I deficiency is among the four leading micronutrient deficiencies in the world, which can produce detrimental effects, particularly in neonates and young infants. Consequences of I deficiency during these critical periods are mainly developmental delays, irreversible brain damage and mental retardation( Reference Bath, Steer and Golding 1 , Reference Zimmermann, Jooste and Pandav 2 ). Despite global progress in elimination of I deficiency, today over thirty-five million newborns remain unprotected from the lifelong consequences of brain damage associated with I deficiency( 3 ).
In order to evaluate the efficiency of national programmes implemented for elimination of I deficiency and to monitor I status of populations, median urinary I concentration (UIC), thyroid size, neonatal thyroid stimulating hormone (TSH) and thyroglobulin concentrations can all serve as indicators( 4 ). On a population basis, there is a negative association between maternal UIC and neonatal TSH values, specifically in countries with moderate-to-severe I deficiency( Reference Li and Eastman 5 ); however, in countries with mild-to-borderline I deficiency, decline in UIC has not been accompanied by change in blood spot TSH concentration, indicating that neonatal TSH may not have the sensitivity needed to detect mild I deficiency( Reference Li and Eastman 5 ). Moreover, there is evidence indicating an inconsistent relationship between maternal I status and neonatal UIC levels. In I-deficient countries such as Ireland( Reference Smyth, Hetherton and Smith 6 ), Portugal( Reference Costeira, Oliveira and Ares 7 ), Turkey( Reference Simsek, Karabay and Kocabay 8 ) and Niger( Reference Sadou, Moussa and Alma 9 ), median neonatal UIC was inadequate along with I deficiency among postpartum women. However, findings of a few studies conducted in I-sufficient areas are inconclusive: in some, I adequacy among neonates was also accompanied by I sufficiency among lactating mothers( Reference Hashemipour, Nasri and Hovsepian 10 , Reference Wang, Zhang and Ge 11 ); others, however, report decreased-to-suboptimal I status among postpartum women, despite I adequacy among their neonates( Reference Ordookhani, Pearce and Hedayati 12 ).
The Islamic Republic of Iran was once known as an area of I deficiency. Production and nationwide consumption of iodised salt containing 20–40 parts per million I began in 1990 and became mandatory for household consumption by 1994. In 2000, Iran was declared I deficiency disorders (IDD) free, and national surveys conducted every 5–7 years have shown sustainable elimination of IDD in schoolchildren( Reference Delshad, Amouzegar and Mirmiran 13 ). However, I sufficiency in the general population as assessed by the median UIC among school-aged children was not accompanied by the adequate median UIC among pregnant and lactating women and their infants. Previous studies conducted in Iran, comparing urinary I values of postpartum mothers and their infants, have demonstrated that median UIC in postpartum mothers ranged from low to borderline levels, whereas urinary I of their neonates was within optimal range and no definitive correlations between maternal and neonatal UIC were reported( Reference Ordookhani, Pearce and Hedayati 12 , Reference Bazrafshan, Mohammadian and Ordookhani 14 ).
Therefore, considering the limited data and controversial results available regarding the correlation between maternal urinary I levels during postpartum periods and I nutrition status of their infants in countries with different I status, the present study was designed to explore whether urinary I of the postpartum mothers residing in an area with I sufficiency can be used to estimate I status of newborns using UIC and TSH values.
Methods
Subjects
For this cross-sectional study conducted from April to December 2014 in Tehran, the capital city of Iran, four healthcare centres responsible for screening newborns with congenital hypothyroidism were randomly selected; in each centre, on the first visit, within 3–5 d postpartum, mother–newborn pairs were included to the study based on the following inclusion criteria: healthy mothers with no history of thyroid disorders and currently not using I-containing supplements and disinfectants; mothers having a singleton birth and who were exclusively breast-feeding; and neonates born full-term (gestational age, 37–42 weeks), aged 3–5 d and with normal birth weight (2500–4200 g). Using an interviewer-administered questionnaire, maternal information on age, education, occupation, last pregnancy, gravidity, parity, history of abortion in previous pregnancies, use of I-containing supplements during last pregnancy and the type of delivery were documented, and newborn demographic information including birth date, sex and birth weight, height and head measurements was obtained. Written informed consent was obtained after the study protocol and objectives had been fully explained to all postpartum mothers and/or their husbands. The present study was approved by the Ethics Committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences. Of a total of 262 mother–newborn pairs initially enrolled, newborns without urine samples (n 16) as well as mothers (n 22) and newborns (n 77) with urinary I levels >800 µg/l (considered outliers, defined as more than 3 sd from the mean) were excluded; hence, 147 mother–newborn pairs remained for the current analysis.
Thyroid stimulating hormone concentration
Heel-prick blood samples were obtained by trained nurses from all newborns within 3–5 d after birth, as part of routine newborn screening, spotted on filter paper ((Schleicher & Schuell NO 903) S&S 903) and air-dried for 2–3 h. The samples were sent to the reference screening laboratory by express mail service for TSH values to be assayed within 7–10 d. Neonates with abnormal TSH levels on screening, that is, TSH level ≥5 mIU/l in the heel prick test, were recalled for confirmatory tests on the basis of the serum TSH and thyroxine concentrations.
Urine collection
At the first visit, labelled plastic bottles and adhesive paediatric urine bags (SUPA medical services) were provided to collect spot urine samples of each postpartum mother and her newborn according to the detailed instructions provided. Mothers were asked to collect a casual urine sample at random at any time during the day; they were also instructed to clean the genital region of their newborns and to place the entire penis in the bag and attach the adhesive to the skin for boys and to fit the bag over the labia for girls. If urine samples of newborns could not be collected using adhesive urine bags after three attempts, mothers were asked collect samples by holding a specimen bottle in the urine stream. All samples were collected and sent to the iodine laboratory of the Research Institute for Endocrine Sciences, where they were transferred into screw-top labelled plastic vials. The aliquots were kept frozen at −20°C until I concentrations were measured.
Laboratory measurements
I concentration in urine samples was analysed using the Sandell–Kolthoff (acid digestion) reaction( Reference Hedayati, Khazan and Yaghmaee 15 ), and the results are expressed as micrograms of I per litre of urine. Intra-assay CV at UIC values of 8·5, 17·5 and 36·0 µg/l were 8·5, 6·2 and 8·0 %, respectively. The inter-assay CV at concentrations of 8·5, 17·4 and 36·4 µg/l were 10·3, 9·7 and 8·0 %, respectively; TSH concentration was determined by ELISA using available neonatal TSH kits (Kimia Pajouhan Co.). The minimal detectable concentration of TSH in this assay is estimated to be 1·2 μIU/ml. Intra-assay CV at TSH concentrations of 5·1, 8·6, 17·9 and 30 mIU/l were 11·6, 8·0, 7·4 and 6·6 %, respectively. The inter-assay CV for different methods at TSH concentrations of 4·7, 8·8, 18·6 and 31·7 mIU/l were 13·2, 9·8, 7·2 and 7·3 %, respectively.
Definition of terms
In postpartum women and newborns, according to WHO/International Council for the Control of Iodine Deficiency Disorders (ICCIDD)/UNICEF criteria, median UIC values <100 and ≥100 µg/l were representative of deficient and sufficient urinary I, respectively( 4 ). The TSH cut-off point was set at 5 mIU/l, and a frequency <3 % for TSH>5 mIU/l was considered population I sufficiency( Reference Delange 16 ).
Statistics analysis
Frequency distribution (percentage), mean values and standard deviations and medians and interquartile ranges were expressed for categorical and continuous variables. Normality of the variables was assessed by the Kolmogorov–Smirnov test and histogram chart. χ 2 and Mann–Whitney U test or t tests were used to assess significance of differences for categorical and continuous variables in postpartum mothers and newborns and also among mothers with median UIC<100 and ≥100 µg/l. The skewed variables were log-transformed before analysis. To identify factor(s) affecting neonatal UIC and TSH values, multiple linear regression was used. Factors considered in this analysis were as follows: maternal UIC, mothers’ occupation and educational grade, gravidity, parity, delivery type, infant sex and birth weight. Statistical analyses were carried out using IBM SPSS for windows (version 20.0, 2011; IBM Corp.), P values<0·05 were considered to be significant.
Results
A total of 147 postpartum women and newborns, aged 27·8 (sd 5·3) years and 4·2 (sd 0·6) d, respectively, participated in this study. Table 1 shows the basic characteristics of the postpartum women and their neonates. The mean grade of education of the women was 11·1 (sd 3·4) years; over 90·0 % of the mothers were housewives; over half of the postpartum mothers had multigravidity (62·6 %) and multiparity (55·1 %); only about 5·0 % of the mothers had used I-containing supplements during pregnancy; and approximately 60·0 % of them had had caesarean sections. In all, 54 % of newborns were male and 46·3 % were female; mean values for birth weight, height and head circumference among newborns were 3345 (sd 421) g, 50·3 (sd 2·0) cm and 35·0 (sd 1·4) cm, respectively.
Table 1 Baseline characteristics of mothers and newborns 3–5 d postpartum (Numbers and percentages; mean values and standard deviations)

NVD, natural vaginal delivery; CS, caesarean section.
Data on neonatal UIC and TSH levels according to maternal urinary I are shown in Table 2. The median UIC was 68·0 (interquartile range (IQR) 39·4–133·5) µg/l in postpartum mothers and was 212·5 (IQR 92·3–307·3) µg/l in newborns, respectively. The median UIC values in mothers with deficient and sufficient urinary I were 46·6 (IQR 35·2–70·8) and 173·6 (IQR 144·5–211·4) µg/l, respectively; values of UIC of neonates born to mothers with deficient and sufficient urinary I were 192·8 (IQR 85·0–284·3) and 243·0 (IQR 109·3–331·2) µg/l, respectively (P=0·100). Urinary I of mothers and neonates did not differ between those who had natural vaginal delivery (NVD) and those who had a caesarean section.
Table 2 Urinary iodine and neonatal thyrotropin concentrations of 3–5 d old newborns according to maternal urinary iodine concentration (UIC) (Numbers and percentages; median and interquartile range (IQR))
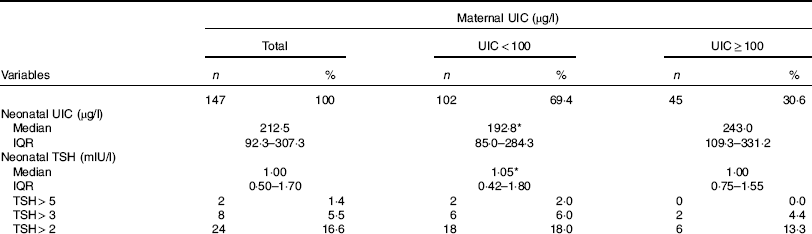
TSH, thyroid stimulating hormone.
* No significant difference between the two maternal UIC groups.
The median neonatal TSH value was 1·00 (IQR 0·50–1·70) mIU/l; median TSH values were 1·05 (IQR 0·42–1·80) mIU/l for neonates born to mothers with deficient UIC and 1·00 (IQR 0·75–1·55) mIU/l for neonates born to mothers with sufficient UIC (P=0·930). Frequency distributions of TSH values are presented in Table 2. Of neonates, 1·4, 5·5 and 16·6 % had TSH levels >5, >3 and>2 mIU/l, respectively. There was no difference in neonatal TSH in mothers who had NVD, as compared with those who had a caesarean section (1·1 v. 0·9 mIU/l, P=0·158).
Factors associated with neonatal UIC and TSH values are presented in Table 3. Maternal UIC was not associated with neonatal UIC (β=0·156, P=0·094) and TSH values (β=0·129, P=0·236) using univariate analysis; however, in the multiple linear regression, neonatal UIC value was significantly associated with maternal urinary I (β=0·191, P=0·048) and parity (β=0·408, P=0·039). In addition, a significant association was found between TSH concentration and infant sex (β=−0·348, P=0·038) and birth weight (β=0·391, P=0·049). Neither neonatal UIC nor TSH values were associated with mothers’ occupation and education, gravidity and type of delivery.
Table 3 Factors associated with neonatal urinary iodine and thyroid stimulating hormone (TSH) concentrations by multiple linear regressionFootnote * (β Coefficients and 95 % confidence intervals)

UIC, urinary I concentration.
* Neonatal UIC and TSH and maternal UIC values log-transformed.
Discussion
Findings of the current study indicate that Tehranian postpartum mothers, but not their newborns, had marginally suboptimal I status, as defined by the median UIC established by the World Health Organization( 4 ); however, on the basis of newborn TSH concentration as an index of population I status (a frequency <3 % for TSH>5 mIU/l considered as I sufficiency), mothers and newborns were both classified as I sufficient. In addition, a decline in maternal urinary I was not accompanied by alteration in neonatal UIC and TSH levels.
