INTRODUCTION
Annually, over 4 million patients within the 27 European Union member states acquire a nosocomial infection, of whom approximately 37 000 will die as a result [1]. Surgical site infections (SSIs) are the third most common form of healthcare-associated infection (HAI), behind urinary tract (27%) and lower respiratory tract (24%) infections, comprising 17% of cases [1]. The economic impact of SSIs is considerable, with an estimated burden on European healthcare providers of €7 billion in 2008 [2]. Costs are driven predominantly by increased length of hospital stay, estimated to be an average 9·8-day increase compared to patients not experiencing a SSI [Reference Leaper3].
In France, surveillance of HAIs has been in place since the early 1990s. It has been estimated that the crude incidence of SSIs in France, between 1996 and 2006, was 1·54% in patients undergoing either elective or emergency surgery [Reference Astagneau4]. The incidence varied depending on the surgical intervention, from 0·49% for knee prosthesis to 9·24% for colon surgery [Reference Daniel and L'Hériteau5]. Despite an increase in the number of patients undergoing surgical procedures, in particular patients deemed to be at high risk of SSI, such as elderly patients, analysis of temporal trends has shown that the incidence of nosocomial infections in France has decreased in recent years [Reference Daniel and L'Hériteau5–Reference Couris7]. The reduction in SSI incidence has been attributed, in part, to the successful introduction of active surveillance programmes such as the Infection du site Opératoire – Réseau Alerte Investigation Surveillance des Infections (ISO-RAISIN) and Incidence des Infections du Site Opératoire (INCISO), and the utilization of infection control strategies that have accompanied surveillance [Reference Astagneau4, Reference Rioux, Grandbastien and Astagneau8]. The majority of SSIs are preventable; with a recent USA-based study finding that up to 55% of cases could be avoided [Reference Umscheid9].
ISO-RAISIN data from 2007 showed that the most common infectious causative agents of SSIs were Escherichia coli (25%), Staphylococcus aureus (19%) and Pseudomonas aeruginosa (10%) [10]. Of the identified strains, 10% of E. coli isolates were cefotaxime- or ceftriaxone-resistant, 19% of S. aureus were methicillin-resistant and 25% of P. aeruginosa were ceftazidime-resistant. The frequency of infection by resistant strains of bacteria significantly adds to the burden of SSIs as more expensive second-line treatments become necessary.
Published data describing the total economic burden of nosocomial infections and SSIs in France are currently lacking. A recent single-centre study found that the mean additional cost per patient experiencing a SSI, taking into account laboratory tests, radiology, surgery, exploratory examinations and antimicrobial agents, was €1814 [Reference Defez11]. The aim of the present study was to estimate the healthcare burden associated with SSIs, both nationally and by region, in France and to explore the potential impact of infection control strategies on the overall clinical and economic burden.
METHODS
Identification of surgical procedures and incidence of HAI
The frequency of 12 preselected surgical procedure categories (Table 1) performed in France between 1 January 2010 and 31 December 2010 was quantified through searching of the Programme de Médicalisation des Systèmes d'Information (PMSI) using 507 appropriate Classification Commune des Actes Medicaux (CCAM) procedure codes, selected from about 8000 codes used to index hospital procedures in France. The 12 procedure categories were chosen to provide an overview of SSIs in France, but do not encompass all surgical procedures performed in 2010, and therefore the total incidence is underestimated. PMSI is a comprehensive database of hospital visits in France since all hospitals, both public and private, are legally obliged to report activity for collation, otherwise remuneration for the procedure performed is withheld by the local health authority [12, 13]. It is recommended as an epidemiological tool by the French guidelines for health economic evaluation [14]. As well as detailing the procedure performed using CCAM codes, the database contains details of length of hospital stay, patients’ details (such as age) and an anonymous patient identifier to allow future hospitalizations to be followed.
