Introduction
Treatment and management of difficult-to-treat depressionReference Rush, Aaronson and Demyttenaere 1 as represented by treatment-resistant depression (TRD) are clinically challenging.Reference Cowen 2 –Reference Voineskos, Daskalakis and Blumberger 4 Among the difficult-to-treat depressions, clinically challenging conditions in particular include not only so-called TRD but also treatment-resistant recurrent depressive disorder (RDD)Reference Pezawas, Angst and Kasper 5 and treatment-resistant bipolar disorder (BD).Reference Bowden, Perlis and Thase 6 –Reference Judd, Akiskal and Schettler 10 The average cumulative recurrence rate for patients with major depressive disorder is estimated to be 13% at 5 years, 23% at 10 years, and 42% at 20 years,Reference Hardeveld, Spijker, De Graaf, Nolen and Beekman 11 and when it is limited to specialized psychiatric institutions that see a large number of patients with TRD, the recurrence rate for major depressive disorder is even higher, with an estimated 60% at 5 years, 67% at 10 years, and 85% at 15 years for patients with major depressive disorder.Reference Hardeveld, Spijker, De Graaf, Nolen and Beekman 12 On the other hand, observational studies of BD (follow-up period 2.1 years) reported an overall recurrence rate of approximately 55% (26%/year), and randomized controlled trails (follow-up period 1.9 years) reported an overall recurrence rate of about 39% (22%/year) in the mood stabilizer treatment group and 61% (31%/year) in the placebo group.Reference Vazquez, Holtzman, Lolich, Ketter and Baldessarini 13 Thus, in the case of BD, more than half of the patients are likely to have a recurrence after about 2 years of follow-up, and the recurrence rate is generally higher than that of unipolar depression. As such, these data are exactly indicative of the fact that some patients with difficult-to-treat depression present with RDD and have difficulty in managing their condition, even if they respond to acute phase treatment. Furthermore, Senova et al. reported that the percentage of patients with TRD who could maintain response after responding to acute transcranial magnetic stimulation (TMS) treatment was approximately 67% after 3 months, 53% after 6 months, and 46% after 12 months of an acute course of treatment.Reference Senova, Cotovio, Pascual-Leone and Oliveira-Maia 14
TMS is a noninvasive treatment that stimulates specific areas of the brain with focused magnetic fields. The evidence is well established, especially for medication-resistant depression. Repetitive TMS (rTMS) treatment improves depressive symptoms in up to 70% of patients with TRD. New approaches and techniques have been developed in this area to reduce treatment time and to optimize and maximize the clinical outcomes. For example, theta burst stimulation protocols now have evidence showing that they are non-inferior to standard rTMS and offer significant advantages in optimizing limited healthcare resources.Reference Li, Nahas, Anderson, Kozel and George 15 In the context of difficult-to-treat depression, previous studies have repeatedly discussed the possibility of maintenance treatment with rTMS as an effective recurrence prevention strategy for difficult-to-treat depression including TRD.Reference O’reardon, Blumner, Peshek, Pradilla and Pimiento 16 Prior studies have shown that maintenance TMS protocols as a recurrence prevention strategy for TRD are considerably varied, with the majority demonstrating their benefits from a clinical perspective with open-label trials and case series.Reference Langguth, Landgrebe, Zowe, Gerst, Hajak and Eichhammer 17 Indeed, to date, a total of 11 case reports and case series on maintenance TMSReference Voineskos, Daskalakis and Blumberger 4 –Reference Senova, Cotovio, Pascual-Leone and Oliveira-Maia 14 have been reported from around the world (see Table 1 for details). Currently, for patients with TRD who have responded to an acute course of rTMS therapy, maintenance rTMS may be beneficial,Reference Hardeveld, Spijker, De Graaf, Nolen and Beekman 12 , Reference Fukuda, Tirrell, Gobin and Carpenter 29 regardless of protocol differences, as there are few effective and reliable noninvasive alternative treatment options available in the outpatient setting in many countries today, including Japan, other than pharmacotherapy. Furthermore, few studies have focused on and clinically evaluated the potential of maintenance TMS treatment after the successful acute TMS treatment for mood disorders such as RDD and depressive episodes of BD other than typical form of TRD.
Table 1. Summary of Case Reports and Case Series on Maintenance TMS for Depression
One of the challenges currently facing clinics specializing in TMS treatment is how to provide clinically meaningful maintenance treatment and follow-up management for patients with refractory mood disorders, including RDD and BD, who have successfully responded to acute TMS treatment. In particular, treatment-resistant RDD and treatment-resistant BD have high recurrence rates and are likely to relapse weeks to months after responding to an acute course of electroconvulsive therapy (ECT)Reference Judd, Akiskal and Schettler 10 as well as TMS treatment.Reference Tundo, De Filippis and Proietti 3 In practice, maintenance pharmacotherapy is continued in many such cases, but given that patients with medication-resistant RDD and BD undergo TMS treatment as an alternative or add-on therapy to medication, there may be little clinical rationale for just continuing maintenance pharmacotherapy, which was relatively ineffective for these patients.
With this background, this case series aimed to examine the efficacy of maintenance intermittent theta burst stimulation (iTBS) on a real-world basis by retrospectively extracting data on cases meeting the criteria described below using the clinical TMS registry data in Japan.Reference Noda, Kizaki, Takahashi and Mimura 30 Moreover, although numerous studies have already been reported from Europe and North America on the potential of maintenance rTMS treatment for TRD,Reference Senova, Cotovio, Pascual-Leone and Oliveira-Maia 14 no coherent report has yet been published from Japan on the treatment strategy using maintenance iTBS for such patients with RDD and BD in the context of difficult-to-treat depression. Furthermore, previous maintenance TMS studies have used maintenance treatment with rTMS, and no case series using a maintenance iTBS protocol after the successful acute treatment with iTBS has yet been reported.Reference D’andrea, Mancusi and Santovito 28 The present study is a retrospective observational study without a control group, but we report this case series here as part of the TMS registry study because we believe it is of clinical importance in terms of a real-world study in actual clinical settings.
