In species where differential mortality between sexes occurs under certain environmental constraints, biased sex ratios can be observed (House et al. Reference House, Simmons, Kotiaho, Tomkins and Hunt2011). External factors such as seasonal changes (Charnov et al. Reference Charnov, Los-Den Hartogh, Jones and van Den1981), disease (Jiggins et al. Reference Jiggins, Hurst and Majerus1998), predators (Tabadkani et al. Reference Tabadkani, Ashouri, Rahimi-Alangi and Fathi-Moghaddam2013), protandry (Wiklund et al. Reference Wiklund, Wickman and Nylin1992), and food availability (Charnov et al. Reference Charnov, Los-Den Hartogh, Jones and van Den1981) can play a role in determining sex ratios. In outbreaking insects that significantly compromise their host, nutritional variation may directly affect insect biological performance such as body size and fecundity (Awmack and Leather Reference Awmack and Leather2002). Differences in fitness costs and metabolic requirements between males and females suggest that nutritional quality would differentially affect the survival of the two sexes (Mopper and Whitham Reference Mopper and Whitham1992; Carisey and Bauce Reference Carisey and Bauce2002). Therefore continuous nutritional stress over several generations may result in sex ratio distortion and eventually, a local reduction in population size (Robinson Reference Robinson1983; Mauffette and Jobin Reference Mauffette and Jobin1985; Lobinger Reference Lobinger1996). The eastern spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Torticidae) is one of the most destructive outbreak insects in North America (Rauchfuss and Ziegler Reference Rauchfuss and Ziegler2011). This pest attacks balsam fir (Abies balsamea (Linnaeus) Miller; Pinaceae), white spruce (Picea glauca (Moench) Voss; Pinaceae), black spruce (Picea mariana (Miller); Pinaceae) and red spruce (Picea rubens Sargent) (Montgomery Reference Montgomery1983). Continuous severe defoliation significantly reduces growth of the host and can result in mortality (Morris Reference Morris1963). Following an outbreak, foliage production decreases considerably because removal of foliage through defoliation causes growth loss of trees and decreased wood production (MacLean Reference MacLean1984). Nutritional quality of foliage in heavily defoliated trees is diminished in terms of nitrogen, carbon, and defensive compounds (White Reference White2004). Lack of food availability accompanied by changes in natural enemy composition and increase in incidence of diseases due to overcrowding can cause local decline of outbreaks (Morris Reference Morris1963; Royama Reference Royama1984). There is evidence of a slight sex ratio bias in the western spruce budworm, Choristoneura occidentalis (Walsingham), in sites where defoliation of current-year shoots was >50% (Campbell et al. Reference Campbell, Torgersen, Hosman and Srivastava1983). In this study, we evaluated the effect of nutritional stress on sex ratio in the eastern spruce budworm. We tested the hypothesis that poor nutritional quality favours survival of males because females have higher fitness and metabolic costs.
We reared a total of 14 000 insects over three consecutive generations under controlled conditions, at 23 °C, 60 ± 5% relative humidity in a 16:8 hour light:dark photoperiod cycle (Robertson Reference Robertson1985). Two artificial diets were formulated differing only in nitrogen and sugar content (Bidon Reference Bidon1999). They are hereafter referred to as “normal” and “stress” diet and contain 12% sugar, 5% nitrogen and 1.5% sugar, 7% nitrogen, respectively. This stress diet was chosen because it has a negative impact on larval survival, development, and growth that is representative of food quality deterioration in outbreak conditions (Bidon Reference Bidon1999). Rearing was initiated with 2000 individuals in each diet in the first generation. Insects were reared in the laboratory in Petri dishes (100 × 15 mm) with 10 individuals per dish. Pupae and adults were separated according to sex (Robertson Reference Robertson1985). Mortality was recorded every day. Couples were installed in plastic cages of 11 × 7.5 cm. Adults were fed a 5% sugar water solution and allowed to mate. Eggs were collected two or three days after females died. Substrates provided were wax paper for oviposition and cheese cloth for overwintering. Larvae that hatched were held at 18 °C for two weeks and were then transferred to 2 °C for 25 weeks to overwinter in dark growth chambers (Robertson Reference Robertson1985). The laboratory culture was maintained for three generations and laboratory conditions were kept stable to minimise environmental variability. We did not consider larval mortality because sex of individuals is not evident in all larval stages. Individuals used for mating were selected randomly. A χ2 test and a logistic regression were performed to assess treatment effects using log-likelihood ratios (Zar Reference Zar2010). We tested the null hypothesis that sex ratio is maintained at 1:1 across generations. Data were analysed using PROC FREQ and PROC LOGISTIC modules of SAS 6.12 (SAS Institute Inc. 2003).
We observed equal sex ratios for pupae and adults reared on normal diet in all three generations. However, when larvae were reared under stress diet conditions, from the second generation onwards, strong distortions in favour of males were observed both for pupae (Table 1) and adults (Table 2). It appears that sex ratio distortion in favour of males was caused by higher mortality of females reared on the stress diet. Poor nutritional quality leads to differential mortality (Charnov et al. Reference Charnov, Los-Den Hartogh, Jones and van Den1981) with greater impact on the larger-sized sex because larger individuals require more energy for development (Caswell and Weeks Reference Caswell and Weeks1986). Female spruce budworms are larger and have a longer lifespan than males. They also require additional resources to produce progeny (Miller Reference Miller1975). Sex distortion on stress diet found in this study may be related to higher energy costs for female development compared with males. Females may not have been able to derive enough energy from the stress diet for their development and therefore failed to reach the pupal and adult stages. From the second generation onwards, sex ratio distortion was significant. Our results indicate that mortality of females would increase during the course of an outbreak if nutritional stress, as caused by severe defoliation over consecutive years, continues for several generations. Decline in host nutritional quality can trigger emigration from outbreak areas resulting in moth dispersal into uninfested areas (Greenbank et al. Reference Greenbank, Schaeffer and Rainey1980) causing outbreaks to spread.
Table 1 The effect of diet on sex ratios of three generations of spruce budworm pupae.

Notes: Normal diet: likelihood-ratio χ2 = 3.12, df = 2, P = 0.2097; stress diet: likelihood ratio χ2 = 39.05, df = 2, bold values: P < 0.0001.
*Number of individuals in the three generations.
†Departure of sex ratio from 1:1 with the χ2 test, P < 0.0001.
Table 2 The effect of diet on sex ratios on three generations of adult spruce budworm.
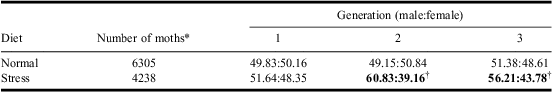
Notes: Normal diet: likelihood-ratio χ2 = 1.85, df = 2, P = 0.3959; stress diet: likelihood-ratio χ2 = 21.65, df = 2, bold values: P < 0.0001.
*Number of individuals in the three generations.
†Departure of sex ratio from 1:1 with the χ2 test, P < 0.0001.
The sex ratio difference that we observed was interpreted as a deficit of females and not as an excess of males. Hamilton's (Reference Hamilton1967) local mate competition theory posits that when a population is subject to environmental stress, sex ratio distortion can be beneficial because when there are more males the likelihood of fertilising females increases. Variance in reproductive success is expected to be greater among females in polygynous animals because males have more than one mate available (Godfray and Werren Reference Godfray and Werren1996). High reproductive performance by males as opposed to females may increase overall reproductive success in local populations because males are more likely to successfully mate with several females than vice versa. A nonmating female would represent an energy loss to the population.
From an ecological point of view, defoliation during spruce budworm outbreaks causes significant variability in food resources (Morris Reference Morris1963). Under these conditions, unequal sex ratios may offer an advantage for auto-regulation of the population. Linear decreases in sex ratios were observed in several insect species when population densities increased or when food quality and quantity decreased (Lobinger Reference Lobinger1996) and this negative feedback may contribute to auto-regulation of a population (Dingle Reference Dingle1966). Female forest insect pests generally tend to produce an equal sex ratio under favourable conditions (Robinson Reference Robinson1983). In this study we observed that the lower food quality results in a higher mortality of females from the second generation onwards, so it was less likely that females reached pupal and adult stages. For outbreak insects that attack their host over the course of several years, causing foliage quality to deteriorate over time, a high proportion of males could indicate that the population is in decline (Lobinger Reference Lobinger1996). Decline in foliage quality has been documented as a factor contributing to population crashes during spruce budworm outbreak cycles (Nealis and Régnière Reference Nealis and Régnière2004; Régnière and Nealis Reference Régnière and Nealis2007), but sex ratio bias has neither been considered as an effect of deteriorating resources nor as a cause for population decline. Our study provides evidence that consumption of poor quality resources over a few generations causes male-biased sex ratios suggesting that occurrence of this phenomenon should be tested under natural conditions.
Acknowledgements
This work was supported by the iFor Insectes forestiers consortium. The authors particularly thank anonymous reviewers for their invaluable contributions to improving the manuscript, and Dr. W.F.J. Parsons for correcting the English text.




