In recent years, several reports in the veterinary literature have associated low vitamin D status with many different disease processes in dogs, including chronic kidney disease( Reference Gerber, Hassig and Reusch 1 ), congestive heart failure( Reference Kraus, Rassnick and Wakshlag 2 ), inflammatory bowel disease( Reference Gow, Else and Evans 3 ), mast cell tumour( Reference Wakshlag, Rassnick and Malone 4 ) and cancer( Reference Selting, Sharp and Ringold 5 ). Whether low vitamin D status is causative or a consequence of disease has not been established. Nonetheless, studies such as these have brought attention to vitamin D status and health in adult dogs.
Dogs are unable to adequately synthesise vitamin D3 (D3) in their skin in response to UV light( Reference How, Hazewinkel and Mol 6 ) and, therefore, are reliant upon their diet to supply their vitamin D needs. The dietary vitamin D requirement for adult dogs is not clearly established. The current adequate intake of vitamin D recommended by the National Research Council (NRC) for dogs in all life stages is based on findings of studies for the prevention of skeletal abnormalities in puppies( 7 ). This vitamin D recommendation, though supporting of normal bone growth and maintenance in a puppy, may not be sufficient for other health outcomes in adult dogs.
It is widely accepted that the best indicator of vitamin D status is serum 25-hydroxyvitamin D (25(OH)D), as it is the most abundant circulating metabolite of vitamin D, and its concentration is determined by vitamin D intake( Reference Stocklin and Eggersdorfer 8 ). Reports of serum 25(OH)D concentrations amongst apparently healthy dogs are quite varied( Reference Selting, Sharp and Ringold 5 , Reference Fairweather, Eason and Elder 9 – Reference Young and Backus 11 ). One study attempted to define vitamin D sufficiency in healthy, adult dogs by comparing the relationship between serum 25(OH)D concentrations and intact parathyroid hormone (iPTH)( Reference Selting, Sharp and Ringold 5 ). Investigators found that the median and variance in iPTH observations among dogs declined to a plateau when 25(OH)D concentrations were at 100 ng/ml. They also found a significant drop in variability of serum mean C-reactive protein concentrations, a marker of chronic inflammation, when 25(OH)D concentrations were 100–120 ng/ml. The authors concluded that vitamin D sufficiency is indicated when serum 25(OH)D is 100–120 ng/ml, and that many apparently healthy dogs are vitamin D insufficient.
The present authors found in a previous cohort survey of forty-six adult, healthy dogs( Reference Young and Backus 11 ) that 71·7 % had serum 25(OH)D concentrations below 100 ng/ml. A subsequent D3 supplementation trial was conducted in thirteen of the dogs deemed vitamin D insufficient. Seven dogs received D3 in an olive oil solution on their food at 2·3 µg/kg body weight (BW)0·75 per d, an amount that was 5·1 times the NRC recommended allowance but not in excess of the safe upper limit for maintenance of adult dogs (2·6 µg/kg BW0·75)( 12 ). Six dogs received an olive oil placebo. Unexpectedly, we found that D3 supplementation, at an oral dosage that we believed to be substantial, did not significantly increase serum 25(OH)D concentrations above baseline. At the end of 9 to 10 weeks of supplementation, only a modest difference (12 %) in vitamin D status resulted between the treated and control dogs.
The cause of the poor response to vitamin D supplementation was not apparent, but it prompted us to investigate the use of 25(OH)D3 as a supplement in dogs. The objective of this study was to determine the relative potency of 25(OH)D3 as compared with D3 for increasing vitamin D status in dogs deemed vitamin D insufficient. Based on a previous report in dogs( Reference Dusso, Lopez-Hilker and Rapp 13 ), we hypothesised that vitamin D status, as measured by serum 25(OH)D3 and 24R,25-dihydroxyvitamin D3 (24R,25(OH)2D3) concentrations, would be significantly more responsive to 25(OH)D3 than D3.
