INTRODUCTION
A diverse range of microbes are associated with infections of wounds. Recently, particular concern has emerged for community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA), particularly strain USA300, as the latter has been linked with the development of severe skin and soft tissue infections [Reference Ma1–Reference Saravolatz, Pohlod and Arking3], including necrotizing fasciitis [Reference Miller4]. According to the guidelines from the Centers for Disease Control and Prevention, CA-MRSA can be epidemiologically defined as a diagnosis of MRSA when the infection is (i) acquired outside of a hospital or within the first 48 h of hospital admission, and/or (ii) the individual has not been hospitalized or had a medical procedure that breached the skin (e.g. surgery or dialysis) in the past year [5]. In North America the prevalent CA-MRSA strains (i.e. USA300 and USA400) have a characteristic pulsed-field gel electrophoresis (PFGE) pattern and there is a high correlation of the presence of the Panton–Valentine leukocidin (PVL) gene with the USA300 strain [Reference Deurenberg6]. Most CA-MRSA isolates also exhibit preserved susceptibility to non-β-lactam antibiotics [Reference Kowalski, Berbari and Osmon7].
Individuals who inject illicit drugs are more likely to be positive for CA-MRSA [Reference Young2, Reference Saravolatz, Pohlod and Arking3, Reference Huang8, Reference Saravolatz9]. This is not surprising given the intrinsic host factors related to bacterial transmission and infection, such as compromised skin integrity, as well as extrinsic factors of poor personal hygiene or environmental conditions such as crowding [Reference Young2, Reference Saravolatz, Pohlod and Arking3, Reference King10], residing in shelters or single room occupancy hotels [Reference Shannon11].
It is important to examine the prevalence of MRSA infections in injection drug users (IDUs) as these organisms may cause severe morbidity and a greater mortality compared to methicillin-sensitive S. aureus (MSSA) strains [Reference Nelson and Masters Williams12, Reference Huang13]. In addition, it is of value to have a measure of MRSA prevalence from a community-based sample as it provides an indicator of the level of CA-MRSA, compared to a sample of IDUs recruited from within a hospital or emergency department (ED), which would capture IDUs requiring medical attention and may therefore give higher rates of MRSA infection than seen in the community at large. Furthermore, CA-MRSA remains susceptible to a limited number of antibiotics and their inappropriate use may promote development of resistance to these agents [Reference Bradley14]. Finally, IDUs could be a potential source for the importation of CA-MRSA into the hospital setting which may result in secondary nosocomial transmission.
This paper describes the microbiological distribution and related antibiotic susceptibilities of wound cultures in a community-recruited cohort of IDUs and provides data on the characterization of S. aureus recovered from these subjects.
METHODS
Study sample
Vancouver's supervised injection facility (SIF) has been evaluated through the Scientific Evaluation of Supervised Injection (SEOSI) cohort, which has been described in detail [Reference Wood15]. Briefly, the cohort was assembled through random recruitment of IDUs from within the SIF [Reference Wood15].
From 7 July to 28 November 2008, a sub-study was conducted in SEOSI participants who were recruited during visits to the SEOSI research office. All SEOSI participants were eligible for the sub-study. A brief questionnaire was completed by the subject and a swab of any open wound was collected by a physician or a study nurse. Only one wound swab was collected per participant. If wounds appeared infected and were thought to require additional medical treatment, participants were referred to the ED or their primary-care physician. Informed consent was obtained for all participants. The University of British Columbia–Providence Health Care Research Ethics Board approved the study.
Microbiology
Wounds were swabbed for culture of aerobic organisms using Venturi transystem culture swabs (Copan, Italy). In order to identify all aerobic organisms, swabs were streaked onto 5% sheep's blood agar and MacConkey agar plates (Columbia agar, PML Microbiologicals, USA). A wound was considered culture negative if no bacterial colonies were recovered, or if only normal skin flora grew. MRSA was identified using a selective, chromogenic medium (MRSASelect, Bio-Rad, France) and confirmed by a combination of cefoxitin disk testing and/or penicillin-binding protein 2a detection. Isolates were confirmed by PCR for the presence the mecA and nuc genes [Reference Costa16]. CA-MRSA were classified by the presence of the LukS-PV and LukF-PV genes encoding PVL, tested by PCR [Reference Lina17]. Antimicrobial susceptibility of isolates was determined by automated micro-broth susceptibility testing according to current Clinical and Laboratory Standards Institute (CLSI) guidelines [18]. Kirby–Bauer susceptibility testing was performed when needed as a supplementary test. Confirmatory disk-diffusion testing for clindamycin resistance by D-test was also performed.
