Introduction
Xiphidiocercariae is a non-taxonomic group of small free-living digenean larvae. They were early subdivided into subgroups according to their morphology (Lühe, Reference Lühe1909). However, their taxonomic position cannot usually be established based on morphology only, even at the family level. Identification is especially problematic for Cercariae virgulae and Cercariae microcotylae subgroups (Faltýnková & Literák, Reference Faltýnková and Literák2002).
The position of cercariae in the taxonomic system of digeneans can be established with the use of molecular phylogeny. Nevertheless, the gap between the morphological and the molecular phylogenetic studies persists. To date, taxonomic identity has been established only for several virgulate and microcotylous cercariae (e.g. Hall, Reference Hall1959; Heneberg et al., Reference Heneberg, Faltýnková, Bizos, Malá, Žiak and Literák2015; Kudlai et al., Reference Kudlai, Cutmore and Cribb2015a, Reference Kudlai, Stunzenas and Tkachb; Chontananarth et al., Reference Chontananarth, Tejangkura, Wetchasart and Chimburut2017). Recent data indicate that the key morphological characters used to classify xiphidiocercariae need to be reassessed (Kudlai et al., Reference Kudlai, Cutmore and Cribb2015a, Reference Kudlai, Stunzenas and Tkachb).
In this study, we present the morphological descriptions of ten new virgulate and microcotylous xiphidiocercariae and redescribe one cercaria studied previously (Wergun, Reference Wergun1957). Their taxonomic position was clarified with the use of 28S rDNA gene sequencing. Based on the obtained results, we reconsidered the morphological features useful for taxonomic identification of virgulate and microcotylous cercariae. The set of morphological characteristics identified in this way allows a reliable identification of newly described cercariae as members of the families Lecithodendriidae, Pleurogenidae, Prosthogonimidae and Microphallidae.
Materials and methods
Host snails Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae) were collected in the Kristatell'ka River (St. Petersburg: 59°53′30.5″N, 29°50′05.9″E), Baushi Pond (St. Petersburg: 59°53′10.3″N, 29°51′10.4″E), and the Vorskla River (Belgorod Oblast: 50°33′52.6″N, 36°04′08.5″E). Host snails Viviparus viviparus Linnaeus, 1758 (Caenogastropoda: Viviparidae) were collected in the Kristatell'ka River (St. Petersburg: 59°53′30.5″N, 29°50′05.9″E). Collections were made in the period from the spring of 2012 to the summer of 2018.
The snails were kept in separate dishes and checked for invasion. Cercariae emerged from infected snails were placed on glass slides in a small water drop and studied in vivo with the help of Leica DM1000 (Wetzlar, Germany) and LOMO MBR-1 (St. Petersburg, Russia) microscopes. Morphological descriptions were made with RA-7 (LOMO, St. Petersburg, Russia) drawing apparatus. Nile blue sulphate and neutral red vital dyes were used to clarify cercarial morphology, when necessary. The mucoid apparatus was studied by staining with toluidine blue (Kruidenier, Reference Kruidenier1951, Reference Kruidenier1953a, Reference Kruidenierb, Reference Kruidenierc, Reference Kruidenierd; Ito & Watanabe, Reference Ito and Watanabe1958; Shchenkov et al., Reference Shchenkov, Smirnova and Kremnev2016). The presence of fat droplets was verified with Sudan A and Sudan Schwarz dyes. All measurements were made on the cercariae fixed in hot formaldehyde. They are given in micrometres as the range, with the mean in parentheses.
Samples for DNA isolation were fixed with 96% ethanol. Total DNA was isolated from the single specimens using a ZymoBead Genomic DNA Kit™ (Irvine, California, USA). The d1–d3 domain of about 1200 bp localized at the 5′ end of 28 rDNA was amplified using the BIO-RAD C1000 Thermal Cycler (Hercules, California, USA). Forward primers LSU-5 (5′-TAG GTC GAC CCG CTG AAY TTA AGC A-3′), 28Sy (5′-CTA ACC AGG ATT CCC TCA GTA ACG GCG AGT-3′), dig12 (5′-AAG CAT ATC ACT AAG CGG-3′) and reverse primers 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′), 28Sz (5′-AGA CTC CTT GGT CCG TGT TTC AAG AC-3′) were used (table 1). Polymerase chain reactions were performed in a total volume of 20 µl (11.5 µl H2O, 2.5 µl Taq buffer, 2 µl dNTP at a concentration of 10 pM, 0.5 µl of each primer at a concentration of 10 pM, 1 µl of Syntol Taq polymerase, 1 µl of the DNA template). The thermal cycle parameters were as follows: initial denaturation at 95°C (3 min); 35 cycles of 20 s at 95°C; 20 s at 56°C for LSU5/1500R primers, 120 s at 72°C; or 20 s at 50°C for dig12/1500R primers, 100 s at 72°C; or 20 s at 62°C for 28Sy/28Sz primers, 100 s at 72°C; 5 min at 72°C for the final extension.
Table 1. General information about newly obtained sequences.
Amplicons were purified using a Cleanup Mini Purification Kit™ (Evrogen, Moscow, Russia). All amplicons were sequenced using the equipment of the Research Park of St. Petersburg State University (Centre for Molecular and Cell Technologies). Sequences from both forward and reverse primers were assembled using Chromas Pro 1.7.4 (Technelysium Pty Ltd).