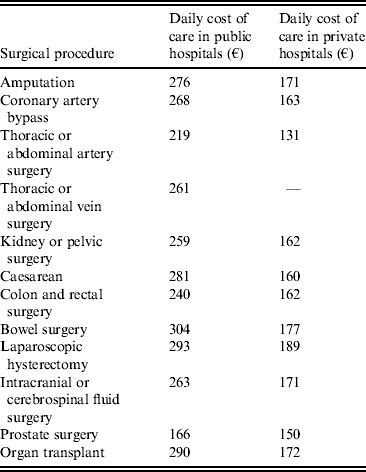
Table 1. Daily cost of hospital care following healthcare-associated infection by surgical procedure, in public and private hospitals

€ = 2009 euros.
Having identified patients undergoing surgical procedures in France, the incidence of SSIs was examined by inspection of patients’ history subsequent to surgery for the Classification Statistique Internationale des Maladies et des Problèmes de Santé Connexes (ICD-10) codes relating to infection, namely T814 (Infection following a therapeutic or diagnostic procedure, not classified elsewhere), L02 (Cutaneous abscess, furuncle, carbuncle), and Y95 (Nosocomial infection). Since these codes are not specific to infection of the surgical site, data shown is for HAI incidence following surgery.
Estimation of daily cost of care
Daily costs of care were calculated for both public and private hospitals based on resource use and cost data from the 2009 Echelle Nationale de Coûts à Méthodologie Commune (ENCC). The ENCC provides costs of care, taking into account costs of a range of factors, including medical professionals, drug costs, and running costs of the medical unit (e.g. cleaning and laundry). It is possible to evaluate daily physician costs for public hospitals, but this is not the case for private hospitals since physicians are reimbursed per CCAM procedure performed rather than per day of care administered, and therefore physician costs are captured in public, but not private, hospitals. In this analysis only costs allocated to the days of stay following surgical procedures were included. The mean daily cost of care, in 2009 euros (€), following each category of surgical procedure is summarized in Table 1.
Health economic model
Having collected data on the length of stay in patients with and without HAI, a health economic model was constructed to evaluate cost differences between the infection control scenarios. A bespoke model was coded, with SQLite and Adobe Air used to manage the database and present the results. A 1-year time horizon was used with outcomes evaluated from a hospital perspective. In this model HAI incidence could be varied to examine the clinical and economic impact of infection control interventions used. Model inputs were length of stay associated with each procedure with and without HAI, daily cost of hospital care following each procedure, and HAI incidence following each procedure. The model reported outputs of number of hospital stays, number of hospital days of care, and cost of care.
As well as examining the burden at current rates of infection, three scenario analyses were performed in which HAI incidence was varied. In the first scenario HAI incidence was reduced by 8%. This was based on a recent study of the use of triclosan antibacterial sutures, which found that SSI incidence was reduced by 8% (7% vs. 15%) compared to the control arm in which conventional sutures were used [Reference Gala and El-Hindawy15]. To examine the impact of less focused approaches that could be used to reduce SSIs, scenarios with 20% and 30% reduction in incidence were also analysed. These were based on the upper and lower limits of an estimate of the number of preventable nosocomial infections, made by the European Centre for Disease Prevention and Control [1].
Statistical analysis
Descriptive statistics were used to summarize the clinical and economic data from the analysis. Statistical analysis was conducted to examine differences between clinical outcomes in patients experiencing HAI and those not experiencing HAI, based on the data collected from PMSI. Hypothesis testing of the difference between normally distributed variables was performed using χ2 tests, while Mann–Whitney U tests were used for non-normally distributed variables. Normality tests, to determine whether the data were well modelled by a normal distribution, were performed using Kolmogorov–Smirnov non-parametric tests.
