine. This is supported by the 23 % reduction in serine-derived glycine from the first to the third trimester in the pregnant adolescents.
An obvious interpretation of these findings is that there is an overall shortage in the supply of serine to support glycine synthesis. In the present study, although serine flux tended to be lower by 17 % in trimester 3 in the adolescent group, the change was not statistically significant. Serine flux in the adult group in trimester 3 was not different from trimester 1, but it was slightly lower (approximately 6 %). However, in a study by Kalhan et al. ( Reference Kalhan, Gruca and Parimi 17 ), they reported that serine flux in adult women was significantly lower in late pregnancy compared with early pregnancy. The authors suggested that the lower serine kinetics in late pregnancy may be part of a general down-regulation of α-amino N turnover related to a decreased rate of branched-chain amino acid transamination to facilitate N conservation and accretion. In agreement, in an earlier report of data from this project( Reference Thame, Hsu and Gibson 11 ), urea flux, an index of protein and amino acid catabolism, decreased from the first to the third trimester in both pregnant adolescent girls and adult women, indicating that they were conserving more N in the third trimester. However, leucine oxidation in the third trimester was similar to the first trimester, which does not support a decrease in branched-chain amino acid transamination in late pregnancy. Therefore, the lower serine kinetics in late pregnancy was not because of down-regulation of α-amino N turnover in branched-chain amino acid transamination as suggested by Kalhan et al. ( Reference Kalhan, Gruca and Parimi 17 ).
In summary, pregnant adolescents cannot maintain glycine flux in late pregnancy compared with early pregnancy. One factor contributing to the slower glycine flux is decreased synthesis from serine. It is possible that inability of the adolescent girl to maintain glycine synthesis makes her fetus vulnerable to impaired cartilage synthesis, and thus linear growth.
Acknowledgements
The authors are grateful to the nursing staff of the Obstetrics ward at the University Hospital of the West Indies for their care of the subjects.
This research was supported with federal funds from the US Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6001 and funds from the International Atomic Energy Agency.
All the authors contributed to different aspects of the study, including the design of the study, data collection, sample analysis, data interpretation and writing of the manuscript as follows: F. J., M. M. T. and A. A. J. designed and supervised various aspects of the study; M. M. T., R. G. and T. M. B. recruited the participants, conducted the experiments, processed samples and took care of the subjects; J. W. H., G. J. T. and S. K. C analysed samples and calculated data; and M. M. T., A. A. J., F. J. and J. W. H. analysed and interpreted the data and wrote the manuscript.
None of the authors has any conflicts of interest with the funding agencies.







