Accurately estimating the dietary protein and amino acid (AA) digestibility of food products is necessary( Reference Moughan 1 ). The protein digestibility-corrected amino acid score (PDCAAS) has been adopted by the Joint FAO/WHO Expert Consultation since 1991 and has since been used for the evaluation of protein quality in food products( 2 ). However, this method has several limitations( Reference Rutherfurd, Fanning and Miller 3 , Reference Schaafsma 4 ). The main difference between the newly recommended digestible indispensable AA score (DIAAS) and PDCAAS is that the true ileal AA digestibility for the dietary indispensable AA is used in DIAAS rather than a single faecal crude protein (CP) digestibility value. AA are absorbed from the small intestine only and are metabolised extensively by the microbiota of the hindgut. Terminal ileal digestibility is more accurate than faecal digestibility in estimating AA bioavailability( Reference Moughan and Stevens 5 – Reference Rowan, Moughan and Wilson 8 ). Moreover, PDCAAS underestimates the comparatively high nutritional values of some proteins by truncation and overestimates the quality of proteins containing anti-nutritional factors and limiting AA( Reference Schaafsma 9 – Reference Gilani, Xiao and Cockell 14 ). In contrast, DIAAS is not truncated for a single-source protein and is preferred to PDCAAS for the evaluation of protein quality by the FAO( 15 ). Digestibility should be based on the true ileal digestibility (TID) of each AA, which is preferably determined in humans, but if this is not possible, TID can be determined in growing pigs or rats( Reference Wolfe, Rutherfurd and Kim 16 ).
Cereal grains are often the main component of the human diet and provide a large proportion of the dietary protein for humans, especially in developing countries( Reference Bwibo and Neumann 17 ). Thus, accurately assessing the protein nutritional value of cereal grains is essential( Reference Lee, Weisell and Albert 11 ). Cereal grains and grain by-products are usually cooked before human consumption. Directly determining ileal AA digestibility in humans is difficult and expensive; thus, the Expert Consultation (FAO, 2013) recommended the use of pigs, which are the best models for adult humans; alternatively, growing rat can also be used( 15 , Reference Deglaire and Moughan 18 , Reference Butts, Monro and Moughan 19 ). In this study, we aimed to determine the apparent ileal digestibility (AID), TID values of AA and DIAAS values in nine cooked cereal grains fed to growing rats.
Methods
Materials
Brown rice, polished white rice, oats, tartary buckwheat, buckwheat, foxtail millet, proso millet, adlay and wheat were used. Adlay was purchased from the Guizhou Province, while oats and foxtail millet were procured from Inner Mongolia. The other cereal grains were obtained from Northwest A&F University. Wheat was baked into wheat bread according to the national standard (LST 3204-1993). The other cereal grains were soaked for 30 min with 25°C deionised water. The cereals were then cooked using a commercially available cooker as described by the manufacturer. The respective proportions of brown rice, polished white rice, oats, tartary buckwheat, buckwheat, foxtail millet, proso millet or adlay to water were 1:1·6, 1:1·6, 1:2·3, 1:20, 1:20, 1:1·8, 1:1·9 or 1:1·4 (w/v), respectively. All the cooked materials were freeze dried after cooking, and all the materials were ground through a size-60 mesh before inclusion into the diets.
Animals and diets
The animal experiments used 150 male Sprague–Dawley rats that were approximately 240 g in weight and were purchased from the Beijing Vital River Laboratory Animal Center. Rats were caged individually and were maintained under controlled temperature (22±2°C), humidity and airflow condition, with a 12-h on–off light cycle as described by Rutherfurd et al. ( Reference Rutherfurd, Fanning and Miller 3 ). Adequate measures were taken to minimise the pain or discomfort of the rats, and we used the smallest possible number of animals. The study was reviewed and approved by the Institutional Animal Ethics Committee at Jiangnan University (JN. No. 20170930k1201105 [36]). All animals were maintained according to local regulations and guidelines.