RESULTS
Incidence of SSI
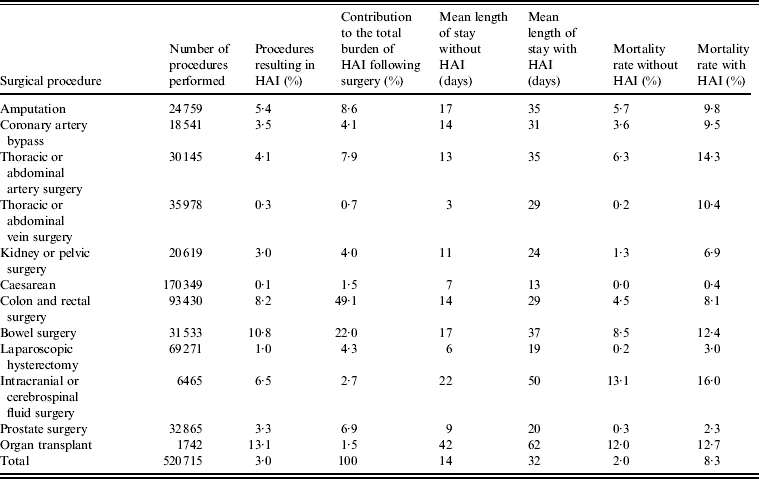
The PMSI data showed that of 520 715 selected surgical procedures performed between 1 January 2010 and 31 December 2010, 3·0% resulted in infection (Table 2). Frequency of HAI was correlated with age, with 43·9% of cases occurring in patients aged ⩾70 years, while only 15·1% of cases involved patients aged <50 years (Fig. 1). The frequency of HAI also varied with the type of surgery performed (Table 3). Of the selected surgical procedures, the group with the highest incidence of HAI was organ transplant, with 13·1% of surgeries resulting in HAI. Caesarean section had the lowest incidence of HAI, with only 0·1% of procedures being followed by infection. The clinical burden of HAI was greatest following colon and bowel surgery, comprising 49·1% and 22·0%, respectively, of the total HAI cases. Incidence also varied by region (Fig. 2). It was found that the highest incidence of infection was in Martinique (5·1%) and the lowest in French Guiana (0·7%). Of the mainland French regions, the highest incidence was found in Languedoc-Roussillon (4·2%) and the lowest incidence in Alsace (2·2%). However, when corrected for case mix it was found that incidence rates were similar across regions. This suggests that differences found between regions were due to the number of high-risk procedures performed, rather than other factors influencing HAI incidence. The incidence rates in public and private hospitals showed minimal variation, with 2·9% and 3·1% of procedures resulting in HAI, respectively. Incidence rates by procedure were also found to be comparable for public and private hospitals.

Fig. 1. Distribution of hospital stays by age. HAI, Healthcare-associated infection.

Fig. 2. Incidence of healthcare-associated infection (HAI) following surgery in the 27 regions of France.
Table 2. Summary of episodes of hospital care included in the analysis (with and without HAI)

HAI, healthcare-associated infection.
Table 3. Burden of HAI by procedure

HAI, Healthcare-associated infection.
Some procedures were classified as more than one type of surgery. Therefore the total number of procedures is not the sum of the individual procedures.
Clinical burden of SSI
HAI was associated with a statistically significant increase in mortality. The mortality rate in patients experiencing a HAI was 8·3%, compared to 2·0% in patients who did not (P < 0·001), corresponding to a 4·15-fold relative increase (Table 3). The largest increase in mortality rate was seen in the thoracic or abdominal vein and thoracic or abdominal artery surgery groups, where mortality rates increased over 50-fold, from 0·2% to 10·4%, and 2·3-fold, from 6·3% to 14·3%, respectively. Mortality rates did not vary in public and private hospitals. Length of hospital stay was found to be non-normally distributed (Kolmogorov–Smirnov D = 0·253, P < 0·01). Patients experiencing HAI had a threefold longer hospital stay than those who did not, with median length of stay increasing from 7 days to 22 days (P < 0·001). When mean length of hospital stay following each procedure was evaluated, it was found that HAI was associated with at least a twofold longer stay following all procedures (Table 3). Of particular note was the almost tenfold increase following thoracic or abdominal vein surgery, with mean length of stay increasing from 3 days to 29 days following the procedure if HAI occurred.
Cost of SSI
The health economic modelling analysis suggested that in 2010, HAIs led to an additional cost to public and private hospitals of €43 019 936 and €14 872 779, respectively (Table 4). This difference was driven not by the rate of infection, since incidence rates were found to be comparable, but by the increased number of procedures performed in public hospitals and the increased daily cost of care. A total of 86 861 more procedures were performed in public hospitals, resulting in 1856 further HAIs. The daily cost of care was found to be significantly higher in public hospitals for all procedures, ranging from €122 per day increase for care following intracranial surgery (€293 vs. €171) to €88 per day increase following thoracic and abdominal artery surgery (€219 vs. €131). This was, at least in part, driven by the exclusion of physicians’ costs in the private sector.
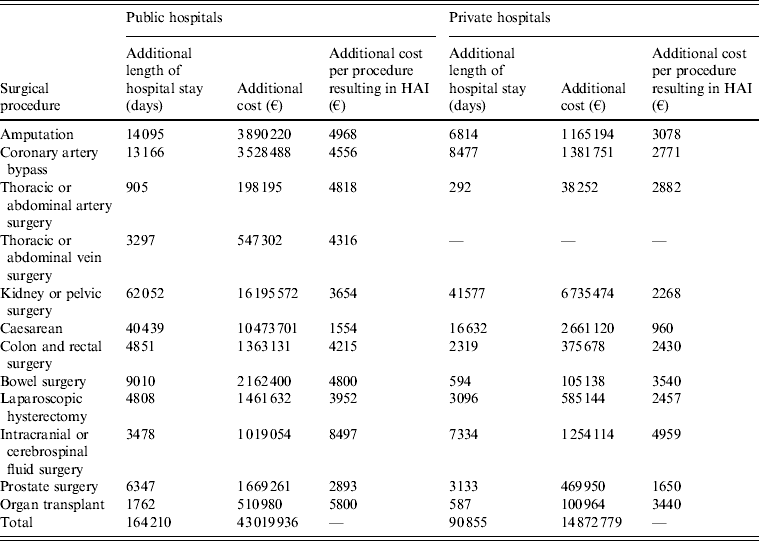
Table 4. Additional length of hospital stay and additional costs associated with healthcare-associated infection following surgery for public and private hospitals in France

HAI, Healthcare-associated infection.
€ = 2009 euros.
The largest cost burden was that of HAI following kidney or pelvic surgery, costing public hospitals €16 195 572 per year and private hospitals €6 735 474 per year. An increased number of patients experienced HAI in public hospitals, despite similar rates of infection (∼3% in both), due to the increased number of procedures performed in public hospitals (12 365 in public hospitals vs. 8283 in private hospitals). Furthermore, daily costs of care following kidney or pelvic surgery were €99 higher in public hospitals than in private hospitals. Expenditure following kidney or pelvic surgery equated to 38% and 45%, in public and private hospitals, respectively, of additional expenditure as a result of HAI. HAIs had a particularly large cost burden following caesarean (€13 134 821), amputation (€5 055 414) and coronary artery surgery (€4 910 239), comprising 40% of additional expenditure, across public and private hospitals, between these three procedures.
Exploratory infection control scenarios
An 8% reduction in HAI incidence, based on a recent study into the reduction in SSIs associated with the use of triclosan antibacterial sutures [Reference Gala and El-Hindawy15] was predicted to result in a saving of 20 205 hospital days per year, across both public and private hospitals, resulting in annual cost savings of €3 406 520 in public hospitals and €1 178 999 in private hospitals. The bulk of the cost savings came from reduced cost of HAI following kidney or pelvic surgery, with public hospitals saving €1 294 299 per year and private hospitals saving €537 516 per year, 40% of total savings (Figs 3, 4).

Fig. 3. Cost saving by reducing the incidence of healthcare-associated infections (HAIs) following surgery by 8%, 20% and 30% in public hospitals. € = 2009 euros.

Fig. 4. Cost saving by reducing the incidence of healthcare-associated infections (HAIs) following surgery by 8%, 20% and 30% in private hospitals. € = 2009 euros.
The analysis in which a 20% reduction in HAI incidence was applied, based on the lower limit of an estimate of the number of preventable nosocomial infections by the European Centre for Disease Prevention and Control [1] suggested that 3185 HAI cases could be avoided annually, resulting in 50 871 hospital days saved. This was made up of 32 758 days in public hospitals and 18 113 days in private hospitals. Cost savings of €8 583 202 per year and €2 964 855 per year could be made in public and private hospitals, respectively (Figs 3, 4). Healthcare spending as a result of HAIs was reduced from €57 892 715 to €46 344 658.
When a 30% reduction in HAI incidence was applied, based on the upper limit of a European Centre for Disease Prevention and Control estimate of the proportion of nosocomial infections that could be prevented [1], analysis suggested that 76 373 hospital days per year could be saved as a result of 4781 HAI cases being avoided. Cost savings of €12 880 036 per year could be made in public hospitals, and €4 454 660 per year in private hospitals (Figs 3, 4). This saving of €17 334 696 represents a 30% reduction in healthcare spending as a result of HAIs.
DISCUSSION
The present study has quantified part of the considerable clinical and economic burden of SSIs on the French healthcare system, where about 3% of the included surgical procedures resulted in infection. The incidence of HAI following surgery increased with patients’ age and varied depending on the surgical procedure performed. Included HAIs led to a 4·15-fold increase in post-surgery mortality, a threefold increase in length of hospital stay and an annual cost burden of €57 892 715. The largest costs were seen in patients experiencing HAI after kidney or pelvic surgery. The current analysis underestimates the total burden of SSIs on the French health economy for two reasons. First, only a predefined set of surgical procedures were included in the analysis, not all the surgical procedures performed in France in 2010. Furthermore, the ICD-10 codes used captured only infections reported during the initial hospital stay. Since not all SSI cases were identified in this analysis, the clinical and economic estimates are conservative and the burden to the French health economy is expected to be greater. Scenario analysis suggests that reduction in HAI incidence by 8%, 20% and 30% will result in savings of €4 588 519, €11 548 057 and €17 334 696, respectively. These are substantial cost savings, made predominantly in public hospitals due to the increased number of surgical procedures performed and the increased daily cost of care. The frequency of surgical procedures involving patients at high risk of SSI is increasing, and therefore the use of infection control strategies is likely to become increasingly important in the future.
One of the major strengths of the present study is the robust epidemiological data on which the health economic model is based. PMSI provides a detailed account of hospital stays and allows the number of procedures and treatments performed in a given time-frame to be accurately determined. Furthermore, the age of the patient and the post-operative clinical consequences can be accurately assessed using the anonymous patient identifier. In this analysis, 507 CCAM codes relating to preselected surgical procedures were used to identify treatments of interest, giving a comprehensive list of procedures performed, while the use of ICD-10 codes relating to HAI allows accurate quantification of HAI incidence following surgery during the initial hospital stay. The bespoke health economic model developed for this analysis, based on these data, may be a valuable tool for future economic evaluations of infection control strategies and interventions in the French setting.
An assumption of this analysis is that the daily cost of care following surgical procedures is the same in patients experiencing HAI and those not experiencing HAI. It may be the case that patients with HAI would require not only a longer period of care, taken into account through the increased length of hospital stay, but also require more intense and expensive care during hospitalization. This may be through increased volume of diagnostic tests to identify the causative pathogen, intensified antimicrobial treatments (including more expensive second-line therapy if the causative agent is resistant to first-line treatments), and increased nursing staff time. Given this conservative assumption, cost savings reducing SSI incidence may be greater than those suggested in this analysis.
An important limitation of the present analysis is that it does not capture the costs of achieving 8%, 20% and 30% reductions in HAI incidence following surgery. However, the analysis does show the threshold at which an intervention to achieve this reduction will be cost saving. For example, if an infection control strategy resulting in an 8% reduction in HAI could be introduced in public hospitals at an annual cost of less than €3 400 000, healthcare providers would have reduced expenditure. While including the costs of interventions to achieve reductions in HAI following surgery was beyond the scope of the present analysis, the potential for significant cost savings as a result of interventions that reduce HAI incidence following surgery is worthy of further investigation.
The risk of SSI is dependent on the type and virulence of the infecting bacteria, and the immunological status of the host. Patients’ characteristics and comorbidities play a key role in determining the likelihood of SSI. Factors increasing the susceptibility of patients to SSI include advanced age, poor nutritional status, diabetes, smoking, obesity, infection remote to the surgical site and lengthy preoperative hospitalization. Due to the increased resource use and cost of hospital care for patients experiencing a SSI, infection control strategies to reduce the incidence are important, particularly in patients at the highest risk.
While it is acknowledged that not all SSIs can be prevented, evidence suggests that the current level of incidence can be reduced [1–Reference Leaper3]. The majority of SSIs occur at superficial sites around foreign material left in the wound [Reference Katz, Izhar and Mirelman16]. One potential avenue for improving infection control is in the choice of sutures, which are known to provide a focal point for bacterial colonization, act as a vehicle to transport bacteria into the wound and afford a repository for bacteria in the suture knot. Use of triclosan-coated sutures reduces SSIs by preventing bacterial colonization of the wound around the suture, due to the presence of an antimicrobial. Triclosan is a broad-spectrum antimicrobial agent, possessing mostly antibacterial, but also some antifungal and antiviral properties. Use of triclosan-coated sutures has been shown to reduce SSI incidence by 8% (7% vs. 15%) in a comparison with conventional sutures [Reference Gala and El-Hindawy15]. Examination of this benefit in the present scenario analysis suggests that this reduction in SSI incidence may have a substantial impact on both the clinical and economic burden, reducing the number of hospital stays, length of stay and cost of care by as much as €4 588 519 per year. Further economic evaluation of the benefit of introduction of infection control interventions such as triclosan antibacterial sutures in the French setting may be warranted based on these observations.
The World Health Organization and the Centre for Disease Control and Prevention have issued guidelines detailing pre-, peri- and post-operative infection control actions that can be used to reduce the incidence of SSIs [17, Reference Mangram18]. Preoperatively patients should be treated to resolve infections remote from the surgical site and appropriate disinfection of the skin at the surgical site should take place, with shaving only performed if absolutely necessary [17, Reference Mangram18]. If the patient is considered at high risk of SSI, a prophylactic dose of an appropriate antimicrobial should be administered, ideally intravenously, at a time to establish a bactericidal concentration at the operational site [17, Reference Mangram18]. Surgical team members should follow good practice guidelines for hand washing and preoperative aseptic procedures [17, Reference Mangram18]. During the surgical procedure, surgical team members should use surgical masks, caps, gowns, gloves and drapes and replace these items when they become soiled [17, Reference Mangram18]. The operating theatre should be cleaned using a hospital-approved disinfectant and good practice used to maintain sterility of surgical instruments [17, Reference Mangram18]. Air within the operating theatre should be changed at least 15 times per hour, with three of these being with fresh air, and filtering should take place using an appropriate filter [17, Reference Mangram18]. In the post-operative period, sterile dressing should be used and sterile techniques should be adhered to during dressing changes [17, Reference Mangram18]. Patients should undergo post-operative surveillance, including post-discharge if required, with any SSI assessed and reported appropriately [17, Reference Mangram18]. SSI rates should then be conveyed to surgical team members [17, Reference Mangram18]. All of these steps offer opportunities to reduce SSI incidence. Some estimates have suggested that up to 55% of SSIs can be prevented [Reference Daniel and L'Hériteau5]. The present study used conservative estimates of an overall reduction in incidence of 20–30% in SSIs. The analyses suggest that substantial reductions in healthcare expenditure could occur if SSI incidence is reduced. With greater decreases in SSI incidence larger cost savings can be expected, as the required number of hospital days of care will be reduced beyond the levels estimated in this study.
The present study has quantified the substantial economic burden that SSIs following a limited selection of procedures place on the healthcare system in France to be €57892715 per year. However, our analysis was conservative and the total burden is expected to be greater, both clinically and economically. New infection control interventions which reduce SSI incidence have the potential to lessen the clinical and economic burden, providing valuable cost savings to healthcare providers and should be the subject of future health economic evaluations.
DECLARATION OF INTEREST
Ludovic Lamarsalle is an employee of HEVA, and Barnaby Hunt and William Valentine are employees of Ossian Health Economics and Communications. These institutions received unrestricted funding from Ethicon, EMEA to perform the present study. Marion Schauf and Karine Szwarcensztein are employees of Johnson and Johnson.