Methods
Case series setting
In this case series, data from patients with RDD or BD, in the context of difficult-to-treat depression, who received 15 to 30 sessions of maintenance iTBS after the successful acute course of iTBS treatmentReference Ekman, Popiolek, Boden, Nordenskjold and Lundberg 31 at the Shinjuku-Yoyogi Mental Lab Clinic in Tokyo were extracted from the real-world clinical TMS database registry. The data included in this case series were collected between January 2020 and September 2023. Specifically, case series data of 21 outpatients (including 10 patients with RDD and 11 patients with BD type II) who had received a total of 15 to 45 sessions of acute iTBS treatment and had at least a clinical response and then transitioned to weekly (15 to 30 sessions) maintenance iTBS treatment were extracted and analyzed retrospectively. Note that the diagnosis of RDD was based on the following definitions: 1) The patient must have had at least two depressive episodes in their life, 2) each episode must have lasted for at least 2 weeks, 3) there must not be enough mood elevation or hyperactivity to be diagnosed as mania, and 4) in women, the episodes must not be directly related to the menstrual cycle. In addition, even if there was elevated mood or hyperactivity, it should be mild.
Here, clinical response in this study was defined as a case in which score improved by 50% or more after an acute course of iTBS treatment from the start of acute iTBS treatment, based on the Montgomery–Åsberg Depression Rating Scale (MADRS) or the Hamilton Depression Rating Scale–17 items (HDRS-17) score. In all cases of iTBS treatment at the clinic, general informed consent for examination and treatment (ie, iTBS) in private practice was given to the patients by the physician in charge. This TMS registry and retrospective observational analysis study was approved by the ITO Yoyogi Mental Clinic Research Ethics Committee (ID: RKK319) and conducted in compliance with the norms and guidelines of the “Ethical Guidelines for Medical and Health Research Involving Human Subjects” and the Declaration of Helsinki (revised October 2013) at Shinjuku-Yoyogi Mental Lab Clinic. In accordance with the TMS registry study protocol, informed consent was obtained from the majority of patients, but an opt-out procedure was applied for cases where retrospective consent could not be obtained because of past data. Note that the opt-out procedures refer to the specific disclosure of information regarding the study on the notice board and/or website of the hospital, and if the patient does not wish his or her clinical data to be used in the study, he or she can inform the clinic so that the clinic cannot use the data for research purposes.
Data extraction criteria for this case series