A total of eleven semisynthetic wheat starch-based diets (Table 1) were formulated to contain 100 g/kg CP, which was the sole protein source. To meet the requirements for growing rats, we added vitamins and minerals. A total of 3 g/kg of titanium dioxide was included in each diet as an indigestible marker. Purified sucrose, soyabean oil and cellulose were mixed in a ratio of 10:5:3 (180 g/kg DM). To maintain a dietary CP concentration of 100 g/kg for low-protein foods with CP concentration of <150 g/kg DM, the test ingredient was diluted with cellulose and soyabean oil (1:0·6); for foods with CP concentration of <100 g/kg DM, the test diet consists of the test ingredient, vitamin/mineral mixture and titanium dioxide( 20 ). The ingredient compositions of the basal and test diets are shown in Table 1. A protein-free-based diet was prepared for rats to determine the amount of endogenous loss of AA in the ileal content( Reference Moughan and Rutherfurd 21 ). A basal diet containing 100 g/kg protein was also formulated using casein as the sole source of protein( Reference Rutherfurd and Moughan 22 ).
Table 1 Ingredient composition of the experimental diet (g/kg DM)
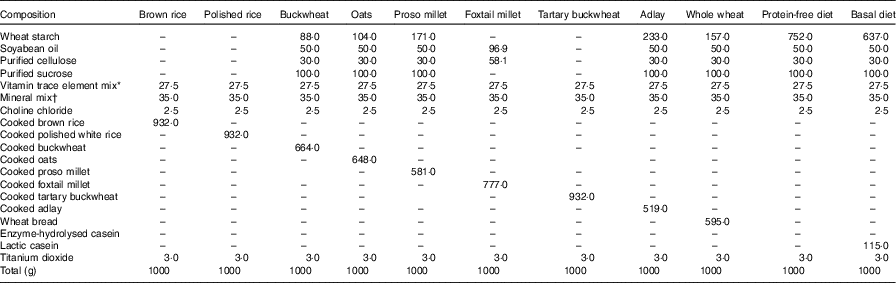
* The vitamins and trace elements are as follows: 250 mg retinol; 1·8 mg cholecalciferol; 1185 mg α-tocopherol; 1808 mg thiamine; 312 mg riboflavin; 2338 mg niacin; 2058 mg pantothenic acid; 312 mg pyridoxine; 1·8 mg cyanocobalamin; 125 mg phylloquinone; 93·9 mg folic acid; 4·56 g Mn; 10·29 g Fe; 904 mg Cu; 3273 mg Zn; 41 mg iodine; 7·5 mg Se; 39 mg Co.
† The mineral mix of the diet includes 25 g CaPO4, 5·3 g CaCO3, 3·6 g NaCl, 12·5 g KCl and 3·6 g MgSO4.
Experimental design
The rats (n 150) were randomly divided into 10 groups (n 15/group) as follows: brown rice group, polished rice group, buckwheat group, oats group, proso millet group, foxtail millet group, tartary buckwheat group, adlay group, whole wheat group and protein-free-based diet group. All rats were initially fed a standard basal diet for 7 d. After 1 week of acclimatisation period, the experimental groups received each diet in Table 1 for 7 d. Each rat received its respective diet in nine hourly meals (08.30–16.30 hours) daily. The diet was freely available for 10 min at each meal time. Water was also freely available. On the 14th day of the study, each rat was killed 5 h after the first meal through asphyxiation with CO2 gas( Reference Rutherfurd, Fanning and Miller 3 , Reference Rutherfurd and Moughan 23 ). Ileal contents were immediately collected from the terminal 20 cm of ileum. Given that the ileal content of each rat is insufficient for the AA detection through HPLC( Reference Rutherfurd and Moughan 22 ), three ileum contents were mixed into one sample in each group (n 5). All ileal content samples were freeze-dried and frozen (–20°C) while awaiting chemical analysis.
Chemical analysis
CP content was determined by rapid N cube (NY/T 2007–2011) using a N-to-protein conversion factor of 6·25. The AA contents were determined in triplicate 5-mg samples following hydrolysis in 500 µl of constant-boiling HCl (6 mol/l) for 24 h at 110±1°C in a hydrolysis tube( Reference Rutherfurd and Gilani 24 ). The liberated AA were derived with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and α-aminobutyric acid was used as the internal standard. The derivatives were separated on a Waters E2695 HPLC system equipped with a C18 column (150 mm×4·6 mm, 5·0 µm; Agilent) and quantified using Waters 2475 fluorescence detector at 395 nm emission and 250 nm excitation. To determine cysteine and methionine, we used performic acid oxidation at 0°C for 16 h, followed by neutralisation with HBr; then, we applied hydrolysis as described above. The concentration of titanium in the diets and ileal samples was determined through the method described by Short et al. ( Reference Short, Gorton and Wiseman 25 ). The samples were ashed, then digested in 60 % (v/v) sulfuric acid and finally added to 30 % hydrogen peroxide. Absorbance at 410 nm was measured. Tryptophan (Trp) was determined using the method described by Rutherfurd & Gilani( Reference Rutherfurd and Gilani 24 ). Free AA molecular weights were used for the calculation of the weight of each AA.
Data analysis
AA and CP contents in the terminal ileal digesta and the TID of AA were calculated by using the equation given by Rutherfurd et al. ( Reference Rutherfurd, Bains and Moughan 26 ). In addition, the endogenous ileal AA flows were determined for rats fed the protein-free diet( Reference Rutherfurd, Cui and Goroncy 27 ).
Apparent and true ileal AA digestibility was calculated using the following equations (units are g/kg DM intake)( Reference Stein, Seve and Fuller 6 , Reference Cervantes-Pahm, Liu and Stein 28 ):
where AIDAA is the AID of AA (%), AAdigesta is the concentration of AA in the ileal digesta DM, AAdiet is the concentration of AA in the diet DM, Tidiet is the concentration of Ti in the diet DM and Tidigesta is the concentration of Ti in the ileal digesta DM.
where IAAend is the ileal endogenous AA losses.
where the reference protein indispensable AA profile was the AA requirement pattern for the 0·5–3 years old child( 15 ).
DIAAS was calculated using the following equation( 15 , 20 ):
Statistical analysis
Calculation of sample size was performed using the ‘resource equation’ method, as described by Charan & Kantharia( Reference Charan and Kantharia 29 ), with a power of 80 % and significance of 5 %. Results were expressed as mean values with their standard errors. The Shapiro–Wilk comparison normality test was used to assess the distribution of all variables. Comparisons for normally distributed data between the two groups were conducted using two-tailed t test and one-way ANOVA followed by Tukey’s significance test for multiple comparisons. Mann–Whitney U and Kruskal–Wallis tests were used for non-parametric analysis when data were non-normally distributed. A P value of <0·05 was considered significant. All statistical calculations were performed on SPSS 21.0 data processing software (SPSS Inc.).
Results
Crude protein and amino acid compositions of nine cooked cereal grains
A total of eighteen AA were detected in nine cooked cereal grains. The total AA concentrations of the nine cooked cereal grains on an as-fed basis ranged from 8·3 % (polished rice) to 18·5 % (adlay; Table 2). The CP contents of the cooked cereal grains ranged from 9·15 % (polished rice) to 19·28 % (adlay). The CP contents of buckwheat, oats, proso millet, foxtail millet, adlay and whole wheat were higher (P<0·05) than those of brown rice, polished rice and tartary buckwheat. The cooked cereal grains had indispensable AA contents, ranging from 30·5 (brown rice) to 66·3 g/kg DM (adlay). The AA compositions in the diets based on the nine cooked cereal grains are shown in Table 3.
Table 2 Determined crude protein (CP) and amino acid (AA) compositions of cooked brown rice, polished rice, buckwheat, oats, proso millet, foxtail millet, tartary buckwheat, adlay and whole wheat (g/kg DM)Footnote * (Mean values with their standard errors)
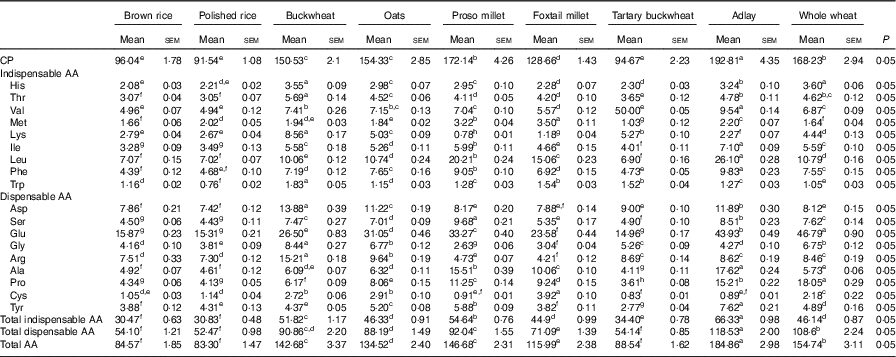
a,b,c,d,e,f,g,h Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Based on triplicate determinations. CP was based on a N-to-protein conversion factor of 6·25.
Table 3 Determined amino acid (AA) compositions of brown rice, polished rice, buckwheat, oats, proso millet, foxtail millet, tartary buckwheat, adlay and whole wheat-based diets (g/kg DM)* (Mean values with their standard errors)
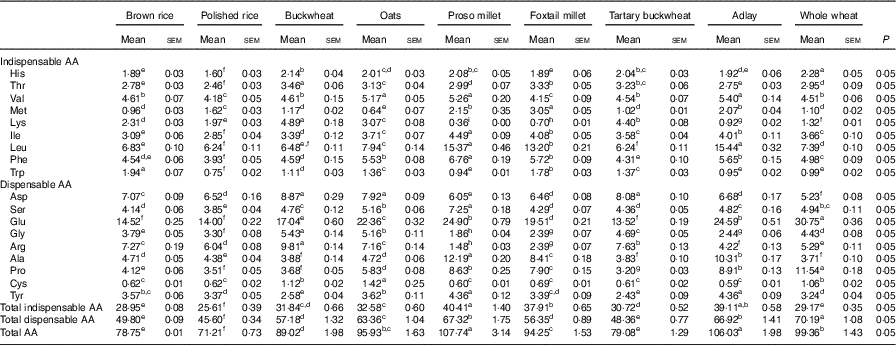
a,b,c,d,e,f,g,h Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Based on triplicate determinations. Crude protein was based on a N-to-protein conversion factor of 6·25.
Mean apparent ileal digestibility of amino acids in nine cooked cereal grains
The mean AID of indispensable AA in whole wheat was greater than that in any of the other cooked cereal grains (Table 4). The AID values of most AA in whole wheat were nonsignificantly different from those in adlay, except that the AID of leucine (Leu) in whole wheat was lower than that in adlay (P<0·05), whereas the AID of lysine (Lys) in whole wheat was higher than that in adlay (P<0·05). The mean AID of the indispensable AA and AID of Lys and Trp in proso-millet were the lowest among the values obtained for all cooked cereal grains. The mean AID of the indispensable AA and AID of all indispensable AA in proso millet were significantly lower (P<0·05) than that in foxtail millet. The mean AID of all AA in adlay were all greater than those in all the other cooked cereal grains, except whole wheat. Meanwhile, the mean AID of all AA in polished rice and proso millet were lowest among the values obtained for all cooked cereal grains (P<0·05).
Table 4 Mean apparent ileal digestibility of amino acid (AA) in brown rice, polished rice, buckwheat, oats, proso millet, foxtail millet, tartary buckwheat, adlay and whole wheat (%) (Mean values with their standard errors)
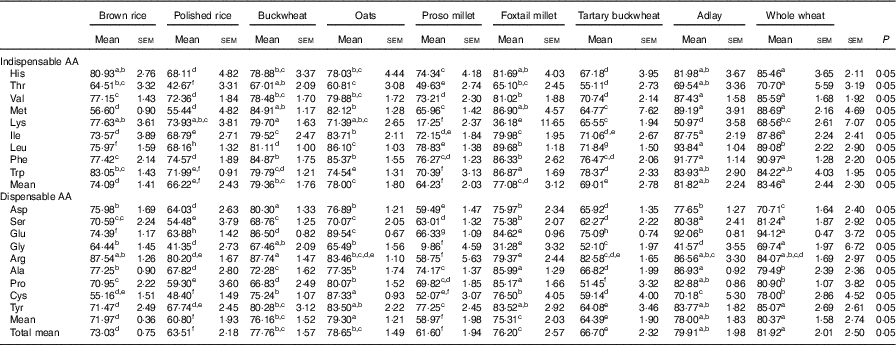
a,b,c,d,e,f Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Mean true ileal digestibility of amino acids in nine cooked cereal grains
The mean TID of indispensable AA in whole wheat and adlay were greater than those for other cooked cereals (P<0·05; Table 5). Furthermore, no difference was observed in the mean TID of indispensable AA between adlay and whole wheat. No difference was observed between the indispensable AA TID values of buckwheat and foxtail millet, although Leu and Trp TID values were greater (P<0·05) in foxtail millet than in buckwheat. The mean TID of the indispensable AA in polished rice, proso millet and tartary buckwheat were lower (P<0·05) than those of the other cooked cereal grains.