On the basis of the frequency of neonatal TSH values >5 mIU/l in determining population I status, monitoring intervention programmes have been successful in some countries such as Poland, Thailand, Belgium, Ireland and Switzerland( Reference Li and Eastman 5 ); however, on the basis of the latest recommendations of the WHO/UNICEF/ICCIDD, newborn TSH level per se is not included among the indicators of I nutrition status( 4 ), which is because many confounding issues (such as maternal I status, prematurity, type of delivery, exposure to I-containing antiseptics, time of sampling and TSH assay methodology) may alter newborn TSH, making this variable a less sensitive and reliable monitoring tool for determining I deficiency( Reference Li and Eastman 5 ). It has been suggested that median UIC in conjunction with neonatal TSH can provide a more reliable assessment of population I status( Reference Li and Eastman 5 , Reference Burns, Mayne and O’Herlihy 17 ). As shown in Belgium and the UK, considered both as mildly I-deficient areas, neonatal TSH may lack the sensitivity required to detect mild I deficiency( Reference Vandevijvere, Coucke and Vanderpas 18 , Reference Evans, Barry Nix and Hillier 19 ). Our study also demonstrated inconsistent I statuses of postpartum mothers and newborns assessed by median UIC and neonatal TSH values, due to the aforementioned reasons. Although, data assessing blood spot TSH from neonates revealed no evidence to support the notion that the population studied is I deficient (only 1·4 % of neonates had TSH>5 mIU/l), I status (expressed as the median UIC) of Tehranian postpartum mothers, but not newborns, was below at 100 µg/l.
Data on newborn screening revealed a negative correlation between neonatal TSH concentration and UIC in their mothers. In Zaire and India, neonatal TSH concentration was significantly elevated in the cord blood of offspring of mothers suffering from moderate-to-severe I deficiency( Reference Delange 20 ). A study comparing maternal and neonatal thyroid status in Nigeria demonstrated that mean plasma TSH was significantly higher in neonates whose mothers had significantly lower UIC levels and higher goitre rates( Reference Ojule and Osotimehin 21 ). In countries with moderate I deficiency (e.g. Turkey, Thailand and Hong Kong), the frequency of serum TSH>5 mIU/l was inversely related to maternal UIC( Reference Simsek, Karabay and Kocabay 8 , Reference Jaruratanasirikul, Sangsupawanich and Koranantakul 22 , Reference Kung, Lao and Low 23 ). However, studies from countries with mild I deficiency such as Australia and Denmark, where the frequency of TSH>5 mIU/l is often <3 %, did not find the expected negative correlation between neonatal whole blood TSH and maternal UIC( Reference McElduff, McElduff and Gunton 24 , Reference Nohr and Laurberg 25 ). Our finding of neonatal TSH values within normal range, despite mild maternal I deficiency, is in agreement with several studies, which have demonstrated that a decline in maternal urinary I is not always accompanied by alteration in I status as assessed by neonatal TSH levels( Reference Li and Eastman 5 ). However, consistent with our results, most studies have reported that male infants generally have higher TSH levels and their birth weight is also positively associated with TSH levels( Reference Herbstman, Apelberg and Witter 26 ).
The main challenge in using median UIC in newborns is the difficulty of sample collection; hence, the best criteria for assessing the newborns’ degree of I deficiency have not yet been established due to lack of sufficient data for urinary I in this age group. The WHO recommends that a median UIC>100 µg/l is adequate for assessing the I status of children aged <2 years( 4 ); however, the study by Zimmermann( Reference Zimmermann 27 ) demonstrated that the current WHO median UIC cut-off for I sufficiency in infancy may be too high for the 1st week after birth. Dorey & Zimmermann( Reference Dorey and Zimmermann 28 ) reported the following median UIC in children <2 years from I-sufficient countries: Canada (n 81), 148 µg/l; the Netherlands (n 64, n 36), 162 and 150 µg/l, respectively; Sweden (n 39, n 61) 112 and 96 µg/l, respectively; and Czech Republic (n 181), 92–109 µg/l. Moreover, there are limited studies that simultaneously include both neonatal TSH and median UIC in screening programmes for newborns. In Switzerland, despite a national survey of healthy, term, euthyroid breast-fed infants, aged 0–5 d (n 634), indicating a median UIC of 77 µg/l, the frequency of TSH>5 mIU/l in the newborn screening programme between 1999 and 2004 was 1·7 %( Reference Zimmermann, Aeberli and Torresani 29 ); however, in the present study, the median UIC of newborns was higher (n 147, 212·5 µg/l) than that reported from the I-sufficient areas, and the TSH concentrations of the newborns met the WHO criteria recommendations.
As the requirement of I increases substantially during pregnancy and lactation, it is conceivable that – if reserves and dietary I intake are low at baseline – subsequent gestations and lactations may deplete the maternal I reserves further, and hence produce relationship with increased parity. Although there are no studies specifically demonstrating an association between parity and I status, our study showed that median urinary I in mothers who had three or more children was lower compared with mothers with only one child (61·3 µg/l, n 26 v. 81·2 µg/l, n 65) (data not shown). On the other hand, maternal I sufficiency is particularly important for exclusively breast-fed infants, in whom breast milk is the sole source of I nutrition during a critical period of growth and development. Therefore, on a biological basis, it might be expected that a positive association between urinary I status in mothers and exclusively breast-fed infants be found; however, our findings indicate that despite maternal I deficiency I status of their infants was adequate. There is some evidence of transfer of I from mothers to newborns as shown in the current study, in which higher UIC of neonates was associated with maternal urinary I – a finding compatible with our previous study conducted in Tehran( Reference Ordookhani, Pearce and Hedayati 12 ) and other investigations from Turkey( Reference Simsek, Karabay and Kocabay 8 ) and Ireland( Reference Smyth, Hetherton and Smith 6 ). These studies have indicated that maternal UIC in breast-feeding mothers was significantly lower than their infants as follows: in Tehran (107 v. 271 µg/l), Turkey (40 v. 85 µg/l) and Ireland 76·5 v. 100 µg/l); a similar pattern was observed in the recent study from Turkey (84 v. 279 µg/l); however, I deficiency in nursing mothers and I excess in their newborns was attributed to the use of antiseptics containing I( Reference Yaman, Demirel and Ermis 30 ).
The main strengths of this study are the estimation of newborn I nutrition status based on postpartum maternal UIC, for the first time in an area of I sufficiency, and assessment of I status among newborns simultaneously by two variables (UIC and TSH) in the early neonatal period. However, among limitations, using a single random urine sample may be a poor indicator to determine an individual’s I status, because of significant day-to-day variation in salt intake, which is the main source of dietary I in many countries, and also variations in hydration in individuals. Previous studies on Na intake have indicated that at least five 24-h urine collections are required to obtain a representative estimate of individual Na excretion. Therefore, approximately ten repeat spot urine samples or 24-h collections are needed to estimate individual I intakes at a precision of 20 %( Reference König, Andersson and Hotz 31 ). Moreover, as discussed above, caution should be taken in interpreting these results due to categorisation of maternal I status on the basis of a single spot urinary I excretion, which raises the potential risk of misclassification. Another potential limitation of this study is related to the cross-sectional design of this study that cannot establish causality relationships.
In conclusion, although on a biological basis, it could be expected that a positive association between urinary I status in mothers and exclusively breast-fed infants be found; however, our findings indicate that despite Tehranian postpartum mothers being mildly I deficient the I status of their infants was adequate as defined by the median UIC values. Furthermore, a decline in maternal urinary I was not accompanied by alteration in I status of neonates, as assessed by TSH levels. It seems that factors other than maternal urinary I may influence UIC and TSH values, both indicators of I status in newborns.
Acknowledgements
This study was supported by a financial grant from the Research Institute of Endocrine Sciences, Shahid Beheshti University of Medical Sciences. The authors express their appreciation and gratitude to Niloofar Shiva for critical editing of English grammar and syntax of the manuscript.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
P. N. contributed to the design, data analysis; P. M. and F. A. contributed to the reading and final approval of the manuscript; P. M., F. A. and M. H. contributed to the design of the study; Y. M. contributed to the statistical analysis; P. N., P. M., M. H., F. A. and Y. M. contributed to the writing of the manuscript.
None of the authors has any personal or financial conflicts of interest to declare.