In total, 54 sequences were used for the alignment (supplementary table S1, after Tkach et al., Reference Tkach, Pawlowski and Mariaux2000, Reference Tkach, Snyder and Swiderski2001, Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003; Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003; Galaktionov et al., Reference Galaktionov, Blasco-Costa and Olson2012; Lord et al., Reference Lord, Parker, Parker and Brooks2012; Greiman et al., Reference Greiman, Tkach and Vaughan2013; Heneberg & Literák, Reference Heneberg and Literák2013; Kanarek et al., Reference Kanarek, Zaleśny, Sitko and Tkach2014, Reference Kanarek, Zaleśny, Czujkowska, Sitko and Harris2015, Reference Kanarek, Zaleśny, Sitko and Tkach2017; Kudlai et al., Reference Kudlai, Cutmore and Cribb2015a, Reference Kudlai, Stunzenas and Tkachb, Reference Kudlai, Cribb and Cutmore2016). The sequences were aligned using the Muscle algorithm (Edgar, Reference Edgar2004) as implemented in SeaView 4.0 (Gouy et al., Reference Gouy, Guindon and Gascuel2010) followed by a manual alignment verification. In total, about 1100 sites were selected for further analysis (sequences were trimmed manually in order to exclude positions with missing data). The phylogenetic analysis was performed using the maximum likelihood (ML) method with the GTR + G + I model. An ML tree was constructed by using the RAxML program (Stamatakis, Reference Stamatakis2006) at the CIPRES Science Gateway (www.phylo.org; Miller et al., Reference Miller, Pfeiffer and Schwartz2010). The stability of clades was assessed using a non-parametric bootstrap with 1000 pseudoreplicates. Bayesian inference analysis was performed using the MrBayes 3.1.2 (mrbayes.sourceforge.net) GTR model with gamma correction for inter-site rate variation (eight categories) and the covarion model. Tree calculations were run as two separate chains (default heating parameters) for 15 million generations, the point at which they ceased converging. The quality of chains was estimated with built-in MrBayes tools and Tracer 1.7 (Rambaut et al., Reference Rambaut, Drummond, Xie, Baele and Suchard2018). Altogether, 5000 generations were discarded as the burn-in phase.
Our phylogenetic analysis included only the taxa with microcotylous and virgulate cercariae. Sequences of digeneans with other types of cercariae (such as Renicolidae Dollfus, 1939, Faustulidae Poche, 1926, Zoogonidae Odhner, 1902, etc.) were not used.
In the Remarks sections, we discuss only the morphological features of newly obtained cercariae in order to confirm their differences from the previously described forms.
Results
Molecular phylogenetic analysis
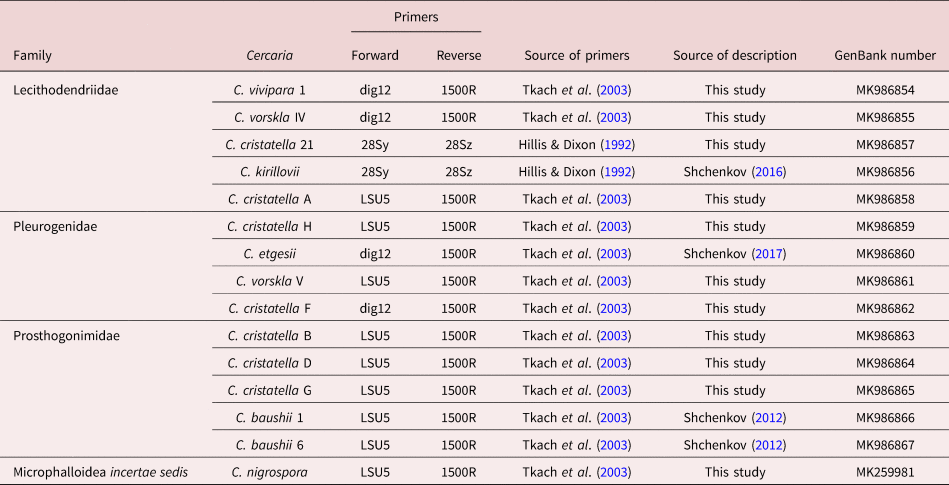
Microphallidae and Lecithodendriidae are sister taxa with a high nodal support (fig. 1). Five cercariae were found to belong to the Lecithodendriidae clade: Cercaria vivipara 1, to the genus Prosthodendrium; Cercaria vorskla IV, to the genus Pycnoporus with a high posterior probability; and Cercaria cristatella 21, C. cristatella A and Cercaria kirillovii Shchenkov, 2016, to the genus Lecithodendrium (fig. 1b). Cercaria nigrospora formed a separate clade with a high posterior probability (fig. 1b).
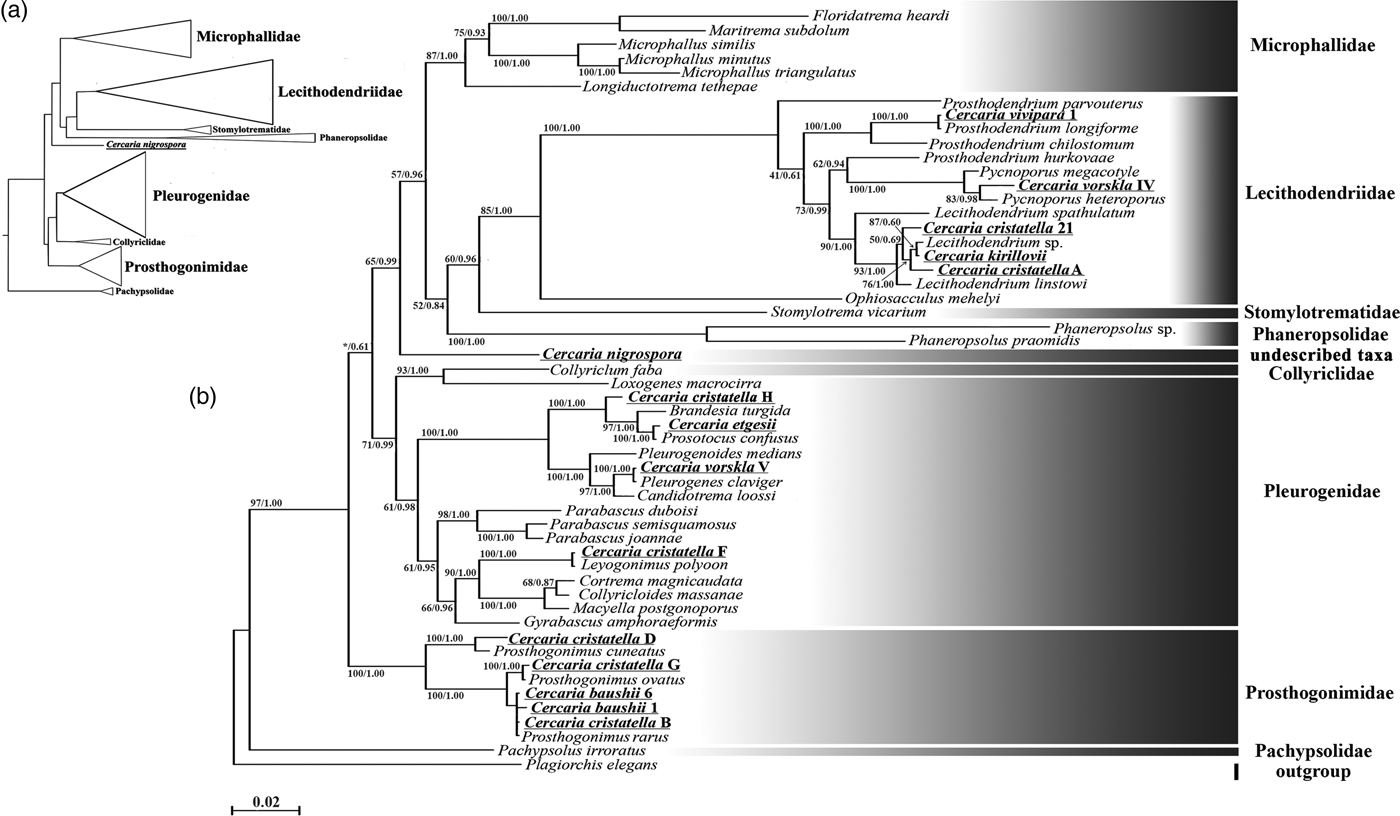
Fig. 1. Phylogenetic position of newly and previously described cercariae (highlighted in bold and underlined, respectively) based on partial 28S rDNA data. (a) General topology of clades resulting from maximum likelihood (ML) analyses; (b) results of Bayesian inference (BI) analyses. Nodal support: ML/BI.
Four cercariae belonged to Pleurogenidae (C. cristatella H, C. vorskla V, C. cristatella F and Cercaria etgesii Shchenkov, 2016). Almost all the Pleurogenidae subclades were highly supported, with the exception of Cortrema magnicaudata and Collyricloides massanae nodes.
C. etgesii was close to the genus Prosotocus, while C. cristatella H formed a separate clade close to Prosotocus and Brandesia. C. vorskla V belonged to the genus Pleurogenes. C. cristatella F belonged to the Leyogonimus clade with the maximum Bayesian support (fig. 1b).
A close proximity of Pleurogenidae to the Microphallidae + Lecithodendriidae + C. nigrospora clade was not supported by Bayesian analysis (fig. 1b). In the ML tree, Pleurogenidae is close to Prosthogonimidae (the general topology of the ML tree is shown in fig. 1a).
Prosthogonimidae is one of the basal clades among the taxa under consideration with the maximum Bayesian support. Three newly described cercariae (C. cristatella D, C. cristatella G, C. cristatella B) and two previously described ones (Cercaria baushii 6 Shchenkov, 2012, C. baushii 1 Shchenkov, 2012) belong to this clade (fig. 1b).
The basal clade is formed by Pachypsolus irroratus.
Morphological data
Family: Lecithodendriidae
Cercaria vorskla IV
(fig. 2a)
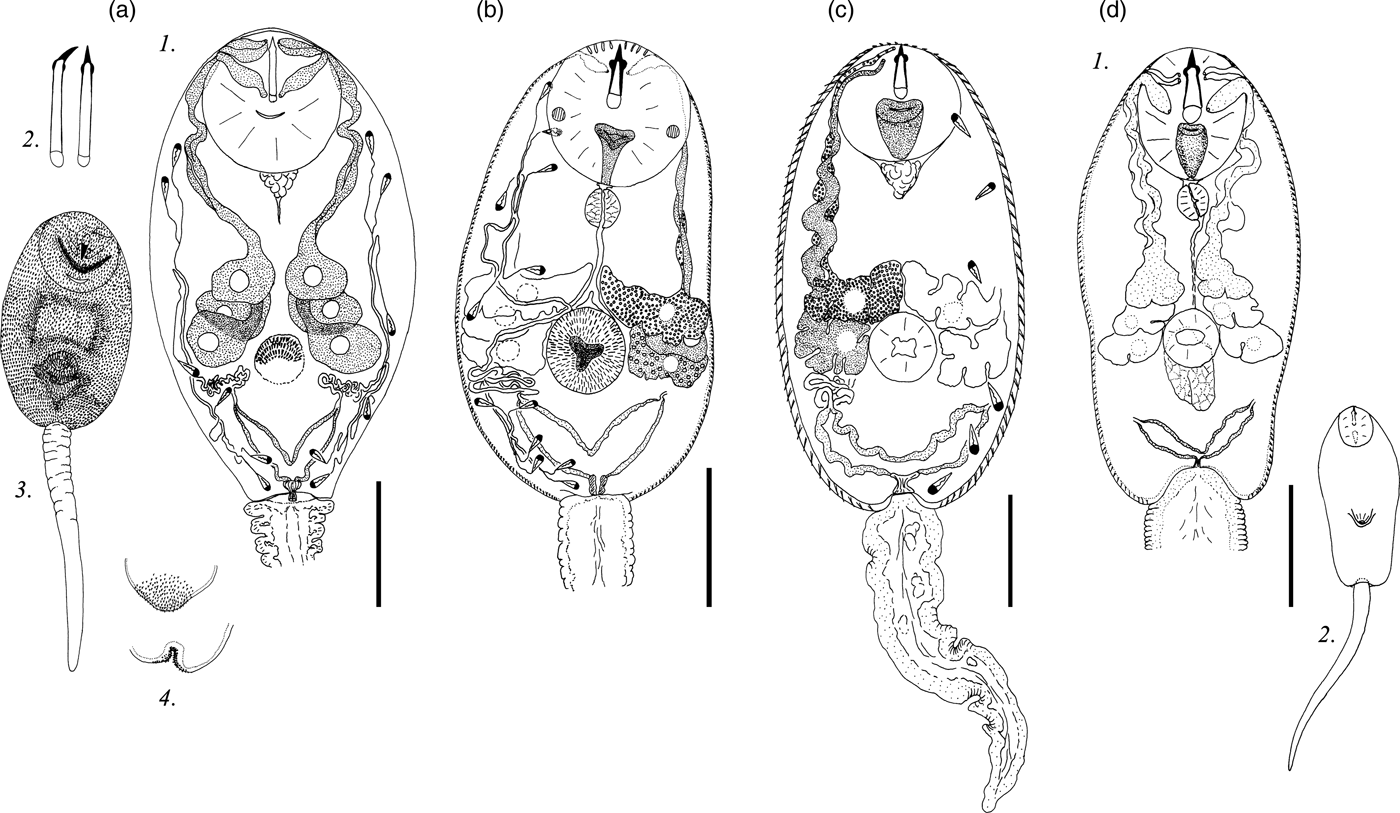
Fig. 2. Cercariae of Lecithodendriidae. (a) Cercaria vorskla IV (1, general morphology; 2, stylet; 3, body with tail; 4, tail tip); (b) C. cristatella A; (c) C. cristatella 21; (d) C. vivipara 1 (1, general morphology; 2, body with tail). Scale bars: 30 µm.
Type localities. Vorskla River, Belgorod Oblast (50°33′52.6″N, 36°04′08.5″E); Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 97–119 (111) μm long, 39–46 (41) μm wide. Tail 70–97 (91) μm long. Oral sucker 28–35 (33) μm in diameter. Ventral sucker underdeveloped, post-equatorial, 18–21 (20) μm in diameter. Oral/ventral sucker ratio: 1:0.6. Stylet 14–15 (14) μm. Body tegument spinose, spines oriented backwards. Three rows of larger spines covering anterior part of ventral sucker, almost all tail tegument without spines. Tail tip, used as sucker for adhesion to water surface, covered with small spines (fig. 2a4). Stylet with thin parallel walls, tip bending onto ventral side (fig. 2a2).
Prepharynx absent, pharynx primordium triangular. Oesophagus lumen short and narrow, intestine bifurcation and caeca branches not detected.
Virgula absent. Two pairs of penetration glands located anteriorly to ventral sucker, one pair located on its sides. Outlets of one pair of penetration glands opening at stylet bulb level, outlets of the other two pairs opening near stylet tip. Cytons nearly equal in size, filled with fine granular secretory material.
Excretory formula: 2 [(1 + 1 + 1) + (1 + 1 + 1)] = 12. Excretory bladder V-shaped, its walls thin. Dense balls of main excretory tubes located immediately behind posterior cytons of penetration glands.
Genital primordium not detected. Parenchyma lacks fat droplets, cystogenous gland cells not detected.
Remarks. C. vorskla IV has a unique stylet with parallel walls and a bent tip. Only Cercaria indica LI Sewell, 1922 has a similar stylet tip. C. indica LI differs from C. vorskla IV in the presence of four pairs of penetration glands and the virgula organ. There are several other microcotylous cercariae with three pairs of penetration gland such as Cercaria rarissima Tabunari, 1928, Cercaria agstaphensis 16 Manafov, 2010 and C. agstaphensis 21 Manafov, 2010. C. vorskla IV differs from them in having homogenous fine granular material in the penetration glands.
Cercaria cristatella A
(fig. 2b)
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 99–123 (115) μm long, 42–51 (48) μm wide. Tail 31–34 (33) μm long. Oral sucker 27–33 (32) μm in diameter. Ventral sucker well developed, post-equatorial, 21–24 (22) μm in diameter. Oral/ventral sucker ratio: 1:0.68. Stylet 12–13 (12) μm long, with large shoulders. Body tegument spinose, spines oriented backwards. Spines in ventral sucker tegument oriented toward centre. Tail without spines.
Prepharynx short, pharynx oval, well developed, 7–8 (7.5) μm long. Oesophagus lumen broad. Intestine bifurcates in front of ventral sucker, caeca branches reach level of anterior third of ventral sucker.
Virgula small, non-lobed. Mucoid secretion also concentrated in ventral sucker tegument.
Three pairs of penetration glands forming two compact groups on ventral sucker sides. Large anterior pair containing coarse granular material. Second pair small, hyalinized. Third pair middle-sized, containing coarse and fine granular material. Only two openings of outlets of penetration glands detected behind stylet shoulders.
Excretory formula: 2 [(2 + 2 + 2) + (2 + 2 + 2)] = 24. Excretory bladder V-shaped, walls thin. Dense balls of large main excretory tubes located behind penetration glands.
Genital primordium and cystogenous gland cells not detected. Parenchyma without fat droplets.
Remarks. C. cristatella A is morphologically close to Cercaria indica XXXVII Sewell, 1922 but differs from it in the lack of spines in tail tegument. It differs from Cercaria agstaphensis 7 Manafov, 2010 in the size, shape and granulation of penetration glands. Cercaria stenodorya Hall & Groves, 1963, Cercaria blennifera Hall & Groves, 1963, Cercaria pinguisoma Hall, 1960 and Cercaria celatoglandis Hall, Reference Hall1960 have three pairs of penetration glands with a smaller second pair, but all of them have a larger virgula organ than C. cristatella A. A unique character of C. cristatella A is a high concentration of mucin in the ventral sucker tegument.
Cercaria cristatella 21
(fig. 2c)
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 85–90 (87) μm long, 39–71 (60) μm wide. Tail 15–31 (23) μm long. Oral sucker 24–34 (28) μm in diameter. Ventral sucker well developed, post-equatorial, 14–24 (22) μm in diameter. Oral/ventral sucker ratio: 1:0.78. Stylet 13–16 (15) μm long. Body tegument spinose, spines oriented backwards. Tail without spines.
Prepharynx absent, pharynx primordium triangular. Oesophagus lumen, intestine bifurcation and caeca branches not detected.
Virgula small, non-lobed. Two pairs of penetration glands located on ventral sucker sides. Anterior pair containing coarse granular material, posterior pair containing fine granular material. Outlets of penetration glands opening close to stylet tip and shoulders.
Excretory formula not established, 12 flame cells detected on entire body. Excretory bladder V-shaped, walls thin. Two loops of main excretory tubes located behind penetration glands.
Genital primordium and cystogenous gland cells not detected. Parenchyma without fat droplets.
Remarks. C. cristatella 21 differs from Cercaria kirillovii Shchenkov, 2016 in the number of flame cells and the relative position of the openings of the outlets of penetration glands on the ventral surface of the oral sucker. C. cristatella 21 differs from Cercaria indica LVII Sewell, 1922 in having numerous flame cells.
Cercaria vivipara 1
(fig. 2d)
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Viviparus viviparus Linnaeus, 1758 (Caenogastropoda: Viviparidae).
Description. Body 86–92 (88) μm long, 32–64 (51) μm wide. Tail 63–89 (78) μm long. Oral sucker 23–38 (27) μm in diameter. Ventral sucker well developed, post-equatorial, 13–16 (15) μm in diameter. Oral/ventral sucker ratio: 1:0.55. Stylet 14–18 (17) μm long. Body tegument spinose, spines oriented backwards. Tail without spines.
Prepharynx short, pharynx oval, well developed, 7–8 (8) μm long. Oesophagus lumen narrow, intestine bifurcation not detected.
Virgula small, non-lobed. Three pairs of penetration glands located on ventral sucker sides. Cytons of almost equal size. First pair of glands containing fine granular material, second and third pair containing hyalinized material. Outlets of two pairs of penetration glands opening near stylet shoulders and those of the third pair, near stylet bulb.
Excretory formula not established. Excretory bladder V-shaped, walls thin.
Genital primordium located between penetration glands of the last pair, bilobed, elongated posteriorly.
Cystogenous gland cells not identified. Parenchyma without fat droplets.
Remarks. Most of the larvae with the same number of penetration glands, such as Cercaria indica XLIV Sewell, 1922 have a lobed virgula organ. However, C. indica V Sewell, 1922 has similar penetration glands and an identical V-shaped excretory bladder. C. vivipara 1 differs from C. indica V in having fine granular material in the anterior penetration glands and in the shape of cytons. Intestine bifurcation, visible in C. indica V, was invisible in C. vivipara 1. The host snails of C. indica V are Melanoides tuberculatus Müller, 1774 and Bithynia (Digoniostoma) cerameopoma Benson, 1830, whereas C. vivipara 1 is described from V. viviparus.
Family: Pleurogenidae
Cercaria cristatella H
(fig. 3a)
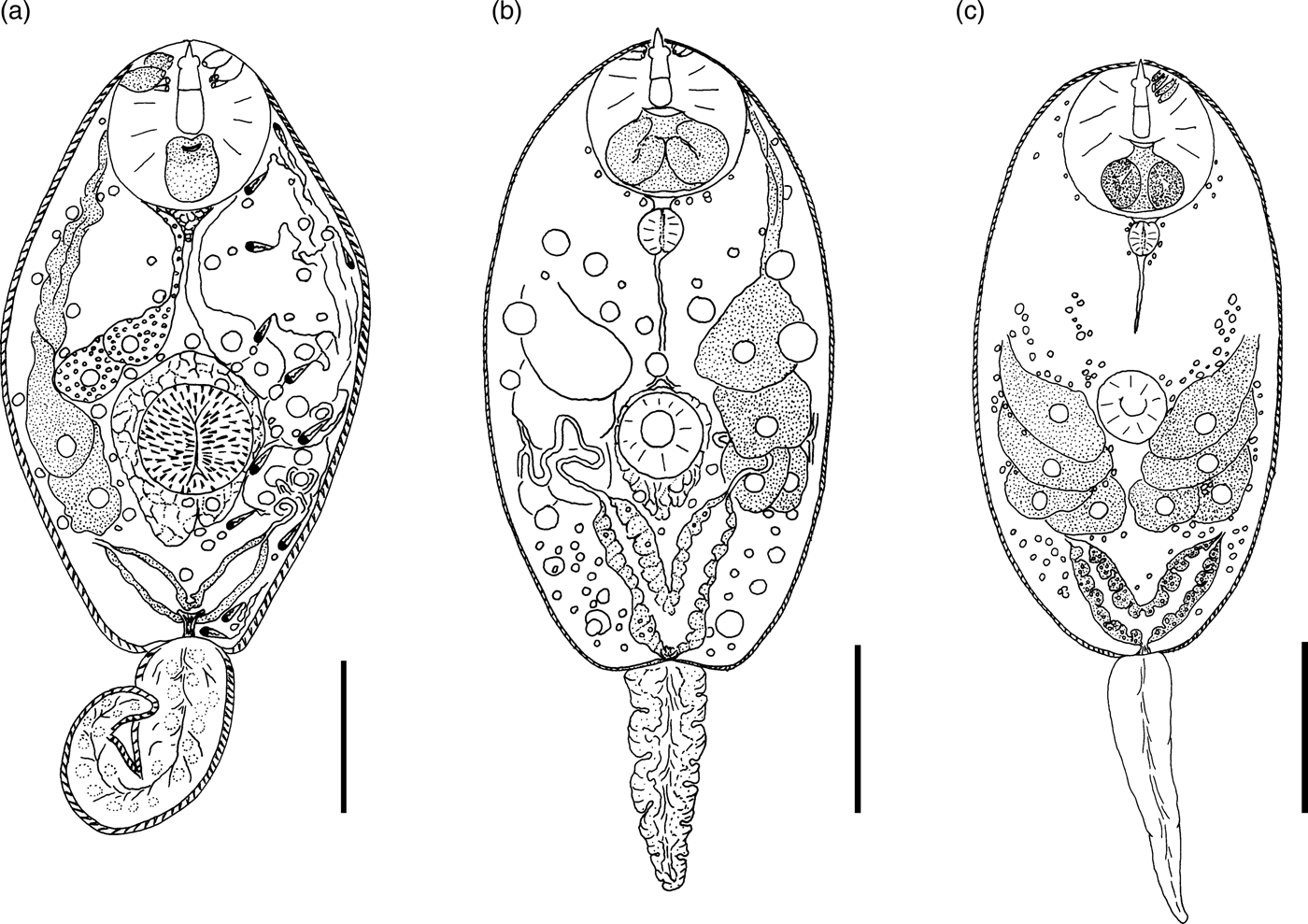
Fig. 3. Cercariae of Pleurogenidae. (a) Cercaria cristatella H; (b) C. cristatella F; (c) C. vorskla V. Scale bars: 30 µm.
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 97–127 (119) μm long, 43–77 (70) μm wide. Tail 29–37 (35) μm long. Oral sucker 27–33 (31) μm in diameter. Ventral sucker well developed, post-equatorial, 15–23 (20) μm in diameter. Oral/ventral sucker ratio: 1:0.64. Stylet 13–17 (16) μm long. Body and tail tegument spinose, spines oriented backwards. Spines in ventral sucker tegument oriented toward centre.
Prepharynx absent. Pharynx primordium triangular. Intestine and caeca bifurcation not detected.
Virgula small, non-lobed. Four pairs of penetration glands located on ventral sucker sides. Two anterior pairs slightly smaller than two posterior pairs. First and second pairs containing coarse granular secretory material. Two posterior pairs of penetration glands containing fine granular matrix. Outlets of penetration glands opening one after another at level of first third of stylet length.
Excretory formula: 2 [(2 + 2 + 2) + (2 + 2 + 2)] = 24. Excretory bladder V-shaped, walls thin. Dense balls of main excretory tubes located behind penetration glands. Genital primordium nearly twice larger than ventral sucker, located between penetration glands, bilobed, elongated anteroposteriorly.
Cystogenous gland cells not detected. Parenchyma containing small and medium-sized fat droplets.
Remarks. C. cristatella H differs from Lecithodendriidae gen. sp. 3 Faltynkova & Literak, 2002 in the granulation, location and relative size of penetration glands, as well as in the stylet shape and the body length.
Cercaria cristatella F
(fig. 3b)
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 87–131 (119) μm long, 39–68 (65) μm wide. Tail 21–32 (27) μm long. Oral sucker 22–33 (27) μm in diameter. Ventral sucker 14–26 (24) μm in diameter, well developed, post-equatorial. Oral/ventral sucker ratio: 1:0.89. Stylet 13–14 (14) μm long. Body tegument spinose, spines oriented backwards. Tail without spines.
Prepharynx short, pharynx oval, well developed, 6–8 (7) μm long. Oesophagus lumen narrow, intestine bifurcation in front of ventral sucker, caeca branches not detected.
Bilobed virgula (pear-shaped organ) located in posterior part of oral sucker. Four pairs of penetration glands located on ventral sucker sides. Second pair located behind first pair, third and fourth pairs smaller, located at the same level. Outlets of penetration glands opening near stylet shoulders.
Excretory bladder V-shaped, walls thick. Loops of main excretory tubes few in number, located between cytons of second and third penetration glands. Genital primordium small, bilobed, elongated anteroposteriorly, located above ventral sucker between penetration glands.
Cystogenous gland cells not detected. Parenchyma with numerous fat droplets. Large droplets located mainly in preacetabular zone, small droplets surrounding excretory bladder.
Remarks. There are many cercariae morphologically similar to C. cristatella F, with four pairs of penetration glands and a large pear-shaped organ. Most of them belong to the genera Pleurogenus or Pleurogenoides. C. cristatella F differs from other pleurogenid cercariae in having a specific distribution of fat droplets in the parenchyma: numerous small fat droplets surround the excretory bladder and single large fat droplets are present in the anterior body part.
Cercaria vorskla V
(fig. 3c)
Type locality. Vorskla River, Belgorod Oblast (50°33′52.6″N, 36°04′08.5″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 85–129 (120) μm long, 33–71 (59) μm wide. Tail 24–37 (31) μm long. Oral sucker 21–34 (29) μm in diameter. Ventral sucker 12–24 (21) μm in diameter, equatorial, well developed. Oral/ventral sucker ratio: 1:0.72. Stylet 12–15 (13) μm long. Body tegument spinose, spines oriented backwards. Tail without spines.
Prepharynx short, pharynx oval, well developed, 5–8 (6) μm long. Oesophagus lumen narrow, intestine bifurcation and caeca branches not detected.
Bilobed virgula (pear-shaped organ) located in posterior part of oral sucker. All penetration glands nearly equal in size. Second pair located behind first pair, third and fourth pairs smaller, located at the same level. Outlets of penetration glands openings one after another behind stylet shoulders.
Excretory bladder V-shaped, walls thick. Excretory formula not established.
Cystogenous gland cells not detected. Parenchyma with numerous small fat droplets, located predominantly in posterior two thirds of body.
Remarks. Similar numerous small fat droplets are characteristic of Collyriclum faba cercariae (Heneberg et al., Reference Heneberg, Faltýnková, Bizos, Malá, Žiak and Literák2015). C. vorskla V differs from this species in having thick walls of the excretory bladder, in the position of the two posterior pairs of penetration glands, and in having a relatively small pear-shaped organ.
Family: Prosthogonimidae
Cercaria cristatella B
(fig. 4a)
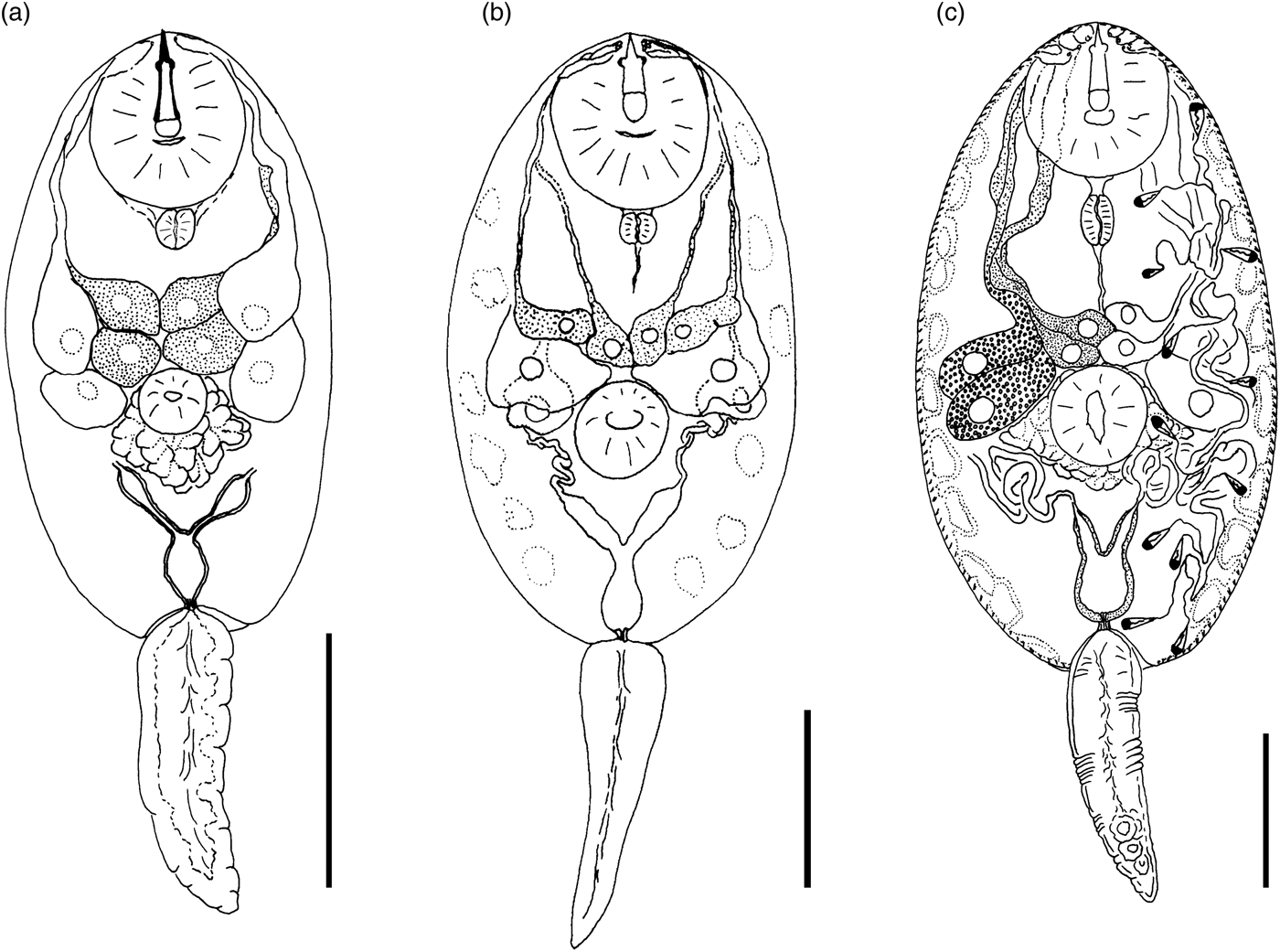
Fig. 4. Cercariae of Prosthogonimidae. (a) Cercaria cristatella B; (b) C. cristatella D; (c) C. cristatella G. Scale bars: 30 µm.
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 85–117 (105) μm long, 33–59 (50) μm wide. Tail 16–29 (24) μm long. Oral sucker 29–39 (37) μm in diameter, ventral sucker 14–23 (19) μm in diameter, nearly equatorial, well developed. Oral/ventral sucker ratio: 1:0.51. Stylet 12–15 (14) μm long. Spines in body and tail tegument not detected.
Prepharynx very short, only visible in fully stretched cercariae. Pharynx oval, well developed, 8–9 (8) μm long. Other details of digestive system structure not detected.
Virgula absent. Penetration glands arranged in two adjacent rhomboid groups, four glands in each group: two lateral glands with hyalinized cytoplasm and two submedian glands with fine granular matrix. Outlets of penetration glands opening near stylet tip.
Excretory bladder Y-shaped, walls thin. Other details of excretory system organization not established. Genital primordium rhomboid, located behind penetration glands and ventral sucker.
Cystogenous gland cells not detected. Parenchyma without fat droplets.
Remarks. C. cristatella B differs from the morphologically close Cercaria helvetica XI Dubois, 1929 in the presence of fine granular material in two submedian penetration glands. Cercaria baushii 1 Shchenkov, 2012 contains a similar fine granular material in all cytons of the penetration glands, dilated outlets of penetration glands and a triangular genital primordium. However, C. cristatella B differs from C. baushii 1 in the shape of the excretory bladder, whose arms are constricted near the connection with the stem.
Cercaria cristatella D
(fig. 4b)
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 85–134 (121) μm long, 35–68 (65) μm wide. Tail 22–34 (26) μm long. Oral sucker 21–33 (28) μm in diameter. Ventral sucker 12–22 (16) μm in diameter, post-equatorial, well developed. Oral/ventral sucker ratio: 1:0.57. Stylet 12–17 (15) μm long. Spines in body and tail tegument not detected.
Prepharynx short, pharynx oval, well developed, 7–8 (8) μm long. Oesophagus narrow, other details not detected.
Virgula absent. Penetration glands arranged in two adjacent rhomboid groups, four glands in each group: two large lateral glands with hyalinized cytoplasm and two small submedian glands with fine granular material. Outlets of penetration glands opening near stylet tip and shoulders.
Excretory bladder Y-shaped, walls thin. Small loops of main excretory tubes located behind penetration glands. Excretory formula not established.
Cystogenous gland cells not numerous, containing fine granular material. Genital primordium not detected. Parenchyma without fat droplets.
Remarks. C. cristatella D differs from other similar cercariae in a smaller fourth pair of penetration glands. It differs from C. helvetica XI Dubois, 1929 in having fine granular material in small submedian penetration glands. Similar penetration glands are characteristic of Cercaria baushii 6 Shchenkov, 2012, but the main excretory tubes in that species merge with the arms of the excretory bladder subterminally, while in C. cristatella D they merge terminally.
Cercaria cristatella G
(fig. 4c)
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Bythinia tentaculata Linnaeus, 1758 (Caenogastropoda: Bythiniidae).
Description. Body 86–129 (120) μm long, 40–65 (59) μm wide. Tail 17–28 (25) μm long. Oral sucker 24–37 (31) μm in diameter. Ventral sucker 11–29 (18) μm in diameter, nearly equatorial, well developed. Oral/ventral sucker ratio: 1:0.58. Stylet 12–19 (16) μm long. Body tegument spinose, spines oriented backwards. Tail without spines.
Prepharynx long, pharynx oval, well developed, 8–9 (9) μm long. Oesophagus narrow, other details not detected.
Virgula absent. Two middle pairs of penetration glands small, with fine granular matrix. Large lateral cytons with coarse granular secretory material. Cytons differing greatly in size. Openings of outlets of penetration glands located from stylet tip level to first quarter of length.
Excretory formula: 2 [(2 + 2 + 2) + (2 + 2 + 2)] = 24. Excretory bladder close to Y-shaped, walls thin, arms short. Dense balls of large main excretory tubes located behind rhomboid genital primordium.
Cystogenous gland cells numerous, containing fine granular material. Parenchyma without fat droplets.
Remarks. C. cristatella G differs from C. cristatella B, C. cristatella D, C. baushii 6 Shchenkov, 2012 and C. helvetica XI Dubois, 1929 in having very short arms of the excretory bladder. A unique feature of C. cristatella G is a coarse granulation of lateral pairs of penetration glands.
Microphalloidea fam. gen. sp.
