Free radical species can be endogenously or exogenously produced(Reference Rice-Evans, Diplock, Symons, Burdon and Knippenberg1). A major target of free radicals is the cell membrane, which is involved in the control of cell function through integral maintenance of the spatial and intermolecular arrangements of its components. Because of their susceptibility to oxidation, erythrocytes have been used as a model to investigate oxidative damage in biomembranes(Reference López-Revuelta, Sánchez-Gallego and Hernández-Hernández2, Reference Li, Qian and Ryu3).
There have been reports of oxidant–antioxidant imbalances and increased free radical activity(Reference Babu, Kumar and Chandra4, Reference EI-Rashidy, AI-Turk and Stohs5), which may damage cell membranes resulting changes in membrane fluidity and cell function(Reference Miguel, Miguel and Linares6). Pathological processes in ageing have been associated with free radical-induced damage(Reference Southorn and Powis7). Elderly people are subject to a decrease in antioxidant defences (25 %) and higher levels of oxidative stress, which causes an increase in plasma hydroperoxide levels (18 %)(Reference Goi, Cazzola and Tringali8). A variety of antioxidant systems exist in vivo to remove free radicals and limit tissue damage. Supplementation with antioxidants, such as vitamin A(Reference Alpsoy, Yildirim and Agar9), ascorbic acid or Se, reduces oxidative stress and enhances total antioxidant status(Reference Seyedrezazadeh, Ostadrahimi and Mahboob10, Reference Mayne11).
Many in vitro and in vivo studies have shown that several parameters related to erythrocyte function and integrity are negatively affected by increased oxidative stress. Data reported in the literature(Reference Brosche and Platt12, Reference Prisco, Rogasi and Paniccia13) concerning membrane characteristics in the elderly are often conflicting, due to the difficulty in examining oxidative stress damage independently of its frequently associated pathologies. Thus, the mechanisms underlying these phenomena are not yet understood, and little information is available about erythrocyte membrane modification resulting from oxidative stress and ageing and about membrane fluidity in children, young and elderly people. Data have been reported(Reference Knight14–Reference Davydov, Dobaeva and Bozhkov16), indicating that senescence is associated with an increase in both oxidant generation rates and in the susceptibility of tissues to oxidative damage during ageing. Therefore, the objectives of the present study were to investigate whether there are any differences in erythrocyte membrane fluidity (EMF) between individuals of various ages and to describe any changes in antioxidant capacity and membrane fluidity in children, young and elderly people after supplementation with multiple micronutrients. We carried out the study in a rural population, where poor nutrition was expected to contribute to lack of micronutrients and increased oxidative stress, and where we could expect to observe bigger changes in the measured variables after supplementation. Nevertheless, the present results will be helpful for improving our understanding whether micronutrient supplementation is effective across all populations in decreasing oxidative stress and improving EMF.
Subjects and methods
Subjects
A total of 255 healthy persons including seventy-four children (aged 8–10 years) from a primary school, ninety young people (aged 18–22 years) from a college and ninety-one elderly (aged 60–75 years) from a community were recruited. A total of seventy-four children were randomly divided into A1 and A2 groups; group A1 (n 36) was the placebo-controlled group and group A2 was supplemented daily with 600 μg of retinol (as retinyl palmitate), 1·0 mg of β-carotene, 100 mg of tocopherol (as dl-α-tocopherol acetate), 300 mg of ascorbic acid and 200 μg of Se (as sodium selenite). A total of ninety young people were randomly divided into B1 and B2 groups, and ninety-one elderly subjects were divided into C1 and C2 groups. Groups B1 (n 45) and C1 (n 48) were placebo-controlled groups, and groups B2 (n 45) and C2 (n 43) were supplemented with 900 μg of retinol, 1·5 mg of β-carotene, 200 mg of tocopherol, 500 mg of ascorbic acid and 400 μg of Se. The capsules used in these treatment groups were labelled and manufactured by Hurun Company, Ypsilanti, MI, USA (a Chinese food additive company in Beijing). The trial participants and the research team were unaware of the treatment assignment. The trial was deblinded after analysis of its primary outcomes. The characteristics of the subjects are shown in Table 1.
Table 1 General characteristics and indicators in children, young and elderly people
(Mean values and standard deviations)
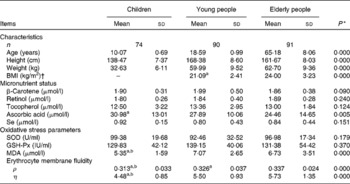
SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; ρ, polarisation; η, microviscosity.
a,b Mean values with unlike superscript letters were significantly different between elderly people and young people.
* ANOVA.
† BMI of children could not be calculated because the formula is not suitable for children.
Potential study subjects were informed about the study content and design via a formal presentation and an individual interview. Written, informed consent was obtained from the adult participants and the parents of child subjects before entering the trial. The subjects underwent a clinical examination to determine their suitability for participation in the study. Exclusion criteria were severe or chronic illness; smoking; a history of gastrointestinal surgery or chronic bowel disease; a current metabolic illness, such as diabetes mellitus, untreated hyper- or hypothyroidism or a severe lipid metabolism disorder; and regular intake of nutritional supplements (i.e. vitamin/mineral supplements or micronutrient-enriched foods), unless discontinued for at least 4 weeks before entry into the study and throughout the investigation. After ascertainment of eligibility, consenting subjects were enrolled in the study, subjected to a baseline interview and were given their allocated supplements once a week, which were to be taken daily for a period of 2 months. The subjects were examined or visited once each week by teachers or co-investigators trained to replenish supplements and to monitor compliance by counting and recording the number of supplements that were taken.
The study was approved by the ethical review committees of the Medical College of Qingdao University, Qingdao, China (QY-ETC20031120). Written consent was given by each subject at the beginning of the trial.
Blood sample treatment
Blood samples were taken from all subjects at baseline and at the end of the trial and processed immediately for storage. Specimens were stored in polypropylene vials at − 80°C for up to 3 months before batch analysis. It has been determined that vitamins C, retinol, β-carotene, vitamin E and Se are stable under these storage conditions(Reference Comstock, Norkus and Hoffman17).
Micronutrient concentrations in plasma
Retinol, β-carotene and tocopherols were determined in plasma samples according to the method of Hess et al. (Reference Hess, Keller and Oberlin18). Analyses were performed using HPLC equipped with an LC-10AD pump. A standard curve for each analyte was constructed from authentic standards (Sigma, St Louis, MO, USA). Standard concentrations were calculated on the basis of their known extinction coefficients. Total ascorbic acid concentrations in the subjects' plasma were determined spectrophotometrically, using the 2,4-dinitrophenylhydrazine method. Ascorbic acid was converted to dehydroascorbic acid in the presence of thiourea and CuSO4(Reference Pressman, Cavanaugh and Mingione19, 20). Plasma Se levels were measured using a standardised fluorometric method(Reference Koh and Benson21).
The activities of superoxide dismutase and glutathione peroxidase (GSH-Px) were determined in plasma(Reference Durak, Canbolat and Kavutcu22) as U/ml and IU/ml, respectively. The malondialdehyde (MDA) concentration was determined using the thiobarbituric acid reaction. MDA, an end product of fatty acid peroxidation, reacts with thiobarbituric acid to form a coloured complex, and the concentrations of this complex were calculated by comparing the absorbance values of the samples with those of standard MDA solutions. Results are expressed as nmol/ml plasma(Reference Avci, Atli and Ergüder23).
Preparation of erythrocyte membranes
Fresh erythrocytes were washed three times with isotonic saline (0·15 m-NaCl, 10 mm-Tris–HCl, pH 7·4), and the buffy coat and plasma were removed each time. Erythrocyte membranes were prepared by haemolysing washed erythrocytes in 5 mm phosphate buffer, pH 8, according to a method described previously(Reference Steck, Kant, Colowkck and Kaplaneditors24). Erythrocyte membranes (ghosts) were prepared by hypotonic haemolysis according to Raccah et al. (Reference Raccah, Dadoun and Coste25). Membrane (ghost) proteins were determined according to Lowry et al. (Reference Lowry, Rosebrough and Farr26), and aliquots were immediately frozen and stored at − 80°C until use for membrane fluidity determination.
Erythrocyte membrane fluidity measurement
EMF can be measured by fluorescence polarisation (ρ) and microviscosity (η). Erythrocytes were washed using physiological saline and suspended in a PBS solution (pH 7·4) at 0·01 mol/l. Then, 1,6-diphenyl-1,3,5-hexatriene (2 × 10− 6 mol/l) was added to the erythrocyte suspension before being incubated in a water-bath at 25°C for 15 min(Reference Chadha and Dhawan27, Reference Aricha, Fishov and Cohen28). The suspension was examined using a spectrofluorophotometer (Perkin–Elmer fluorescence spectrometer, LS-50) with an excitation wavelength of 360 nm and an emission wavelength of 430 nm(Reference Chauhan, Chauhan and Wegiel29), and the ρ and η of the erythrocyte membranes were calculated using the following formula:
The indices V and H indicate the vertical and horizontal position of the polariser in the excitation and fluorescence beams, respectively. G is an instrumental correction factor equal to (I VV/I VH). The subscript H refers to the horizontally polarised excitation beam. I VV and I VH represent the components of the corrected polarised emissions parallel and perpendicular to the vertical direction, respectively(Reference Tang, Xia and Yu30).
Statistical analysis
Data are presented as means and standard deviations. Variables were assessed for normality by the skewness and kurtosis test. The indicators of the vitamin and oxidative stress parameters exhibited a normal distribution. The skewed data of ρ and η were not distributed and were logarithmically transformed for statistical analysis and then back-transformed to their natural units for presentation in tables. The differences of all of the data between groups were determined using a general linear model ANOVA. The baseline variables were compared across treatment groups. P < 0·05 was considered as the significance level for all tests.
Results
In the intervention study, complete data were available for 238 individuals, including seventy-four children, ninety young and ninety-one elderly people representing 93·3 % of the original 255 subjects. For seventeen subjects, insufficient volumes of plasma and whole blood were available for the measurement of micronutrient concentrations, oxidative stress and membrane fluidity analysis. There were no substantial differences in any of the baseline characteristics between the placebo and supplemented groups in children, young or elderly people.
The general characteristics, micronutrient status, oxidative stress and EMF in the children, young and elderly people investigated are shown in Table 1. The average levels of retinol, tocopherol and total ascorbic acid concentrations in the plasma were over the cut-off values for deficiencies and were 0·70, 11·6 and 11·4 μmol/l(Reference Ma, Schoutenm and Wangm31, Reference Drewel, Giraud and Davy32), respectively; however, the plasma level of total ascorbic acid in children was 30·98 μmol/l, which was significantly higher than in young (27·89 μmol/l) and elderly (24·46 μmol/l) people.
At baseline, no differences of the superoxide dismutase or GSH-Px activities were found in the three age populations, and there were higher levels of MDA in young (7·07 μmol/l) and elderly people (6·73 μmol/l) compared with children (5·35 μmol/l). Compared with elderly people (0·337), there was a lower ρ value (0·326) in young subjects (P < 0·001), and the lowest values of ρ and η were 0·313 and 4·48, respectively, in children, which indicates a high EMF, as shown in Table 1.
After 2 months of supplementation, there were significant increases in plasma β-carotene, retinol, tocopherol, ascorbic acid and Se concentrations in the treated groups compared with the control groups in all three age groups. Moreover, there were significant increases in the plasma β-carotene, retinol, tocopherol, ascorbic acid and Se concentrations in the supplemented groups after the trial, compared with the controls and the baseline of children, young and elderly people, respectively (all P < 0·05; Table 2).
Table 2 Comparison of plasma micronutrient levels between the supplemented and control groups* in the three populations
(Mean values and standard deviations)
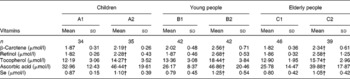
* Six groups: A1, B1 and C1 were the control groups, and A2, B2 and C2 were the supplemented groups of children, young and elderly people, respectively.
† Mean values were significantly different after supplementation from those of controls in children, young people and elderly people (P < 0·05, one-way ANOVA).
The changes in the oxidative stress parameters investigated are presented in Table 3. Compared with the control groups of children, young and elderly people, the increases in GSH-Px activities were 44·91, 90·12 and 107·21 IU/ml in the A2, B2 and C2 groups, respectively; and the levels of MDA decreased by 2·16 and 1·63 μmol/l (P < 0·001) in the B2 and C2 groups, respectively; however, there were no important changes in superoxide dismutase activities following the trial.
Table 3 Comparison of indicator levels between the supplemented and control groups* in the three populations
(Mean values and standard deviations)
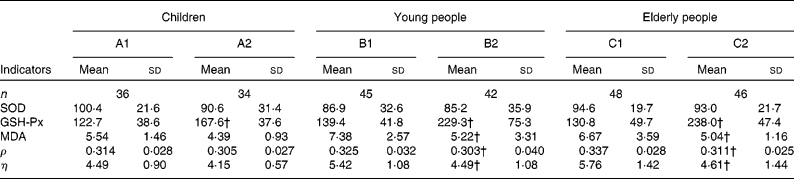
SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; ρ, polarisation; η, microviscosity.
* Six groups: A1, B1 and C1 were the control groups, and A2, B2 and C2 were the supplemented groups of children, young and elderly people, respectively.
† Mean values were significantly different after supplementation from those of controls in children, young people and elderly people (P < 0·05, one-way ANOVA).
EMF was evaluated by measuring the fluorescence ρ and η; lower values indicate better membrane fluidity. After the 2-month trial, the decreases in ρ and η were significantly greater in the supplemented groups of young and elderly people compared with the control groups. The differences in the ρ and η values were − 0·023 and − 0·93 for young subjects and − 0·026 and − 1·15 for elderly people, respectively. However, the differences in the ρ and η values in the supplemented group of children were − 0·009 and − 0·34, respectively, which is not significantly different than the child control group.
Discussion
The present study demonstrated age-related changes in MDA levels and EMF in children, young and elderly people. There was a lower membrane fluidity in young and elderly people, which was related to an age-dependent increase in erythrocyte MDA levels(Reference Rizvi and Maurya33). Plasma MDA concentration and carbonyl content of plasma proteins were used as an index of lipid and protein oxidation, respectively(Reference Berzosa, Gómez-Trullén and Piedrafita34). After 2 months of micronutrient supplementation, there were considerable increases in GSH-Px activity and plasma antioxidant micronutrient concentration and decreases in MDA levels in the three age populations. Moreover, there were significant improvements in the EMF in the supplemented groups of young and elderly people compared with the control groups, which are attributed to an increase in the antioxidant capacity, resulting in significant protection of erythrocytes against oxidative stress(Reference Maurya and Rizvi35).
In the present study, we chose to use moderate doses of antioxidants, i.e. 900 μg retinol, 1·5 mg β-carotene, 200 mg α-tocopherol, 500 mg ascorbic acid and 400 μg Se for adult subjects. These doses were selected mainly based on previous studies taking into consideration the beneficial effect in reducing oxidative stress or the lipid peroxidation process in ageing without obvious adverse effects. Fluorescence polarisation has been used to assess membrane fluidity in the past four decades, but it is now frequently also applied to other areas of study, including investigations of oxidative stress(Reference Soto-Arriaza, Sotomayor and Lissi36), erythrocyte aggregation(Reference Spengler, Bertoluzzo and Catalani37) and membrane protein function(Reference Kaneko, Matsui and Shimokawa38). To date, few studies have evaluated changes in EMF in large populations, possibly because erythrocyte membrane preparation is quite laborious.
Around 70 % of the total Chinese population lives in, often underdeveloped, rural areas(Reference Yang39). The present study was carried out under difficult circumstances in a poor, rural part of China, where nutritional problems are frequent(Reference Ma, Schouten and Zhang40). The people still live in lower socio-economic conditions, with low intake of meat, vegetables and fruit. We selected these subjects knowing that poor diet was likely to contribute to low antioxidant and micronutrient levels, which in turn gives rise to cellular oxidative stress. We would, therefore, anticipate that changes in antioxidant micronutrient status, membrane fluidity and decreasing oxidative stress after supplementation would be clearer in such subjects. Moreover, compliance was excellent, because the study subjects were motivated by the offer of free medical care and all subjects were visited weekly by village nurses, who counted leftover capsules, provided new supplies and gave support in case of any problems or questions related to the study.
Compared with children, the lower membrane fluidity observed in elderly people at baseline should be attributed to a higher rate of peroxidation, which might be due to age-related changes in the glutathione redox system(Reference Rizvi, Pandey and Jha41). The susceptibility of membranes to peroxidation is affected by the nature and physical state of the lipid bilayer(Reference Clemens and Waller42). The present findings are in accordance with reported data in animal models(Reference Oh, Lim and Lee43, Reference Harris44), and it has been observed that there were lower MDA levels and higher EMF in a verbascoside-treated group compared with the placebo group(Reference Liu, Li and Guo45). It has been reported that positive correlations between age and GSH-Px and oxidative stress increase during the ageing process(Reference Erden-Inal, Sunal and Kanbak46). Human studies showed that supplementation of ascorbic acid and α-tocopherol is useful in preventing bone loss linked to oxidative stress in the elderly(Reference Ruiz-Ramos, Vargas and Fortoul Van der Goes47), and might be beneficial in women with a low antioxidant status(Reference McCance, Holmes and Maresh48). An antioxidant micronutrient combination (800 mg dl-α-tocopherol acetate, 24 mg β-carotene, 1·0 g vitamin C, 200 μg selenomethionine, 7·2 mg riboflavin, 80 mg niacin, 60 mg Zn and 5 mg Mn) can modulate biomarkers of oxidative stress and inflammation in human subjects(Reference Hopkins, Fedirko and Jones49), and dietary supplementation with vitamin E (50 mg/d) and vitamin C (150 mg/d) for 6 months decreased plasma oxidative damage and enhanced the erythrocyte activities of catalase and glutathione reductase(Reference Ferrer, Tauler and Sureda50). Additionally, it was found that adequate supplementation with the aforementioned vitamins led to increased quality of life in haemodialysis patients, from some clinical points of view(Reference Mydlik and Derzsiová51). Proanthocyanidin-rich grape seed extract has also been found to be an effective anti-ageing drug in preventing the oxidative stress-associated loss of membrane surface charge, which thereby maintains erythrocyte membrane integrity and functions in elderly individuals(Reference Sangeetha, Balu and Haripriya52). The present results and the reports described earlier show that antioxidant supplementation is beneficial to elderly people for decreasing oxidative stress and improving EMF.
In the young subjects we investigated, who came from a college, there were higher MDA levels (7·07 μmol/l) and lower EMF, with higher ρ and η values at baseline than in children, which may be related to a greater amount of physical exercise. This leads to a decrease in antioxidant levels, which could promote an increase both in the markers of lipoprotein peroxidation and in damage to the erythrocyte membrane, with consequential modifications to membrane fluidity(Reference Brzeszczynska, Pieniazek and Gwozdzinski53). The oxidative stress and EMF of young subjects in the present study was greatly improved following multiple micronutrient supplementations, which is beneficial to young people when they engage in hard exercise. However, there have been few papers published about oxidative stress and micronutrient supplementation in healthy children. Although no change of EMF was found in the supplemented children group compared with the control group, the present results showed that healthy children (aged 8–10 years) exhibit higher EMF than those of young and elderly people, which should be attributed to lower oxidative stress or lower accumulation of oxidative damage.
In conclusion, the present findings indicate that the supplementation of multiple antioxidant micronutrients is more effective in decreasing oxidative stress and improving the EMF of young and elderly people than those of children, suggesting that taking these supplements is beneficial to young and elderly people. Moreover, new hypotheses on the physiological causes of ageing and the physiological outcomes of oxidant–antioxidant imbalances, such as those related to membrane fluidity, are expected to be developed.
Acknowledgements
We thank Chinese Natural Science Foundation (no. 30872103) and the Danone Nutrition Institute, Beijing, China, for financial support. X.-X. H. and A.-G. M. designed the intervention study, analysed the data and wrote the manuscript. M. Z., S. G., X.-X. S. and Y.-Y. S. conducted the investigations at field sites, data collection, analyses and interpretation, and laboratory analyses. Q.-Z. W. and H. L. performed a part of the laboratory analyses. There are no conflicts of interest.