Statistical analyses
Baseline characteristics of the overall SEOSI cohort were compared to the subsample of subjects. Of the subsample, we then examined baseline characteristics stratified by presence or not of a wound, and by a positive or negative culture for CA-MRSA. Baseline variables considered included sex at birth, age, living in unstable housing, residing in the Downtown Eastside (DTES) of Vancouver, requiring assistance with injecting, borrowing a syringe, at least once daily cocaine injecting, at least once daily heroin injecting, presence of a skin abscess, and HIV serostatus. Antibiotic use in the previous 6 months was also documented. Living in unstable housing was defined as living in a single room occupancy hotel, shelter, recovery or transition house, jail, on the street, or having no fixed address as opposed to living in an apartment or house. Variables refer to behaviour from the previous 6 months unless otherwise indicated. Pearson's χ2 test was used for categorical variables and the Wilcoxon rank sum test was used for continuous variables. SAS statistical software version 9.1 was used for these analyses (SAS Institute Inc., USA). A P value of ⩽0·05 was considered significant and all P values were two-sided.
RESULTS
Study sample
The SEOSI cohort included 1090 users of Vancouver's SIF. At baseline, 29% were female [median age 38·1 years; interquartile range (IQR) 32·6–43·9 years], 58% were living in unstable housing, 65% were residing in DTES, 23% were requiring assistance with injecting, 9% were borrowing a syringe, 23% were injecting cocaine at least once daily and 44% were injecting heroin at least once daily, 24% reported having an abscess, and 19% were HIV positive.
Of SEOSI participants, 41 (4%) individuals lacked information on abscesses and 36 (3%) individuals lacked HIV serostatus data. These participants were excluded from further analyses. Fourteen participants of the subsample provided consent for the overall SEOSI cohort but not the subsample and are included in the overall SEOSI data for Table 1. In Table 2, baseline information includes 204 participants. For the SEOSI subsample, five individuals had missing data on the abscess variable and were excluded from this univariate analysis.
Table 1. Baseline characteristics and unadjusted odds ratios of SEOSI cohort compared to SEOSI subsample
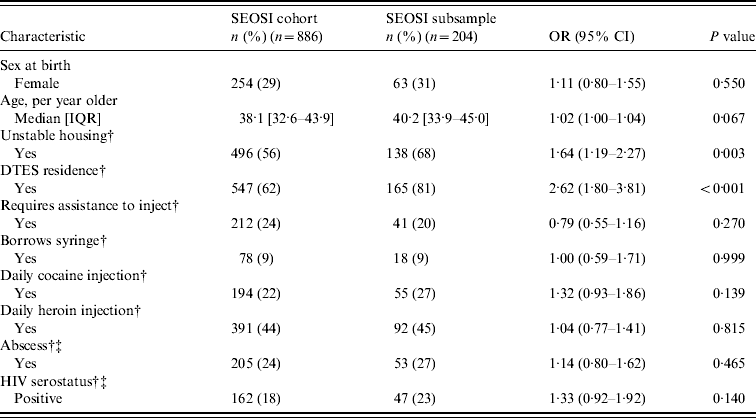
SEOSI, Scientific Evaluation of Supervised Injection; OR, odds ratio; CI, confidence interval; IQR, interquartile range; DTES, Downtown Eastside.
† Relates to previous 6 months.
‡ Missing data on 41 participants for abscess variable and 36 for HIV serostatus variable.
Table 2. Baseline characteristics and unadjusted odds ratios of SEOSI subsample with a wound compared to those without a wound
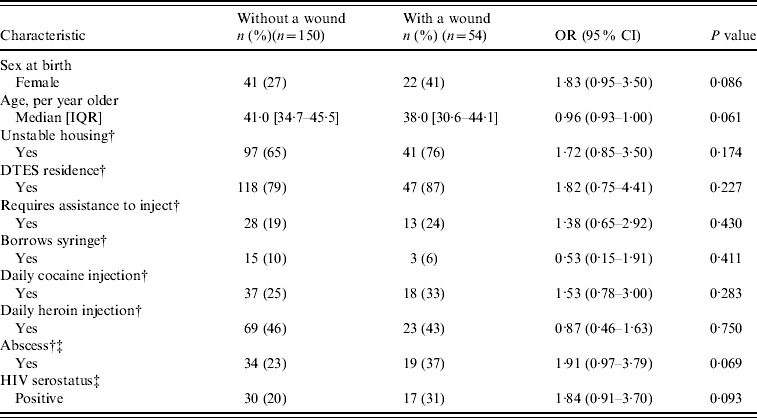
SEOSI, Scientific Evaluation of Supervised Injection; OR, Odds ratio; CI, confidence interval; IQR, interquartile range; DTES, Downtown Eastside.
† Relates to previous 6 months.
‡ Missing information on five participants.
As seen in Table 1, compared to the overall SEOSI cohort, participants of this subsample were more likely to be living in unstable housing [odds ratio (OR) 1·64, 95% confidence interval (CI) 1·19–2·27)] and be residing in DTES (OR 2·6, 95% CI 1·80–3·81). The median age of the subsample was 40·2 years (IQR 33·9–45·0 years).
Our subsample consisted of 218 SEOSI participants, of whom 59 (27%) were found to have a wound. As shown in Table 2, in the subsample, no significant differences were seen between individuals who presented with a wound compared to those who did not present with a wound. Moreover, there were no significant differences between those with a positive or negative CA-MRSA culture except for those who reported having an abscess (OR 2·16, 95% CI 1·31–3·54) (data not shown). Most wounds were located on the extremities: upper extremity (n=26, 44%) or lower extremity (n=19, 32%). Wounds were also located on the head (n=12, 20%) and trunk (n=2, 3%).
Microbiology
Of the 59 (27%) participants who had a wound present, 42 (71%) had a positive wound culture and of those 16 (27%) had a positive wound culture for CA-MRSA (Fig. 1). In the latter, four grew other species (group A and group B streptococcus, Enterobacter and Pseudomonas spp. and MSSA) in addition to CA-MRSA. Eleven (26%) of culture-positive wounds, were polymicrobial. Of those with a positive CA-MRSA culture, 11 (69%) reported antibiotic use for a skin infection in the last 6 months, compared to eight (42%) with MSSA (P=0·07).
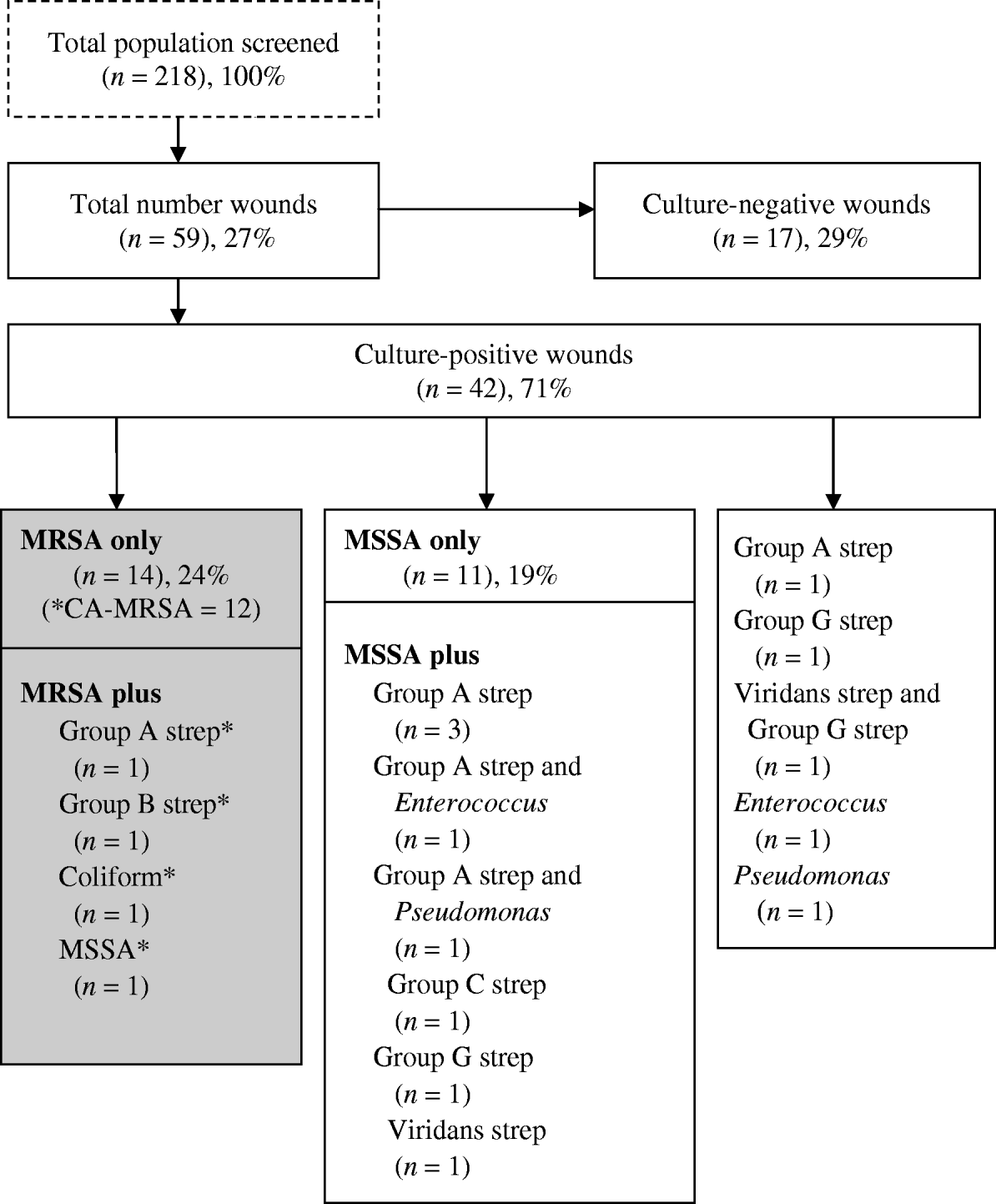
Fig. 1. Microbiology distribution of wounds in a subsample of the Scientific Evaluation of Supervised Injection cohort. * Denotes CA-MRSA (n=16): in all wounds (16/59, 27%); in culture-positive wounds (16/37, 43%); in MRSA (16/18, 89%).