Cercaria nigrospora Wergun, 1957
(fig. 5)
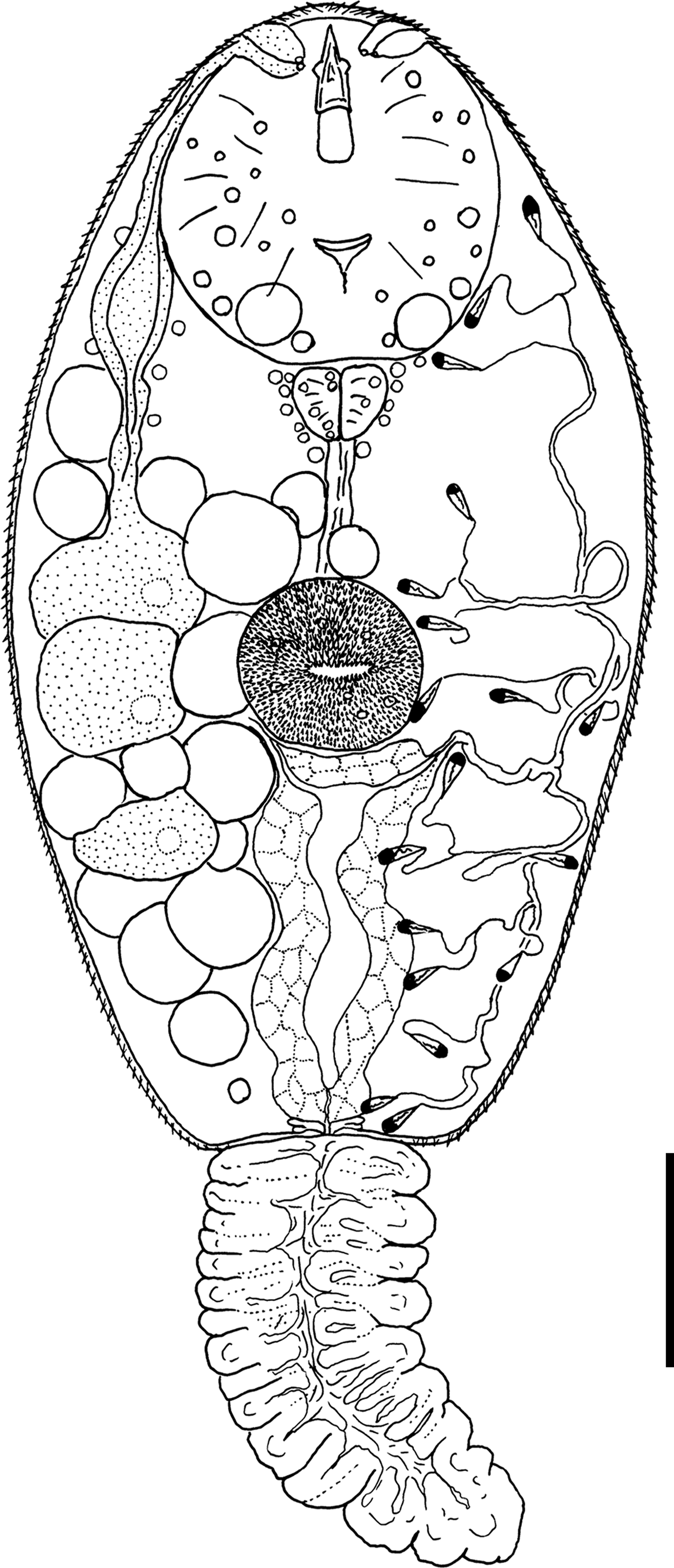
Fig. 5. General morphology of Cercaria nigrospora. Scale bar: 30 µm.
Type locality. Kristatell'ka River, Staryi Petergof, St. Petersburg (59°53′30.5″N, 29°50′05.9″E).
First intermediate host. Viviparus viviparus Linnaeus, 1758 (Caenogastropoda: Viviparidae).
Description. Body 109–155 (132) μm long, 56–67 (61) μm wide. Tail 77–94 (88) μm long. Oral sucker 28–38 (32) μm in diameter. Ventral sucker 18–25 (21) μm in diameter, equatorial, well developed. Oral/ventral sucker ratio: 1:0.65. Stylet 18–23 (20) μm long. Body tegument spinose, spines oriented backwards. Tail without spines.
Prepharynx short, pharynx triangular, well developed, 6–9 (8) μm long. Oesophagus broad, other details not detected.
Virgula absent. Three pairs of penetration glands of nearly equal size. Glands located on both sides of acetabulum. Anterior pairs of cytons close to each other. Posterior cytons separated by a prominent gap, never contacting each other. Secretory material fine granular. Outlets of penetration glands opening at level of stylet shoulders.
Excretory formula: 2 [(3 + 3 + 3) + (3 + 3 + 3)] = 18. Excretory bladder I-shaped, walls thick. Main excretory tubes not forming loops or dense balls.
Parenchyma with numerous large fat droplets. Cystogenous gland cells and genital primordium not detected.
Remarks. This cercaria has the same morphology and the same first intermediate host as C. nigrospora Wergun, 1957. Here we provide its excretory formula, which was not established in the original description.
We combined the morphological descriptions of virgulate and microcotylous cercariae made in this study with those available in the literature (Sewell, Reference Sewell1922; Azim, Reference Azim1936; Seitner, Reference Seitner1945; Hall, Reference Hall1959, Reference Hall1960; Burns, Reference Burns1961; Hall & Groves, Reference Hall and Groves1963; Nasir, Reference Nasir1965, Reference Nasir1972, Reference Nasir1982; Haseeb & Khan, Reference Haseeb and Khan1984; Galaktionov & Malkova, Reference Galaktionov and Malkova1994; Ditrich et al., Reference Ditrich, Scholz, Aguirre-Macedo and Vargas-Vázquez1997; Faltýnková & Literák, Reference Faltýnková and Literák2002; Manafov, Reference Manafov2010; Shchenkov, Reference Shchenkov2012, Reference Shchenkov2016, Reference Shchenkov2017; Kudlai et al., Reference Kudlai, Cutmore and Cribb2015a, Reference Kudlai, Stunzenas and Tkachb, Reference Kudlai, Cribb and Cutmore2016). Based on these data, we identified five morphological types of cercariae (table 2, fig. 6).
Table 2. