Intake of fish has been reported to beneficially influence body weight, blood lipids and glucose homoeostasis, which can protect against type 2 diabetes and CVD( Reference Feskens, Bowles and Kromhout 1 – Reference Kromhout, Bosschieter and de Lezenne Coulander 5 ). The health benefits of fish consumption have traditionally been attributed mainly to the effect of n-3 fatty acids, and, although the TAG-lowering effect of fish oil and long-chain n-3 fatty acids is well documented, there is controversy regarding the cholesterol-regulating as well as glucose-regulating effects of marine n-3 fatty acids( Reference Harris 6 – Reference Balk, Lichtenstein and Chung 14 ). Studies suggests that there are other components in fish such as fish proteins that may be beneficial to human health and affect risk factors leading to CVD, beyond n-3 fatty acids( Reference Jacques 15 – Reference Ouellet, Marois and Weisnagel 18 ). This is supported by results from animal studies showing that fish proteins may have a lowering effect on TAG and cholesterol as well as improve glucose tolerance( Reference Wergedahl, Liaset and Gudbrandsen 19 – Reference Drotningsvik, Mjos and Hogoy 26 ). In line with this, we have recently shown that supplementation with cod protein as tablets reduced fasting glucose and LDL-cholesterol as well as postprandial glucose concentrations in healthy, overweight adults( Reference Vikoren, Nygard and Lied 17 ).
The present study was designed to investigate the impacts of high intake of lean or fatty fish (cod and salmon, respectively) compared with lean meat (chicken) on indicators of CVD and type 2 diabetes risk factors, including lipid and glucose regulation. Serum fructosamine concentration as a measure of the mean glucose concentration over the last 1–3 weeks( Reference Glikmanas, Sarmini and Bigorie 27 ) was examined. In addition, methylglyoxal (MG) and N ε-(carboxymethyl) lysine (CML) were analysed, as changes in lipid and glucose regulation may affect the production of MG from the glycation process by degradation of glucose, Schiff’s bases or from Amadori products such as fructosamine, and the production of CML( Reference Singh, Barden and Mori 28 ). Several intervention studies with fish intake have been conducted by others in overweight, dyslipidaemic, hypertensive, insulin-resistant and/or diabetic subjects( Reference Eslick, Howe and Smith 12 – Reference Nettleton and Katz 13 , Reference Vikoren, Nygard and Lied 17 – Reference Ouellet, Marois and Weisnagel 18 , Reference Telle-Hansen, Larsen and Hostmark 29 – Reference Gross, Zelmanovitz and Moulin 37 ); however, as no studies on the effect of high fish intake in a group of young, healthy, non-smoking, normal-weight adults have been published, little is known about how fish intake may affect lipid and glucose metabolism in this population. As the choice of preparation methods of fish and meat as well as the choices of side dishes and accessories may explain some of the different health effects from fish consumption compared with meat( Reference Mozaffarian, Lemaitre and Kuller 38 – Reference Larsen, Stormo and Dragnes 41 ), participants in the present study were instructed to follow a recipe booklet to prepare fish and lean meat dinners, respectively, with comparable preparation methods and side dishes. In the present study, we wanted to test our hypothesis that a high intake of lean or fatty fish would not affect circulating concentrations of lipids or glucose tolerance in young, healthy, normal-weight adults with serum concentrations of lipids, glucose and insulin within normal reference ranges, when compared with lean meat intake.
Methods
Participants, study setting and ethics
Participants were 1st- to 4th-year students at the Faculty of Medicine and Dentistry, University of Bergen, Norway. The students were invited by members of the research team to participate in the study by using the University’s shared email system for students from April to May 2011. Inclusion criteria were normal body weight (BMI 18·5–24·9 kg/m2), fasting blood glucose ≤7·0 mmol/l and 20–35 years of age. Participants would have to be willing to consume the assigned amount of fish or chicken during the intervention. Exclusion criteria were pregnancy, incompatibility with fish or chicken consumption (allergies, intolerance and/or dislike), diagnosed diabetes mellitus, heart disease or gastrointestinal diseases, use of medications affecting lipid metabolism or glucose homoeostasis, use of anti-inflammatory medications, use of supplements containing long-chain n-3 fatty acids, intentional weight loss and large fluctuation in body weight (>3 kg) over the previous 2 months, and smoking.