Table 5 Mean true ileal digestibility (TID) of amino acid (AA) in brown rice, polished rice, buckwheat, oats, proso millet, foxtail millet, tartary buckwheat, adlay and whole wheat (%)* (Mean values with their standard errors)

a,b,c,d,e,f Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* TID values were calculated by correcting the values for apparent ileal digestibility for the basal endogenous losses. Values used for the basal endogenous losses were follows (g/kg of DM intake): Asp, 1·09; Ser, 0·83; Glu, 1·25; Gly, 1·24; His, 0·26; Arg, 0·42; Thr, 0·73; Ala, 0·43; Pro, 0·76; Cys, 0·09; Tyr, 0·28; Val, 0·44; Met, 0·07; Lys, 0·40; Ile, 0·32; Leu, 0·55; Phe, 0·30; Trp, 0·08.
Mean true ileal digestibility concentrations for amino acids in nine cooked cereal grains
The total TID concentrations of indispensable AA in buckwheat were significantly lower than those for adlay, foxtail millet, proso millet and oats and significantly greater than that for brown rice, tartary buckwheat and polished rice (P<0·05; Table 6). Adlay had the highest TID concentrations of valine, isoleucine, Leu and tyrosine among the cooked cereal grains (P<0·05), and buckwheat and brown rice had the highest TID concentrations of Lys and Trp, respectively (P<0·05). Polished rice had the lowest total TID concentration of indispensable AA (P<0·05).
Table 6 Mean true ileal digestibility concentrations (g/kg DM) for amino acid (AA) in brown rice, polished rice, buckwheat, oats, proso millet, foxtail millet, tartary buckwheat, adlay and whole wheat (Mean values with their standard errors)

a,b,c,d,e,f,g,h Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Digestible indispensable amino acid score for nine cooked cereal grains
The following DIAAS values were obtained: 42, brown rice; 37, polished rice; 68, buckwheat; 43, oats; 7, proso millet; 10, foxtail millet; 47, tartary buckwheat; 13, adlay and 20, whole wheat (Table 7).
Table 7 Digestible indispensable amino acid scores (DIAAS) for brown rice, polished rice, buckwheat, oats, proso millet, foxtail millet, tartary buckwheat, adlay and whole wheatFootnote *
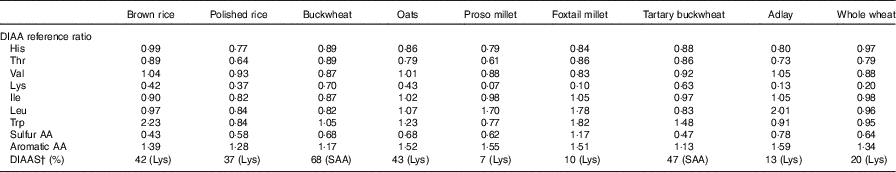
DIAA, digestible indispensable amino acid; AA, amino acid.
* DIAAS were calculated for the 0·5–3 years old child.
† Indispensable AA reference patterns are expressed as mg AA/kg protein: His, 16; Ile, 30; Leu, 61; Lys, 48; sulfur AA, 23; aromatic AA, 41; Thr, 25; Trp, 6·6; Val, 40( 2 ).
Discussion
The nine cereal grains tested in this study are commonly produced in different provinces in China. Buckwheat and tartary buckwheat belonging to Polygonaceae family grow mainly in Russia, China and India( Reference Zhang, Zhou and Tang 30 ). Proso millet (Panicum miliaceum L.) is consumed as a staple food among the majority of people who live in arid and semi-arid tropics of the world, such as Asia, Africa and some parts of Europe( Reference Lu, Zhang and Liu 31 ). Foxtail millet (Setaria italica) is one of the most important food crops of the semi-arid tropics in Asia and Africa( Reference Amadou, Le and Amza 32 ). Adlay (Coix lachryma-jobi L.) is mainly cultivated in China and Japan( Reference Wang, Chen and Su 33 ). Many recent studies indicated that the consumption of these cereal grains are beneficial because they reduce the risk of acquiring chronic diseases( Reference Zhang, Zhou and Tang 30 , Reference Amadou, Le and Amza 32 , Reference Guo, Ma and Parry 34 – Reference Chen, Chung and Chiang 36 ).
The protein and AA contents of protein sources should be determined and the TID of each indispensable AA in the test protein should be used to allow calculation of accurate DIAAS values( Reference Wolfe, Rutherfurd and Kim 16 ). Grain proteins play many important roles in human health; thus, assessing their quality after processing is important. A few decades ago, the FAO established a method for protein nutritional value assessment. AA digestibility determination at the terminal ileum is more accurate than the traditional faecal method( Reference Wielen, Moughan and Mensink 37 ). Although ileal digestibility may not be a perfect measure to determine net AA absorption, it is considerably closer than the AA digestibility determined over the total digestive tract( Reference Fuller 38 ). TID values are usually very accurate unless a protein has been overheated, which may result in reduced digestibility of Lys( 15 ). The variations in the AID values may be a result of the differences among grain varieties and growing conditions of the grains( Reference Nosworthy, Neufeld and Frohlich 39 ). Therefore, protein evaluation can be improved by calculating the TID values of AA and removing the influences of basal endogenous losses of AA on determined digestibility values( Reference Stein, Seve and Fuller 6 ).
In the 2011 Protein Quality Expert Consultation, DIAAS was reported to provide more accurate protein quality scores than the PDCAAS( 15 ). However, nearly all available DIAAS data were obtained from pig models, and those derived from humans remains insufficient( Reference Lee, Weisell and Albert 11 ). In this study, the DIAAS values obtained from polished rice, oats and whole wheat were lower than those reported by Cervantes-Pahm et al. ( Reference Cervantes-Pahm, Liu and Stein 28 ), Mathai et al. ( Reference Mathai, Liu and Stein 40 ) and Abelilla et al. ( Reference Abelilla, Liu and Stein 41 ). According to the DIAAS cut-off value introduced by an FAO Expert Consultation report and the study performed by Cervantes-Pahm et al. ( 15 , Reference Cervantes-Pahm, Liu and Stein 28 ), only dehulled oats are good protein sources for human consumption because its DIAAS is 77. However, the DIAAS was 68 for buckwheat, 47 for tartary buckwheat and 43 for oats in this study. It is possible that buckwheat and tartary buckwheat are better protein sources than oats. However, further work is needed to compare the digestibility in the rat-based assay to that in human-based studies with the use of the same foods when consumed by humans.
In conclusion, diets based on proso millet and foxtail millet require more AA supplementation than those based on buckwheat, tartary buckwheat, oats and brown rice for them to meet the balanced AA based on DIAAS values in this study. DIAAS value obtained from cereal grains can provide comprehensive nutritional information and a scientific basis for the evaluation of the nutritional values of proteins contained in different cereals. Given the DIAAS values obtained from cereal grains, the rational combination of various cereal grains had increased protein quality in human diets and is useful as a scientific basis for formulating balanced diets.
Acknowledgements
The authors thank Shane M. Rutherfurd at Massey University for his skilful technical assistance. The authors also thank Professor Paul J. Moughan at Massey University for his critical reading of this manuscript and helpful suggestions.
This study was financially supported by the Special Funds of Basic Research of Central Public Welfare Institute (no. ZX1731) and the non-profit industry (grain) Scientific Research Special Fund Agreement (no. 201513003-8) from the Ministry of Finance, People’s Republic of China.
F. H. was involved in the design of the experimental protocol and discussion of the results. F. L. H., Y. W., L. P. F., G. S., X. C., P. J., H. J. M. and Y. Y. H. performed the experiments and collected data. Y. W. and F. L. H. wrote the first draft of the manuscript; and all authors critically reviewed the manuscript and approved the final content.
The authors declare that there are no conflicts of interest.