Materials and methods
All procedures were reviewed and approved by our institution's animal care and use committee. Four male and three female institution-owned, 4-year-old, intact, Chinese crested/beagle dogs were studied in a randomised, single cross-over trial. The dogs belonged to the same litter and were in ideal body condition (body condition score 5/9), with BW ranging from 5·9 to 10·7 kg. The dogs were consuming a commercially produced laboratory diet formulated to meet Association of American Feed Control Officials (AAFCO) dog food nutrient profiles for adult canine maintenance fed ad libitum (LabDiet 5006; PMI Nutrition International, Inc.). The vitamin D content of the diet as measured by an independent laboratory (Covance Laboratories, Inc.) was 330 IU/100 g.
Study design
Upon obtaining the dogs for study, jugular venous blood was collected following an overnight food withholding, and serum was harvested for 25(OH)D analysis. Immediately prior to entry into the trial, jugular venous blood was again collected following an overnight food withholding for complete blood counts with manual differentials and clinical serum chemistry analyses to screen each dog for underlying disease. The dogs were subsequently transitioned over a 7-d period to a commercially produced diet identical in ingredients to their previous diet and produced by the same manufacturer, with the exception of no vitamin D supplementation, and maintained on the diet for the duration of the study. The vitamin D content of the diet as measured by the same laboratory was <4 IU/100 g.
Dogs were weighed weekly, fed an amount of diet to maintain ideal BW, and evaluated each week for vitamin D status throughout the study. At each evaluation, jugular venous blood was obtained following an overnight food withholding, and serum was harvested for 25(OH)D3 and 24R,25(OH)2D3 analyses. Following a 1-month run-in period consuming the diet, the dogs voluntary consumed 2–3 g of a treat (Canine Carry Out; Big Heart Pet Brands) to which small volumes (5–10 µl) of ethanolic solutions were applied of D3 (cholecalciferol; Sigma-Aldrich) at a dosage of 2·3 µg/kg BW0·75 per d (n 4), or a molar equivalent dosage as 25(OH)D3 (Sigma-Aldrich) (n 3). The vitamin D content of the treat measured by the same laboratory was <4 IU/100 g. Treatments were given daily until serum 25(OH)D3 concentrations determined on a weekly basis exceeded 100 ng/ml, at which time the washout period began. When serum 25(OH)D3 concentrations returned to baseline, the treatments were resumed in a cross-over assignment.
Laboratory analyses
The clinical haematology (Sysmex xT-2000i; Sysmex America, Inc.) and serum chemistry analyses (Beckman AU 400e; Beckman Coulter, Inc.) were performed at the University of Missouri Veterinary Medical Diagnostic Laboratory, Columbia, MO. Serum concentrations of the vitamin D metabolites were determined using a modification of an HPLC method previously reported( Reference Lensmeyer, Wiebe and Binkley 14 ), but with 25(OH)D2 in place of 3H-labelled 25(OH)D3 as internal standard.
Statistical analysis
Statistical analyses were performed using proprietary software (SAS® 9.3; SAS Institute). All variable observations were found to be normally distributed except 24R,25(OH)2D3 concentrations between the treatments at weeks 2 and 4. For normally distributed observations, the significance of differences within and between treatments was determined with paired t tests. For non-normally distributed observations, significance was tested within and between treatments with a signed rank test.
The fractional rate of decline in serum 25(OH)D3 concentration (k) was determined from the slope of linear-regressed, log-transformed, serum 25(OH)D3 concentrations observed during the 4 weeks following supplement withdrawal using the equation: t ½ = 0·693/k, where 0·693 equals the natural log of 2 and k is the weekly fractional rate of decrease in 25(OH)D3 concentration. This assumes a first-order washout curve as indicated by findings in a previous study( Reference Dougherty, Center and Dzanis 15 ). P values ≤0·05 were considered significant.