The study was designed as a randomised intervention study with a parallel group design, with three intervention arms: lean fish (cod), fatty fish (salmon) or lean meat (chicken) as the control group in weekly doses of 750 g (five meals of 150 g) for 4 weeks. A total of forty-five students were included in the study and were randomly assigned to lean fish (n 14), fatty fish (n 15) or lean meat (n 16) groups. The participants were stratified into the different intervention groups by the project manager on the basis of sex, age and BMI. All examinations were conducted at the Haukeland University Hospital, Bergen, Norway.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving participants were approved by the Regional Committee for Medical and Health Research Ethics (approval no.: 2011/572). Written informed consent was obtained from all subjects. Health professionals performing blood sampling and measuring height and body weight and personnel conducting the laboratory analyses were blinded. All the data were analysed anonymously. This trial was registered at clinicaltrials.gov as NCT02130908.
Interventions
The participants were instructed to eat 5 meals/week containing 150 g of cod, salmon or chicken over a period of 4 weeks, and they were told not to exceed the total amount of 750 g of fish or chicken/week. If 150 g of fish or chicken/d was not sufficient, participants were encouraged to supplement their meal with vegetables, pasta or rice. Participants in the fish-eating groups were instructed not to consume any other fish or seafood during the study period, and participants in the lean meat group were instructed not to consume fish or seafood during the intervention period. All participants were allowed to eat meat, but not along with the meal in which they consumed fish or meat for the study. The participants were instructed to maintain their normal eating habits throughout the study period, apart from eating the mandatory amount of 750 g fish or chicken/week, and not to change their physical activity level during the 4-week intervention period. Use of dietary supplements was not allowed during the study period. Participants were instructed to follow a recipe booklet to prepare fish and lean meat dinners, respectively, and to report recipes that they chose for the study meals to see which methods of preparation and accessories the participants preferred and also as a mean to assess compliance. Dietary intakes before start of the study and before end of the study were assessed using 5-d food records at both time points.
Fish was provided as frozen skin and boneless fillet portions (150 (sd 10) g; Lerøy Seafood Group ASA). Chicken fillets (150 (sd 10) g; ‘Den stolte hane’) were frozen before use in the intervention study. The fish and chicken fillets were supplied free of charge to the participants, and were distributed at baseline or at any time during the study period if preferred. The compositions of the fillets were as follows, presented as mean values and standard deviations for analyses of five samples: proteins (wt%), cod 20·6 (sd 0·5), salmon 20·0 (sd 0·7) and chicken 24·0 (sd 0·5); moisture (wt%), cod 77·4 (sd 0·4), salmon 67·0 (sd 2·0) and chicken 72·6 (sd 0·5); and total fats (wt%), cod 0·67 (sd 0·03), salmon 11·2 (sd 2·2) and chicken 1·7 (sd 0·3). The total content of EPA+DHA was 0·33 (sd 0·02), 1·4 (sd 0·2) and 0·02 (sd 0·01) g/100 g filet of cod, salmon and chicken, respectively. The fillets were analysed by Skretting ARC Laboratory using standard laboratory methods. Protein content (as Nx6.25) was analysed using the Kjeldahl method( 42 ); moisture was analysed gravimetrically after drying to constant weight in an oven at 105°C( 43 ); and fatty acid composition was determined after methylation of the fatty acids in methanolic HCl and extraction in hexane( Reference Grahl-Nielsen and Barnung 44 ). The methyl esters were separated by gas chromatography (Thermo Trace GC with Triplus autosampler; Thermo Scientific) and identified by retention time using standard mixtures of methyl esters (Nu-Chek), using C19 : 0 as the internal standard for quantification. Total fat content was calculated as the sum of fatty acids.
Protocol for study visits
The total study period was 4 weeks, with study visits at baseline and at the end of the study (4 weeks). Examinations were conducted in the morning after an overnight fast. The subjects were instructed not to eat or drink anything except water, not to use substances containing nicotine after 22.00 hours the previous day and to avoid physical exercise and alcohol for 24 h before each sampling day.
Body height was measured at baseline, whereas body weight was measured at baseline and at the end of the study. Blood samples were collected at baseline and at the end point in a fasting state. Blood samples were collected in BD Vacutainer SST II Advance gel tubes (Becton, Dickinson and Company) for isolation of serum and Vacuette K2EDTA tubes (Greiner Bio-one) for isolation of plasma.
After collection of fasting blood samples, glucose tolerance was tested using a standardised breakfast meal containing fat and protein in addition to carbohydrates. A standardised meal test was chosen instead of the traditional oral glucose tolerance test, where the subject is given a measured dose (usually 75 g) of glucose, as the former gives a more physiological description of the body’s response to an oral carbohydrate load( Reference Lefebvre and Luyckx 45 ). The breakfast consisted of two slices (65 g) of white bread, 7·5 g margarine, 50 g strawberry jam and 0·25 litres orange juice, providing a total of 1908 kJ (85 g carbohydrate, 8 g protein and 8 g fat) and had to be consumed within 15 min. The macronutrient and energy contents in the breakfast were calculated using ‘Mat på Data 5.1’ (www.matportalen.no/Emner/matpadata)( 46 ). A second blood sample was collected 120 min after the standardised breakfast. Food records were checked for completeness at both study visits.
Description of recipes
A detailed description of the recipes can be found in the Online Supplementary File 1. The energy and macronutrient contents in the recipes are presented in Table 1.
Table 1 Estimated energy and macronutrient content in the recipes from the provided booklets

CH, carbohydrates.
Estimation of energy and macronutrient intakes from dietary records
Participants completed a dietary record of their food intakes of the 5 preceding days before baseline and the 4-week visit, including at least 1 weekend day. The intakes of energy, carbohydrates, proteins and fats were calculated from the participants’ dietary record using ‘Mat på Data 5.1’ software( 46 ).
Analysis of serum and plasma samples
Analyses of lipids, glucose, total bile acids, C-reactive protein (CRP), insulin and insulin C-peptide in blood serum were performed by routine methods at the Laboratory of Clinical Biochemistry and the Hormone Laboratory at Haukeland University Hospital, and reference values were set according to these laboratories. Serum NEFA and plasma fructosamine were analysed with the Cobas c111 system (Roche Diagnostics GmbH) using the NEFA FS kit (DiaSys; Diagnostic Systems GmbH) and the Fructosamine kit (FRA) for Cobas c systems (Roche), respectively. Adiponectin (HADK1MAG-61K; EMD Millipore) was measured in serum using xMAG®, a bead-based multiplex technique (Luminex Corp.) on a Luminex 100 instrument (Luminex Corp.) using STarStation version 3 software (AppliedCytometry). Plasma concentrations of MG and CML were measured using OxiSelectTM competitive ELISA kits (Cell Biolabs, Inc.) on Spectra Max Plus 384 (Molecular devices LLC), with readings at 450 nm.
Outcome measurements
The primary outcome of the present study was changes in serum concentrations of TAG after a weekly intake of 750-g fillet of either lean or fatty fish, or lean meat. Secondary outcomes were changes in cholesterols, glucose, insulin, insulin C-peptide, body weight and intakes of energy and macronutrients within the groups over time, as well as a comparison of choices of preparation methods, side dishes and accessories between the groups.