The eligibility criteria for this case series are as follows: (1) 18 years of age or older; (2) patients who met the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), and/or International Classification of Diseases, 10th Revision (ICD-10), definitions of the diagnosis of RDD or BD type II with standard psychiatric consultation by certified psychiatrists; (3) patients whose depressive symptoms had not stabilized after a period of standard pharmacotherapy (ie, TRD); (4) patients with no previous history of convulsive seizures; (5) patients with no other apparent contraindications to TMS therapy; and (6) patients who had achieved clinical response or remission with an acute course of iTBS treatment (30 sessions) in the context of TRD. Figure 1 shows a flowchart in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) style.
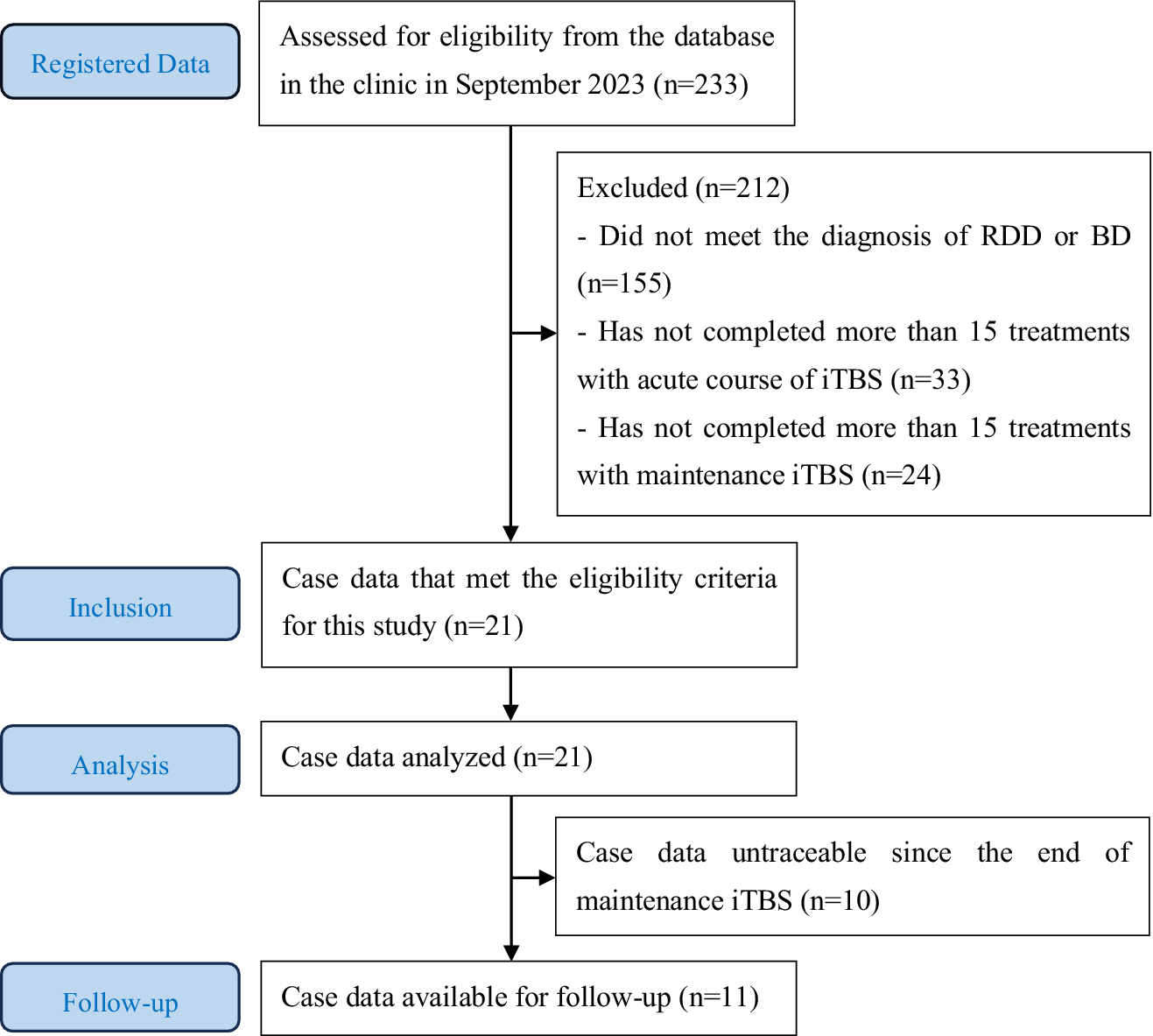
Figure 1. Strengthening the reporting of observational studies in epidemiology (STROBE) flowchart.
Clinical measures
The MADRSReference Montgomery and Asberg 32 and HDRS-17Reference Hamilton 33 scores included in the TMS registry data were used in this case series. In particular, in this study, the MADRS score was used as the primary outcome and the HDRS-17 score as the secondary outcome. The definition of response for both MADRS and HDRS-17 was a decrease by half or less of the pretreatment score with acute iTBS treatment, while the definition of remission was a score ≤ 10 for the MADRS and ≤ 7 for the HDRS-17. In cases where the results for response and remission differed between the two clinical measures, the results from the primary outcome, the MADRS score, were adopted.
Clinical evaluations were performed routinely after every 15 sessions of iTBS treatment by well-trained clinical psychologists. In addition, during the follow-up period after the completion of maintenance iTBS, the MADRS and HDRS-17 assessments were conducted during physician visits at 6-month intervals, as long as the patient could be contacted.
TMS treatment protocol
In the present study, in the context of difficult-to-treatment depression, patients who achieved clinical response to a total of 15–45 sessions of iTBS (double-dose protocol: approximately 6 min with a total of 1200 pulses) as acute treatment were followed by further weekly maintenance iTBS (double-dose protocol: approximately 6 min with a total of 1200 pulses) for a total of 15 sessions (13 patients) or 30 sessions (8 patients) to stabilize the disease and prevent its recurrence. A flow diagram of the acute course of iTBS, maintenance iTBS, and observation period for this case series is shown below (Figure 2).

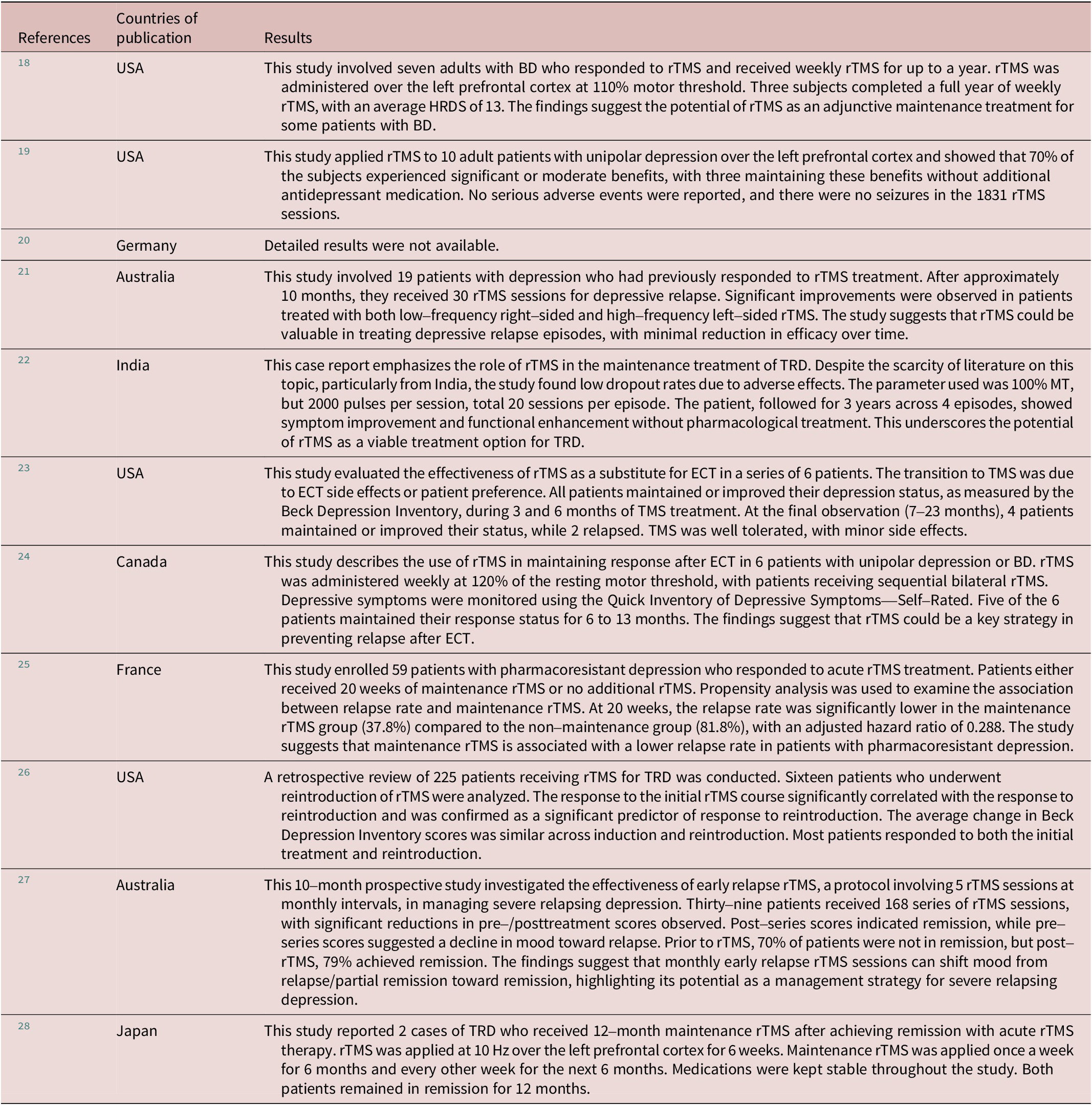
Figure 2. A flow diagram of the acute course of iTBS, maintenance iTBS, and observation period for this case series.