Results
With only a few exceptions, complete blood counts and serum chemistry analyses results among the dogs were within the clinical laboratory reference ranges. The exceptions were not of parameters relevant to the study. The mean 25(OH)D3 concentration for all dogs immediately prior to entry into the trial was 24 (sd 10) ng/ml. At 1 week after supplementations with both D3 and 25(OH)D3, serum 25(OH)D3 concentrations increased significantly above baseline (P < 0·01, P < 0·0001, respectively). However, supplementation with 25(OH)D3 resulted in over two times greater serum 25(OH)D3 concentrations at week 1 (mean 70 v. 31 ng/ml; P < 0·0001). By the second week of supplementation with 25(OH)D3, mean 25(OH)D3 concentration reached 112 (sd 14) ng/ml, which was significantly greater (P < 0·0001) than concentrations with D3 supplementation (41 (sd 13) ng/ml). Supplementations during each phase of the crossover trial were discontinued after 2 weeks when mean serum 25(OH)D3 concentrations were found in excess of 100 ng/ml. Serum 25(OH)D3 concentrations declined to baseline within 4 weeks of discontinuation of supplementations. Throughout the washout period, serum 25(OH)D3 concentrations remained significantly greater (P ≤ 0·02) in dogs when they were given 25(OH)D3 compared with when they were given D3. The rate of decline in serum 25(OH)D3 concentration was approximately twice as rapid in dogs when given 25(OH)D3 (t½ = 1·8 weeks) than when given D3 (t½ = 3·6 weeks) (Fig. 1). Serum concentrations of 24R,25(OH)2D3 were significantly increased (P ≤ 0·02) above baseline following supplementation of 25(OH)D3 but not D3. The increase in 24R,25(OH)2D3 was delayed, occurring 3–5 weeks after initiation of supplementation of 25(OH)D3 and varied among dogs. At 3 and 5 weeks after supplementation began, serum concentrations of 24R,25(OH)2D3 were significantly greater (P ≤ 0·008) when 25(OH)D3 was supplemented as compared with D3 (Fig. 2). Peak serum 24R,25(OH)2D3 concentrations reached 50–119 ng/ml following treatment with 25(OH)D3.
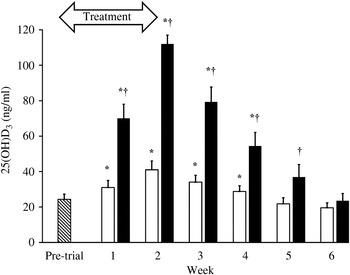
Fig. 1. Serum 25-hydroxyvitamin D3 (25(OH)D3) concentrations prior to entry into the trial (n 7; ![]() ) and weekly throughout vitamin D3 (n 7; □) and 25(OH)D3 (n 7; ■) supplementation and washout period. The treatment period is highlighted by the arrow. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that at pre-trial (P ≤ 0·01, paired t test). † Mean value was significantly different from that for vitamin D3 supplementation (P ≤ 0·02, paired t test).
) and weekly throughout vitamin D3 (n 7; □) and 25(OH)D3 (n 7; ■) supplementation and washout period. The treatment period is highlighted by the arrow. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that at pre-trial (P ≤ 0·01, paired t test). † Mean value was significantly different from that for vitamin D3 supplementation (P ≤ 0·02, paired t test).
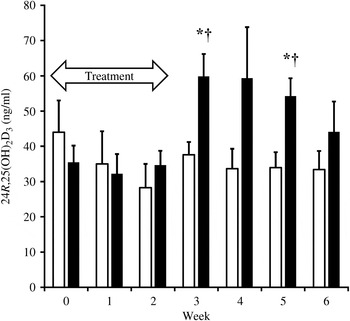
Fig. 2. Serum concentrations of 24R,25-dihydroxyvitamin D3 (24R,25(OH)2D3) during supplementation of vitamin D3 (n 7; □) and 25(OH)D3 (n 7; ■) and washout period. The treatment period is highlighted by the arrow. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that at week 0 (P ≤ 0·02, paired t test). † Mean value was significantly different from that for vitamin D3 supplementation (P ≤ 0·008, paired t test).