Statistical analyses
We consider the present study to be a pilot study, as to our knowledge this is the first study on the effects of high fish intake compared with lean meat intake in a group of young healthy, non-smoking normal-weight adults with serum concentrations of lipids, glucose and insulin within normal reference ranges. Other studies of fish intake in other populations have shown that a sample size of between ten and fifteen was sufficient to observe a lowering effect of fish intake on serum TAG concentration( Reference Telle-Hansen, Larsen and Hostmark 29 , Reference Mori, Bao and Burke 31 , Reference Lindqvist, Langkilde and Undeland 33 ). Therefore, as there is no information available about the necessary sample size, we consider the present study to be hypothesis generating rather than hypothesis testing, and the study will constitute a base for sample size calculations for future studies with similar designs.
Statistical analyses were conducted using PASW Statistics version 22 (SPSS, Inc., IBM Company). Subjects who did not complete the study were excluded from the statistical analyses. For analytes in serum and estimated energy and macronutrient intakes from dietary records, most data were not normally distributed according to the Shapiro–Wilk test, and non-parametric tests were used to investigate changes within groups (Wilcoxon’s signed-rank test). For these non-parametric data, the Kruskal–Wallis test was used to compare values between the three groups at baseline. Changes within the groups were compared using the Kruskal–Wallis test followed by the Mann–Whitney test whenever group differences were detected. Data are expressed as medians and 25th–75th percentiles, with the exception of macronutrient contents in the recipes where the Shapiro–Wilk test revealed that data were normally distributed. Therefore, comparisons of the choices of recipes were conducted using one-way ANOVA, and the results are presented as mean values and standard deviations. All comparisons were two-sided, and P<0·05 was considered to be statistically significant.
Results
Participant characteristics
A total of forty-five students were included in the study and were randomly assigned to one of the three experimental groups eating five dinner meals per week containing 150 g of either lean fish (cod, n 14), fatty fish (salmon, n 15) or lean meat (chicken, n 16). There were two dropouts (both from the lean meat group), and five participants were withdrawn from analysis because they did not comply with the protocol or because of onset of the use of dietary supplements or medications affecting glucose and/or lipid metabolism during the study period. For this reason, the statistical analysis included thirty-eight participants (fifteen men and twenty-three women) who completed the 4-week intervention and fulfilled the criteria (lean fish, n 13 (five men); fatty fish, n 14 (six men); lean meat, n 11 (four men)). All participants were young and apparently healthy adults with normal BMI. The median age was 23·2 (25th–75th percentile 20·9, 24·8) years, and the median BMI was 21·1 (25th–75th percentile 19·5, 22·6) kg/m2. Serum concentrations of lipids, glucose and insulin were within normal reference ranges at baseline. No statistically significant differences were found between the intervention groups at baseline for age (lean fish, 23·2 (25th–75th percentile 21·6, 24·6); fatty fish, 24·5 (25th–75th percentile 22·8, 24·9); lean meat, 20·7 (25th–75th percentile 20·5, 26·0) years) or BMI (lean fish, 21·2 (25th–75th percentile 18·8, 22·8); fatty fish, 20·8 (25th–75th percentile 19·9, 22·1); lean meat, 21·2 (25th–75th percentile 19·7, 23·5) kg/m2). BMI was not affected by 4 weeks of fish intake (data not presented).
Estimated dietary intake
The participants registered their choices of recipes from the provided booklets during the 4-week intervention period. The preferences for preparation methods and side dishes showed considerable variation within each intervention group, but no statistically significant differences were seen between the three intervention groups regarding choice of recipes (Table 2). As presented in Table 1, the estimated energy contents were comparable between Recipes 1–5 and were noticeably higher in Recipe 6. Owing to the high fat content in fatty fish, the energy and fat contents (especially regarding PUFA) were higher in the recipes for fatty fish when compared with those for lean fish and lean meat.