All CA-MRSA isolates were susceptible to tetracycline, rifampin, vancomycin and linezolid. Trimethoprim–sulfamethoxazole (TMP–SMX) susceptibility in CA-MRSA isolates was 94%, while only 13% of the CA-MRSA isolates were susceptible to clindamycin compared to 63% of MSSA isolates (Table 3).
Table 3. Antimicrobial susceptibility patterns of Staphylococcus aureus isolates from wound cultures according to type (% susceptibility)
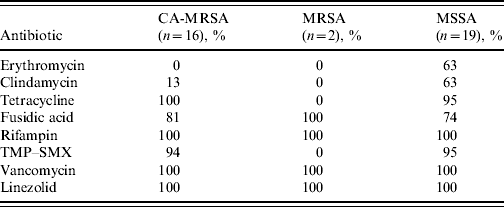
CA-MRSA, Community-associated methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; TMP–SMX, trimethoprim–sulfamethoxazole.
DISCUSSION
Twenty-seven percent of IDUs surveyed in our study had at least one wound, the majority of which were culture positive for S. aureus. Of those with wounds, 27% grew CA-MRSA; 89% of MRSA isolates were classified as CA-MRSA. These results indicate that CA-MRSA is widespread in IDUs in our setting. A recent local investigation of a similar population on more than 300 isolates of S. aureus had shown that over 95% of PVL-positive strains were correlated with the presence of SCCmecIV and the USA300 PFGE profile. Therefore, we used the detection of genes encoding for PVL as a marker for CA-MRSA.
Injection drug use has been associated as a broad risk factor for CA-MRSA infection [Reference Huang8]. Skin and soft tissue infections may be caused by the elaboration of PVL toxin which is capable of destroying leukocytes and leading to severe tissue damage [18]. There may be an additional risk of CA-MRSA transmission during the injection process, such as the preparation of drugs prior to injection, injection paraphernalia or the injecting environment. Microbiological assessments from a hospital in Los Angeles have shown an explosive increase in MRSA prevalence in arm abscesses (5% MRSA of S. aureus cultures in 1999 rising to 82% in 2005) [Reference Allison19].
Consistent with the literature, S. aureus was the most prevalent microbe found in wounds [Reference Young2, Reference Huang8, Reference Brown and Ebright20]. However, the proportion of CA-MRSA in the total S. aureus found in our study (43%, 16/37) is lower than a hospital-based study in Atlanta, where 63% of S. aureus skin and soft tissue infections were due to CA-MRSA [Reference Huang13]. Similarly, a study that examined acute purulent skin and soft tissue infections in the ED of 11 cities across the USA in 2004 found S. aureus in 76% of all wounds and of these 78% were MRSA (59% prevalence overall) and the USA300 strain accounted for 97% of MRSA isolates [Reference Talan, Summamen and Finegold21]. The disparities in proportion may be related to recruitment methods. Hospital-based studies capture more serious wounds that require medical attention compared to the wounds collected in a community-based sample such as ours that did not necessarily require medical treatment.
Our finding that 19% (11/59) of all wounds and 26% (11/42) of culture-positive wounds were polymicrobial aligns with an understanding that cutaneous injection-related infections (CIRI), skin and soft tissue infections in IDUs, are frequently positive for more than one species of bacteria. A randomized control study from a Los Angeles hospital compared the effectiveness of abscess treatment for IDUs and non-IDUs and reported that in IDUs, 50% of abscesses were positive for more than one species and was slightly lower (43%) in those who did not inject drugs [Reference Talan, Summamen and Finegold21]. The fact that the study examined both aerobic and anaerobic bacteria plus infections that required treatment may attribute to the higher polymicrobial proportion compared to our study.
Although susceptibility to antibiotics varies in different settings and longitudinally, it is clinically relevant that only 13% of CA-MRSA isolates here were susceptible to clindamycin as these strains have been reported to be highly susceptible to clindamycin [Reference Moran22]. However, the latter study did report a shift in antimicrobial susceptibility in different settings [Reference Moran22], which may reflect particular bias in antibiotic use. Further, 31% (n=13) of culture-positive wounds contained Streptococcus spp. and 24% (n=10) were polymicrobial wounds with either MRSA or MSSA, which lends support for the inclusion of anti-streptococcal antibiotics when treating CIRI [Reference Ebright and Pieper23]. However, a recent randomized control trial on the treatment of uncomplicated abscesses in a population at risk of CA-MRSA, suggested antibiotic prescription after incision and drainage was unnecessary [Reference Rajendran24].
There are limitations to this research that should be acknowledged. This study was based on a convenience sample from SEOSI participants who visited the research study office during periods of recruitment. It is possible that participants in periods of intense drug use or who had a serious medical condition may have been less likely to attend the research site during office hours. Individual characteristics related to intense drug use are known to be associated with CIRI development [Reference Lloyd-Smith25–Reference Hope28], which may have underestimated the proportion with wounds and CA-MRSA in our study. Information on antibiotic use in the last 6 months was collected in the present study, but did not focus specifically on current use. It is possible that individuals with wounds who were currently on antibiotics at time of specimen collection had culture-negative wounds thus leading to an underreporting of positive wound cultures. In addition, information on size of wounds was not included in this study. Finally, our results are based on a subsample of SEOSI participants and may not be representative of the IDU community in this setting or others. Our results should be confirmed by a larger sample of positive CA-MRSA cultures in wounds of IDUs.
In conclusion, we observed a high prevalence of CA-MRSA in active IDUs in a community-based sample. Given this prevalence it is recommended that antibiotic therapy should include coverage for CA-MRSA and reflect the susceptibility of local strains, which in our setting would restrict the prescription of clindamycin.
ACKNOWLEDGEMENTS
The authors thank the participants in SEOSI and the staff at Insite, the Portland Hotel Society, and Vancouver Coastal Health (Chris Buchner, David Marsh, Heather Hay). We also thank all current and past SEOSI staff as well as staff from the Microbiology Laboratory at St Paul's Hospital. Thanks are due to Dr Sylvie Champagne, Dr Rob Lloyd-Smith, Ben Fischer, Sam Milligan, Leila Sloss, and Myfanwy Bakker for their contributions. We also thank Deborah Graham, Leslie Rae, Caitlin Johnston, Steven Kain and Calvin Lai for research assistance. The evaluation of the SIF was originally made possible through a financial contribution from Health Canada, although the views expressed here do not reflect the official policies of Health Canada. The evaluation is currently supported by the Canadian Institutes of Health Research (grants HPR-85526 and RAA-79918) and Vancouver Coastal Health. Pfizer has generously supported the microbiological and laboratory components for this study through an unrestricted research grant. T.K., M.W.T. and E.L.S. are supported by the Michael Smith Foundation for Health Research; T.K. and E.L.S. are supported by the Canadian Institutes of Health Research.
DECLARATION OF INTEREST
None.