Discussion
Our objective in comparing the relative potency of 25(OH)D3 to D3 for increasing vitamin D status in dogs is based upon our previous finding of a weak response to D3 supplementation in dogs deemed vitamin D insufficient( Reference Young and Backus 11 ). With studies indicating that dogs with chronic disease have low vitamin D concentrations( Reference Gerber, Hassig and Reusch 1 – Reference Selting, Sharp and Ringold 5 ), as well as many healthy dogs( Reference Selting, Sharp and Ringold 5 , Reference Fairweather, Eason and Elder 9 – Reference Young and Backus 11 ), research into an effective and safe means to improving vitamin D status seems warranted. The use of 25(OH)D3 supplementation as a means to improve vitamin D status in the dog has not been evaluated, as it has in people. Earlier work has demonstrated that 25(OH)D3 is absorbed in the human intestine similar to D3 ( Reference Stamp 16 ), yet peak concentrations in serum 25(OH)D after oral administration in humans are reached much more rapidly, as compared with the slow rise in concentrations that are typically observed after D3 administration( Reference Stamp 16 , Reference Haddad and Rojanasathit 17 ). Recently published oral 25(OH)D3 supplementation studies have shown that 25(OH)D3 is much more efficient and rapid at increasing vitamin D status in humans than D3 ( Reference Cashman, Seamans and Lucey 18 – Reference Jetter, Egli and Dawson-Hughes 20 ).
In accordance with this, we have also found that at equivalent doses in dogs, oral supplementation with 25(OH)D3 is much more effective and rapid than D3 in raising serum 25(OH)D3 concentrations above a previously reported minimum indicative of vitamin D sufficiency( Reference Selting, Sharp and Ringold 5 ) (Fig. 1). While both supplementations significantly increased serum 25(OH)D3 concentrations above baseline, oral 25(OH)D3 was at least 5·2 times as potent as D3 after just 2 weeks of supplementation. Due to an unacceptably high trajectory of serum 25(OH)D3 concentrations following supplementation with 25(OH)D3, the trial was discontinued after 2 weeks, before equilibrium could be safely established. Therefore, it is possible that this is an underestimation of the true potency of 25(OH)D3 relative to D3.
Our present finding of a significant increase in serum 25(OH)D3 concentrations following supplementation with D3 is in contrast to our previous study results( Reference Young and Backus 11 ). Although the D3 doses were the same (2·3 µg/kg BW0·75 per d), the vehicle of D3 supplement delivery differed. Bioavailability of D3 may be greater with the treat application presently used compared with our previous top-dressing of food with an olive oil solution of D3. However, dogs in each study willingly accepted both methods of supplement delivery. Studies on factors affecting vitamin D absorption, distribution and metabolism in dogs are lacking. Additionally, the present study should be considered to be much more controlled than our previous work, in which privately owned dogs were studied and D3 supplementation depended on owner compliance.
Concentration of 24R,25(OH)2D in serum is well established to positively correlate with serum 25(OH)D concentration in the dog( Reference Tryfonidou, Oosterlaken-Dijksterhuis and Mol 21 ). Serum 24R,25(OH)2D3 concentrations were significantly increased by supplementation of 25(OH)D3 but not with D3. However, this does not occur until 3 and 5 weeks following initiation of supplementation. The variation in production of this metabolite amongst the dogs probably resulted in the insignificant difference in concentrations between the supplementations at the second week of the washout period (Fig. 2). This probably indicates a lag time in the 24-hydroxylase activity in the kidney necessary to convert 25(OH)D3 to 24R,25(OH)2D3, as has been demonstrated in humans( Reference Wagner, Hanwell and Schnabl 22 ).
A noteworthy limitation of this study is the small number of dogs and lacking of investigation of factors evidenced to influence serum 25(OH)D concentrations in dogs, such as sex (males > females), reproductive status (intact > neutered) and breed( Reference Sharp, Selting and Ringold 10 ). In conclusion, our findings indicated that oral supplementation of 25(OH)D3 is at least 5·2 times as potent as D3 for increasing vitamin D status in dogs with low serum 25(OH)D concentrations. While this work is supportive of the use of 25(OH)D3 as a supplement means to improve vitamin D status in the dog, a safe dosage was not identified and will require further investigation.
Acknowledgements
This work was supported by the Nestlé Purina Endowed Program in Small Animal Nutrition, College of Veterinary Medicine, University of Missouri, Columbia, MO 65211. The funders had no role in the design, analysis or writing of this article.
L. R. Y. was the co-investigator of the study, contributed to the study design, processed and analysed the data, and wrote the manuscript. R. C. B. was the principal investigator of the study, contributed to the study design, processed and analysed the data, and contributed to writing the manuscript.
There were no conflicts of interest.