In the present iTBS treatment, the left dorsolateral prefrontal cortex (DLPFC) was the target site, including the acute and maintenance phases. The target site on the left DLPFC of each patient was identified using the Beam_F3 method.Reference Beam, Borckardt, Reeves and George 34 The resting motor threshold (RMT) was defined as the minimum stimulus intensity to induce muscle contraction in the right abductor pollicis brevis muscle at rest, 50% of the time, with a single pulse of TMS administered to the left primary motor cortex. Stimulation intensity was based on each patient’s RMT, with 120% RMT intensity. The MagPro R30 device with the Cool-B70 coil (MagVenture, Inc., Farum, Denmark) was used for the TMS treatment.
Pharmacotherapy during TMS treatment
In principle, there were no medication changes from at least 1 month prior to the introduction of iTBS treatment until the end of acute iTBS treatment except for medication to be used as needed such as anxiolytics and sleep aids. In addition, during the maintenance iTBS period and the follow-up period, most patients were on the same medication in principle, but some patients were reduced or adjusted to the minimum dose of medication necessary based on discussions between the patient and his/her psychiatrist.
Statistical analysis
IBM SPSS Statistics 29 (IBM, Chicago, IL, USA) was used for statistical analysis. The present case series is a preliminary observational study with the main objective of evaluating the effectiveness of maintenance iTBS on a real-world basis. Thus, the primary outcomes of the present study were as follows: 1) for patients who were already in remission on the MADRS or HDRS-17 score at the start of maintenance iTBS, whether they could remain in remission during the maintenance iTBS period and subsequent follow-up period, and 2) for patients who were not in remission but had clinical response to the acute course of iTBS treatment, whether they could keep that response during the maintenance iTBS and follow-up periods. Here, descriptive statistics were provided with respect to these points. In addition, chi-squared test was performed to evaluate whether there were significant differences in maintenance of response or remission up to the last observation, which is the clinical outcome of maintenance iTBS, across diagnoses or sex. The significance level was set at 0.05 in this study.
Results
The clinico-demographic information on the patient data included in this case series is summarized in Table 2, including a breakdown of each item in the RDD and BD groups. In addition, details of the medications the patients were taking during the iTBS treatment period are summarized in Table 3.
Table 2. Clinico-Demographic Information
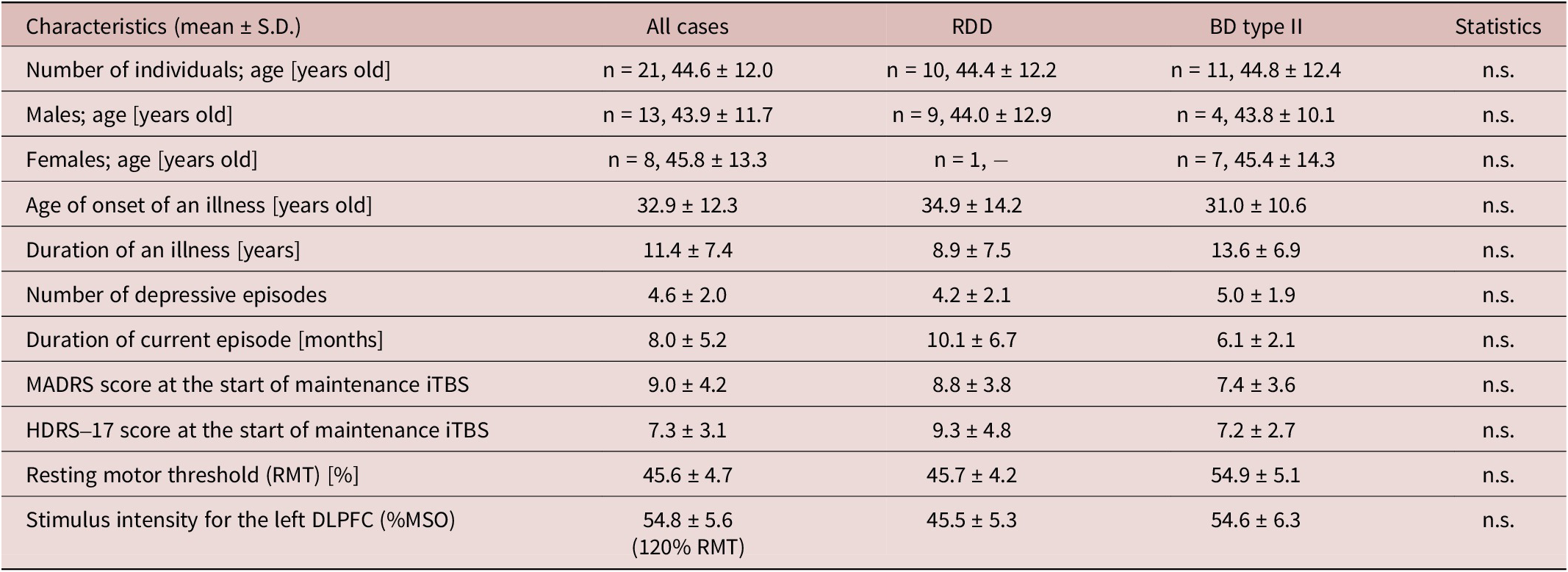
Abbreviations: BD, bipolar depression; HDRS-17, Hamilton Depression Rating Scale–17 items; iTBS, intermittent theta burst stimulation; MADRS, Montgomery–Åsberg Depression Rating Scale; MSO, maximum stimulator output; n.s., no significant; RDD, recurrent depressive disorder; RMT, resting motor threshold; S.D., standard deviation.
Table 3. Medication Information

Abbreviations: BD, bipolar depression; RDD, recurrent depressive disorder.
The present case series study, which utilized TMS registry data to examine the effects of maintenance iTBS on stabilization of mental condition and prevention of relapse and recurrence in patients who have responded to an acute course of iTBS treatment, yielded the following results. First, the longitudinal changes in the MADRS and HDRS-17 scores with maintenance iTBS in this case series, to the extent that they were followable, are presented in Table 4 below. The MADRS and HDRS-17 scores for each patient are depicted graphically as time series data as Figure 3.
Table 4. Longitudinal Changes in Scores of the MADRS and HDRS-17 (mean ± S.D.)
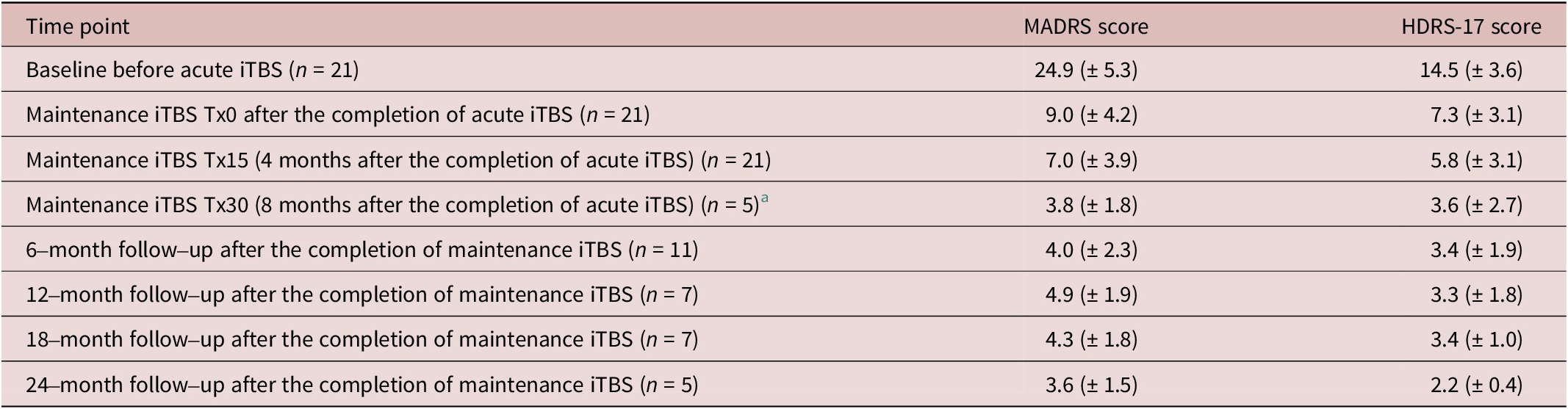
a Five cases were received 30 sessions of maintenance iTBS.
Abbreviations: HDRS-17, Hamilton Depression Rating Scale–17 items; MADRS, Montgomery–Åsberg Depression Rating Scale.