Table 2 Choices of recipes for all participants and for the individual intervention groups, shown as percentage of all choicesFootnote * (Mean values and standard deviations)
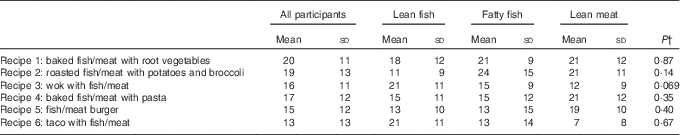
* Results are presented for thirteen participants in the lean fish group, fourteen participants in the fatty fish group and eleven participants in the lean meat group.
† P values between groups. Between-group changes were tested using one-way ANOVA.
Participants completed a 5-d dietary record chart before baseline and end point. No differences regarding energy and macronutrient intakes were observed between the groups at baseline or after 4 weeks, and there were no changes within any of the groups during the course of the study (Table 3).
Table 3 Estimated daily dietary intakes of energy and macronutrients (as percentage of energy intake) based on 5-d dietary records at baseline and after 4 weeksFootnote * (Medians and 25th–75th percentiles)

* No differences were observed between the groups at baseline (Kruskal–Wallis test). Results are presented for thirteen participants in the lean fish group, fourteen participants in the fatty fish group and eleven participants in the lean meat group.
† Within-group changes are tested using the Wilcoxon’s signed-rank test.
‡ Changes within lean fish, fatty fish and lean meat groups are compared using the Kruskal–Wallis test.
Serum lipids
Fatty fish intake for 4 weeks reduced the concentration of TAG and increased the concentration of HDL-cholesterol in serum when compared with the lean meat group (P values of 0·018 and 0·025, respectively, Table 4). The increase in HDL-cholesterol in the fatty fish group was also significant when compared with the lean fish group (P=0·033). No differences were observed between the groups regarding within-groups differences for serum concentrations of NEFA, total cholesterol, LDL-cholesterol and total bile acids after 4 weeks.
Table 4 Fasting concentrations of lipids, NEFA and bile acids in serumFootnote * (Medians and 25th–75th percentiles)

* No differences were observed between the groups at baseline (Kruskal–Wallis test). Results are presented for thirteen participants in the lean fish group, fourteen participants in the fatty fish group and eleven participants in the lean meat group.
† Within-group changes are tested using Wilcoxon’s signed-rank test.
‡ Changes within lean fish, fatty fish and lean meat groups are compared using the Kruskal–Wallis test.
§ Changes within the lean fish group are compared with the lean meat group (A), changes within the fatty fish group are compared with the lean meat group (B), changes within the lean fish group are compared with the fatty fish group (C) using the Mann–Whitney test when the Kruskal–Wallis test showed differences between the groups.
Glucose homoeostasis
A comparison of 4 weeks of intake of lean fish, fatty fish and lean meat showed no effects on fasting concentrations of glucose, insulin, insulin C-peptide and fructosamine in either group (Table 5). We observed no differences between the groups for changes from fasting to postprandial serum concentrations of glucose, insulin and insulin C-peptide after 4 weeks. No differences were observed between the groups regarding the within-group changes in circulating concentrations of MG, CML, CRP and adiponectin.
Table 5 Serum concentrations of glucose, insulin, insulin C-peptide, fructosamine, methylglyoxal (MG), N ε-(carboxymethyl) lysine (CML), C-reactive protein (CRP) and adiponectinFootnote * (Medians and 25th–75th percentiles)

* No differences were observed between the groups at baseline (Kruskal–Wallis test). Results are presented for thirteen participants in the lean fish group, fourteen participants in the fatty fish group and eleven participants in the lean meat group.
† Within-group changes are tested using the Wilcoxon’s signed-rank test.
‡ Changes within lean fish, fatty fish and lean meat groups are compared using the Kruskal–Wallis test.
Discussion
Numerous studies in overweight, dyslipidaemic, hypertensive, insulin-resistant and/or diabetic subjects have been conducted to investigate the effects of fish intake on serum lipids and glucose regulation( Reference Eslick, Howe and Smith 12 , Reference Nettleton and Katz 13 , Reference Vikoren, Nygard and Lied 17 , Reference Ouellet, Marois and Weisnagel 18 , Reference Telle-Hansen, Larsen and Hostmark 29 – Reference Lara, Economou and Wallace 35 , Reference Ouellet, Weisnagel and Marois 47 ); however, no studies on the effect of high fish intake in a group of young, healthy, non-smoking, normal-weight adults have been published to date. In the present study, we investigated the effects of high intake of lean or fatty fish compared with lean meat for 4 weeks on serum lipids and glucose regulation in healthy, normal-weight adults. The results from this study suggest that a high fatty fish intake for 4 weeks as part of the participants’ normal diet was sufficient to affect fasting serum concentrations of TAG and HDL-cholesterol in young, normal-weight adults, but fatty fish intake did not affect glucose regulation. The observed effects on serum lipids seen after high fatty fish intake could neither be explained by weight loss, as body weight was not affected in the fatty fish group, nor could it be explained by change in energy or macronutrient intakes or the choices of preparation of dinner meals and side dishes. No effects were observed on serum lipids and glucose regulation after lean fish intake when compared with lean meat intake.
It has been hypothesised by others that the choice of preparation methods of fish and meat and the choices of side dishes and accessories may explain at least some of the different health effects observed after fish intake when compared with meat( Reference Mozaffarian, Lemaitre and Kuller 38 – Reference Larsen, Stormo and Dragnes 41 ). Therefore, in the present study, it was mandatory for the participants to follow certain recipes with similar preparation methods and side dishes for dinner meals with lean fish, fatty fish and lean meat. In general, the contents of protein and carbohydrates were similar for meals containing fish or meat for each recipe, whereas the fat contents, and thus the energy contents, were always higher in the corresponding meals containing fatty fish. Thus, the intake of fats from dinner meals, especially of MUFA and PUFA, was higher in the fatty fish group. The participants could choose freely between the recipes for preparation of study dinners, and no differences were found between the groups in preferences for the different recipes and side dishes; thus, the current findings regarding serum lipid can probably not be explained by differences in preparation methods or dinner accessories. Despite the higher content of energy and fat in the dinners of the fatty fish group, no differences were observed in intake of energy and fat from baseline to 4 weeks in this group, suggesting that the participants regulated their intake of energy from other meals to maintain a constant intake during the study period.
The beneficial effects of fish (especially fatty fish) and fish oil intake on lipid concentrations have been reported by others( Reference Eslick, Howe and Smith 12 , Reference Nettleton and Katz 13 , Reference Telle-Hansen, Larsen and Hostmark 29 – Reference Lara, Economou and Wallace 35 ). In the present study, in healthy, non-smoking subjects with normal BMI, high intake of fatty fish for 4 weeks resulted in reduced serum TAG and elevated HDL-cholesterol concentrations when compared with high intake of lean meat and higher HDL-cholesterol concentration when compared with lean fish intake. Our results on fatty fish intake resemble reports on n-3 PUFA supplementation showing decreased TAG and increased HDL-cholesterol concentrations in the circulation( Reference Harris 6 , Reference Harris 7 , Reference Popp-Snijders, Schouten and Heine 11 – Reference Balk, Lichtenstein and Chung 14 ). Thus, the observed effects in the fatty fish group may be caused by the high intake of n-3 PUFA, especially as these changes were not induced by lean fish intake. It should be noted that as all participants had serum lipid concentrations within the normal reference ranges the clinical significance of the effects of fatty fish intake on TAG and HDL-cholesterol concentrations in serum is uncertain.
We have recently shown that cod protein supplementation reduced serum LDL-cholesterol concentration in overweight adults, with no effect on serum concentrations of TAG, total cholesterol and HDL-cholesterol( Reference Vikoren, Nygard and Lied 17 ). Reduced serum TAG has been reported in overweight subjects when lean fish was part of an energy-restricted diet( Reference Gunnarsdottir, Tomasson and Kiely 32 ), whereas others found no effect of lean fish intake on serum lipids in overweight, insulin-resistant subjects( Reference Ouellet, Weisnagel and Marois 47 ). In the present study, in subjects with normal BMI, however, lean fish intake did not affect serum TAG and cholesterol concentrations.
Intake of lean meat did not affect serum TAG and cholesterol concentrations in our study, which is in line with findings by others in overweight adults( Reference Murphy, Thomson and Coates 36 ) or patients with type 2 diabetes( Reference Gross, Zelmanovitz and Moulin 37 ) after intake of lean meat.
There is controversy about the glucose-regulating effects of fatty fish or fish oil( Reference Kromhout and de Goede 9 – Reference Popp-Snijders, Schouten and Heine 11 , Reference Mori, Bao and Burke 31 , Reference Lara, Economou and Wallace 35 , Reference Bhathena, Berlin and Judd 48 ); however, studies in rats that are obese and/or fed high-fat or high-carbohydrate diets suggest that intake of fish proteins may improve glucose tolerance( Reference Lavigne, Marette and Jacques 21 – Reference Lavigne, Tremblay and Asselin 24 , Reference Drotningsvik, Mjos and Hogoy 26 ). Clinical studies also suggest that fish proteins may improve glucose regulation in overweight/obese participants( Reference Vikoren, Nygard and Lied 17 – Reference Ouellet, Marois and Weisnagel 18 ). In the present study, there were no changes in fasting glucose, insulin or insulin C-peptide after either fish or meat intake. Postprandial regulation of glucose may be of more interest than solely looking at fasting concentrations of glucose, insulin and insulin C-peptide, as humans spend most of their time in a postprandial state. In our healthy participants, the 2-h glucose concentration was lower than fasting concentrations for almost all participants, whereas the concentrations of insulin and insulin C-peptide increased postprandially. We found no differences between the within-group changes after intakes of lean fish, fatty fish or lean meat regarding fasting concentrations of glucose, insulin, insulin C-peptide, adiponectin and fructosamine, or the fasting glucose:insulin ratio. In addition, the relative increase in concentrations of glucose, insulin and insulin C-peptide from fasting to 2-h concentrations after the standardised breakfast were similar in all groups, strongly suggesting that glucose tolerance was not affected by lean or fatty fish intake. In addition, the unchanged plasma concentrations of MG and CML after fish intake suggest no change in production of advanced glycation end products.
The lack of effect of lean fish intake in the present study is in contrast to our previous study, showing that an intake of 6 g of cod protein as supplement (corresponding to approximately 30 g cod fillet) was sufficient to affect postprandial glucose and insulin concentrations in overweight subjects( Reference Vikoren, Nygard and Lied 17 ). The discrepancy in findings between the present population with normal BMI and the overweight population( Reference Vikoren, Nygard and Lied 17 ) may be caused by differences in the participants’ regulation of glucose metabolism, as glucose regulation was most likely impaired in the overweight subjects.
Elevated circulating NEFA concentration is regarded as a risk factor for the development of insulin resistance in humans( Reference Roden, Price and Perseghin 49 ). In the present study, serum NEFA was significantly reduced after 4 weeks of intake of lean fish or fatty fish; however, no differences were observed between fish and meat groups when the within-group changes were compared. Little is known about how fish and meat intakes affect circulating NEFA concentrations in humans, and future studies are warranted in populations with increased risk of developing insulin resistance and type 2 diabetes.
In the present study, participants were healthy, normal weight subjects without elevated CRP at baseline, and no changes in CRP concentration were observed in any intervention group. The literature on the effects of fish intake on CRP as a marker of inflammation is not consistent, as some studies report no effect of intake of fish on CRP in overweight men( Reference Lindqvist, Langkilde and Undeland 33 ) and non-obese subjects( Reference Lara, Economou and Wallace 35 ), whereas others report an inverse relationship between serum CRP and fish intake in both healthy and insulin-resistant adults( Reference Smith, Barraj and Kantor 8 , Reference Ouellet, Weisnagel and Marois 47 , Reference Pot, Geelen and Majsak-Newman 50 ).
There are some limitations to this study. Participants were given five different recipes to prepare lean fish-, fatty fish- or lean meat-based dinner meals, and they were encouraged to vary between the different recipes. Although great efforts were made to design menus with similar vegetables and other accessories, some differences may have occurred. However, we believe that this would not affect the outcome of the study as the preferences for preparation methods and choice of side dishes and accessories were not different between the groups. Moreover, the groups had similar intakes of energy and macronutrients during the course of the study, which may strengthen the interpretation that high intake of fatty fish caused the observed effects on serum TAG and HDL-cholesterol. As no physiological marker of the compliance was used in this study, participants were interviewed about their intake during the intervention period. More studies are needed to achieve generalisability of our findings. In the present study, the participants were students at the Faculty of Medicine and Dentistry and possibly were more health and nutrition conscious than the general population of the same age. We consider the present study to be a pilot study that is hypothesis generating rather than hypothesis testing, as there is no information available about the necessary sample size; the study will constitute a base for sample size calculations for future studies with similar designs, and no adjustment for multiple testing has been performed. However, other studies of fish intake in other populations have shown that a sample size of between ten and fifteen was sufficient to observe a lowering effect of fish intake on serum TAG concentration( Reference Telle-Hansen, Larsen and Hostmark 29 , Reference Mori, Bao and Burke 31 , Reference Lindqvist, Langkilde and Undeland 33 ).
Conclusions
The hypothesis of this study was that a high intake of lean or fatty fish would not affect circulating concentrations of lipids or glucose tolerance in healthy, normal-weight adults with serum concentrations of lipids, glucose and insulin within normal reference ranges, when compared with lean meat intake. Contrary to our hypothesis, our findings suggests that a high intake of fatty fish reduced serum TAG and increased HDL-cholesterol concentrations when compared with lean meat intake. In addition, fatty fish intake increased HDL-cholesterol when compared with lean fish intake in this population. Our findings may possibly be due to the high content of long-chain n-3 PUFA in fatty fish. This is of particular interest as an improvement in lipid concentrations after fish intake has mainly been explained by others as a result of weight loss when fish was included in an energy-restricted diet( Reference Gunnarsdottir, Tomasson and Kiely 32 , Reference Ramel, Martinez and Kiely 51 ); however, there was no change in body weight in the present study. In addition, there was no change in the intakes of energy or macronutrients within any of the experimental groups or between the groups. The observed changes in lipids in the fatty fish group could not be explained by different preparation methods or side dishes for the dinner meals.
Acknowledgements
The kind contributions of chicken and fish for the intervention trial by ICA Maxi (Bergen, Norway) and Lerøy Seafood Group ASA (Bergen, Norway) are highly appreciated. The authors thank all participants who have contributed to the present study.
The present research has been supported by funding from the Bergen Medical Research Foundation and KG Jebsen Center for Diabetes Research.
G. R., H. S., G. M. and O. A. G. designed the study. I. V. H., A. H., M. B. and O. A. G. conducted the study. I. V. H., A. H., M. B., K. A. B. and O. A. G. analysed the data and performed statistical analyses. O. A. G. drafted the paper and had primary responsibility for the final content. All authors have contributed to the writing and approved the final version of the manuscript.
G. R. and H. S. are employed in Skretting Aquaculture Research Centre AS and Lerøy Seafood Group ASA, respectively. Skretting Aquaculture Research Centre AS is a global leader in providing innovative and sustainable nutritional solutions for the aquaculture industry. Lerøy Seafood Group ASA is the leading exporter of seafood from Norway and the world’s second largest producer of Atlantic Salmon. Skretting Aquaculture Research Centre AS and Lerøy Seafood Group ASA were not involved in on-site data collection. I. V. H., A. H., M. B., K. A. B., G. M. and O. A. G. declare no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516002555