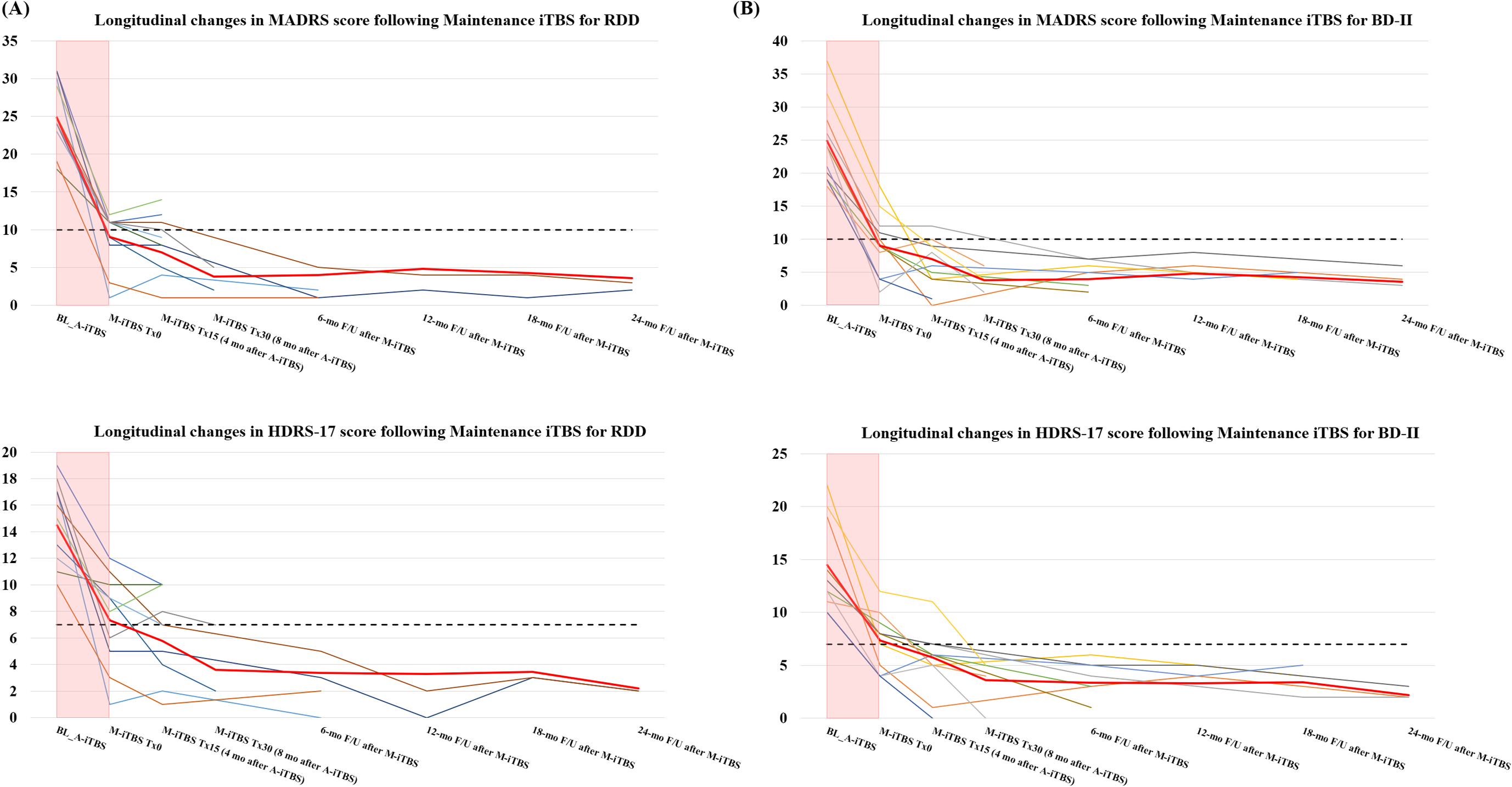
Figure 3. Longitudinal changes in MADRS and HDRS scores in the RDD and BD type II groups from the introduction of maintenance iTBS to the follow-up period. (A) shows the longitudinal changes in MADRS score (upper panel) and HDRS score (lower panel) from the introduction of maintenance iTBS to the follow-up period in the RDD group. (B) depicts the longitudinal changes in MADRS score (upper panel) and HDRS score (lower panel) from the introduction of maintenance iTBS to the follow-up period in the BD type II group. Each color in the line graph indicates a time series change in each patient’s score of depressive symptoms. The bold red lines in the figures show the average trajectory of all cases. The black dashed lines in the upper panel show the cutoff lines (10 points) corresponding to remission in the MADRS; similarly, the black dashed lines in the lower panel show the cutoff lines (7 points) for the HDRS-17 score. Of note, the pink bars indicate score changes during the acute iTBS treatment period. A, acute phase; BL, baseline; F/U, follow-up; iTBS, intermittent theta burst stimulation; M, maintenance phase; mo, months.
With respect to the MADRS score, for 2 of 21 patients (9.5%), response to acute iTBS treatment, followed by a total of 15 sessions of maintenance iTBS over approximately 4 months, was maintained, but remission was not achieved by the time of the last observation. For the other 19 patients (90.5%), further maintenance iTBS on response or remission after acute iTBS treatment not only maintained response in these patients but also achieved remission, and in all of these cases, remission was maintained until the last observation. Thus, in this case series, a total of 15 to 30 weekly maintenance iTBS sessions after the successful acute iTBS treatment resulted in maintaining the response state for at least 4 months during the maintenance iTBS period and up to 28 months including the follow-up period, according to the assessment at the last observation of the cases that could be followed up.
In addition, chi-squared tests were performed to examine whether differences in diagnosis (RDD or BD type II) or sex (males or females) make a significant contribution to the clinical outcome of maintenance iTBS (ie, maintenance of response or remission). The results showed that, although we did not perform the statistical test for response because all patients maintained response at the time of last observation, no significant difference was found for whether remission was maintained (ie, whether recurrence occurred or not) depending on diagnosis (χ2(1) = 2.43, p = 0.214) or sex (χ2(1) = 1.36, p = 0.371).
The only adverse event during the maintenance iTBS period was mild stimulation site pain (28.6%), and no other serious adverse events, including manic switch or convulsive seizures, were observed. Moreover, our maintenance iTBS was tolerated well and adhered comparatively well due to its minimal side effects and the convenience of only receiving the treatment once a week. Also, there were no changes in prescriptions in the majority of patients during the maintenance period, with the exception of a few cases in which the dose of concomitant antidepressants or stabilizers was reduced. In the same period, there were no cases of emergency visits involving self-harm or suicidal behavior. The same was true for adverse events concerning cases that could be followed during the follow-up period after the completion of maintenance iTBS. Moreover, in the BD type II group, no cases of hypomanic/manic episodes, hospitalizations, or suicidal behavior were observed during the follow-up period after the completion of maintenance iTBS.
Discussion
In this case series, 21 patients who had responded to acute iTBS treatment were followed by a total of 15 to 30 sessions of maintenance iTBS, which resulted in sustained response in all patients, with 19 of them also maintaining remission. This case series showed that maintenance iTBS did not worsen depressive symptoms in any case, suggesting that it may contribute to the stabilization of mental condition and the prevention of recurrence. Furthermore, tolerability and safety during the maintenance iTBS period including the follow-up period were also ensured within the observable follow-up range in this case series.
Regarding maintenance rTMS for patients with TRD, the following previous studies have been largely reported.Reference Wilson, Croarkin and Aaronson 27 , Reference Chang, Chu, Ren, Li, Wang and Chu 35 Levkovitz et al. found that in a double-blind sham-controlled RCT of maintenance deep TMS twice a week for 3 months, the active stimulation group (n = 82) maintained a higher percentage of response than the sham stimulation group (n = 77), specifically, immediately after maintenance rTMS response rate for the active group was about 44%, compared to about 26% for the sham group. Regarding the remission rate, the active group had a remission rate of about 32%, while the sham group had a remission rate of only about 22%.Reference Levkovitz, Isserles and Padberg 36 Dunner et al. conducted maintenance rTMS in the framework of an observational study and obtained the following results. Patients who achieved response or remission after an acute course of rTMS treatment and then received maintenance rTMS at least once a week for a certain period of time were shown to maintain response (44% and 61%) or remission (29% and 37%) as measured by the Inventory of Depressive Symptom—Self-Report and 9-Item Patient Health Questionnaire, respectively, with a clinical significance over a 12-month follow-up period.Reference Dunner, Aaronson and Sackeim 37 In addition, Harel et al. conducted a prospective, open-label study of maintenance deep TMS twice weekly for 8 weeks, followed by once weekly for a total of 10 weeks, and found that improvement in depression with an acute course of rTMS treatment was observed over a follow-up period of approximately 6 months.Reference Harel, Rabany, Deutsch, Bloch, Zangen and Levkovitz 38 Richieri et al. also conducted a prospective, open-label study of maintenance rTMS treatment following an acute rTMS treatment tapering regimen. Specifically, a total of 7 sessions of rTMS treatment were administered during the first 3 weeks of the tapering period, followed by weekly rTMS treatment for a total of 2 weeks, then biweekly rTMS treatment for a total of 2 months, and then monthly rTMS treatment for a total of 2 months. The study showed a significantly lower recurrence rate in the maintenance rTMS group (approximately 38%) compared to the non-maintenance rTMS group (approximately 82%). However, the mean time to recurrence was approximately 2.4 months in the maintenance rTMS group versus 2.2 months in the non-maintenance rTMS group, with no significant difference between the two groups.Reference Richieri, Guedj and Michel 22 Fitzgerald et al. conducted a prospective, open-label study of clustered maintenance rTMS therapy. Specifically, they administered clustered maintenance rTMS to patients with depression who had responded to two courses of rTMS treatment, with 5 sessions of rTMS treatment over 2 days once a month after the completion of the second course of rTMS treatment. The study, though preliminary, showed that clustered maintenance rTMS may be effective for a subset of patients (non-relapse rate: 29%) after the successful acute course of rTMS treatment.Reference Fitzgerald, Grace, Hoy, Bailey and Daskalakis 39 Janicak et al. conducted a multicenter, prospective, open-label, symptom-based maintenance rTMS study. In their study, they administered 5 sessions of rTMS treatment per week for up to a total of 4 weeks, followed by 2 sessions of rTMS treatment for a total of 2 weeks during the 6-month follow-up period after acute rTMS treatment, depending on the depressive symptoms of the patients. As a result, approximately 10% of patients developed recurrence during the 6-month follow-up period. Furthermore, the study showed that the safety and tolerability of maintenance rTMS treatment were similar to those of acute rTMS monotherapy and that the therapeutic effects of maintenance rTMS were also durable, indicating its potential effectiveness as a strategy for preventing the recurrence of depression.Reference Janicak, Nahas and Lisanby 40 Connolly et al. conducted a retrospective cohort study in which maintenance rTMS was administered to 42 patients after the completion of acute rTMS treatment depending on the relapse and recurrence of depressive symptoms. In the study, maintenance rTMS was performed in a tapering manner for a total of 6 months, initially once a week for 4 weeks, then twice a month for 2 months, and then once a month for 3 months. Twenty-six patients (62% of them) remained the response at the time of final evaluation during the maintenance rTMS period. No serious adverse events related to rTMS were observed and well tolerated during the maintenance rTMS period in the study.Reference Connolly, Helmer, Cristancho, Cristancho and O’reardon 41
Taken together, compared to these findings of previous studies, the preventive effect of maintenance rTMS on recurrence in the present case series has maintained a high level of both response and remission, despite the limitations of the study design and sample size. In addition, since a recent observational study by Gama-Chonlon et al. showed that patients with BD may respond better to rTMS than patients with unipolar depression,Reference Gama-Chonlon, Scanlan and Allen 42 it is possible that the fact that about half of the cases in this study consisted of patients with BD contributed to the favorable therapeutic as well as preventive effects in this case series. Furthermore, the present maintenance iTBS for the left DLPFC was performed once a week for a total of 15 sessions over a period of approximately 4 months, which is likely to be a promising preventive strategy for relapse and recurrence, in terms of not only its effectiveness but also its feasibility. Furthermore, given the findings of a recent review showing that approximately 54% of patients who were followed up without maintenance treatment after a successful treatment with an acute course of rTMS had recurred at 1 year,Reference Tundo, De Filippis and Proietti 3 the fact that all patients remained the response (ie, none of the cases recurred) during the maintenance iTBS in this case series also indicates that weekly maintenance iTBS is promising.
On the other hand, for the two cases included in this study, the acute course of iTBS treatment did provide the response, but the subsequent maintenance iTBS did not lead to remission (but maintained the response). One had a history of episodic psychotic symptoms associated with BD several months prior to the introduction of acute iTBS treatment, and the severity of the depressive episode was relatively severe at the time of iTBS treatment introduction. The other case had a diagnosis of RDD, but also had a diagnosis of autism spectrum disorder as background pathology, which may have limited the effect of iTBS treatment on the depressive symptoms. Thus, we suspect that the different clinical severity and profile of these two cases compared to the other cases may have prevented maintenance iTBS from having a sufficient stabilizing effect on their symptoms.
In addition, a previous study by Senova and colleagues reported that a higher percentage of female patients and those receiving maintenance therapy had higher response rates at a given time point.Reference Senova, Cotovio, Pascual-Leone and Oliveira-Maia 14 Thus, we examined the impact of sex on recurrence rates in this case series but found no significant effect of female sex on the maintenance of remission. The limited sample size of this study allowed only a preliminary examination but verification with a larger sample is awaited in the future. Regarding the impact of maintenance iTBS on retention of response and remission after acute iTBS treatment, we observed its clinically preventive effect on recurrence, despite the limitation that this study was a retrospective, open-label case series. With respect to the impact of different diagnoses of RDD and BD on response and remission with maintenance iTBS, no significant differences were found in the present study. This may be attributed to the background pathology of both diagnoses, which may form a continuum,Reference Bukh, Andersen and Kessing 43 –Reference Tse, Fok, Yim, Leung and Leung 48 besides the limitation of the small sample size.
Note that the severity of depression in the case series included in this study was for the most part at the mild to moderate level, which was milder than the severity of depression in previous studies. Such differences in clinical background may have contributed to the favorable outcomes of the maintenance iTBS in this study regarding its efficacy in preventing recurrence. More specifically, there are two possible reasons behind this. First, the disparities in clinical practices and depression guidelines between Japan and other countries may contribute to the observed variance in the severity of depression in patients selected for TMS treatment. Secondly, the successful administration of maintenance iTBS over a mid- to long-term period, in terms of preventing relapse and recurrence, may be attributed to the comparatively milder severity of initial depressive episodes relative to prior studies.
The strengths of the present study were as follows. First, we followed up to 28 months with maintenance iTBS after the successful treatment of acute iTBS for a certain number of patients in a real-world clinical setting for the first time. To the best of our knowledge, this case series is the first long-term follow-up observational study beyond 2 years in the world since the few previous studies on maintenance rTMS have usually followed patients for up to 12 months at most. Secondly, in this case series, we focused not on typical cases of TRD, but on more difficult-to-treat depression cases such as RDD and BD, which we believe to be novel. In this sense, this case series is the first challenge of its kind at least in Japan.
One limitation of this study is that, by its nature as an open-label preliminary case series utilizing registry data, it was not an RCT design with a control group, including a sham stimulation condition, and thus, the effect of placebo effect cannot be excluded. However, the placebo effect seems highly unlikely to persist for such a long period of time, since the final observation time point after the completion of acute iTBS treatment in this case series extends to a maximum of 28 months. Next, due to the nature of this report being a case series, the sample size was limited to approximately 20 cases. Therefore, a general conclusion regarding the prevention of relapse and recurrence by maintenance iTBS cannot be drawn from this report by itself, but this study only demonstrated such a possible relapse and recurrence prevention strategy for difficult-to-treat depression. Finally, although we do not have the MADRS and HAMD test data for the patients who responded to acute iTBS treatment but did not receive any maintenance iTBS or who received maintenance iTBS but completed less than 15 sessions because our clinic conducts clinical examinations every 15 sessions, we did not find a single case of clinical worsening of depressive symptoms as a result of receiving maintenance iTBS as far as we could ascertain from the medical records. However, we believe that more detailed follow-up monitoring is needed in the future, to the extent possible, to allow more detailed confirmation of patients’ progress and prognosis after the completion of acute iTBS treatment.
In conclusion, despite the above limitations, this case series report suggests that maintenance iTBS for a certain time period after the successful acute iTBS treatment could be a promising strategy that contributes to the stabilization of depressive symptoms and the prevention of mid- to long-term recurrence.Reference Elsayed, Ercis, Pahwa and Singh 49 Thus, the maintenance iTBS is an approach worth considering more actively not only in Japan but also worldwide in the future. Finally, the findings of this study warrant further investigation in an RCT design with a larger sample size.
Acknowledgments
We would like to thank the patients who participated in this case series and the staff at the Shinjuku-Yoyogi Mental Clinic for their support.
Author contribution
Yoshihiro Noda involved in conceptualization, methodology, investigation, formal analysis, writing the original draft, writing the review and editing, project administration, and supervision. Kyoshiro Fujii involved in investigation and data curation. Shinichiro Nakajima involved in investigation, writing the review and editing. Ryosuke Kitahata involved in investigation and supervision.
Financial support
This research received no external funding.
Disclosure
The authors declare none.









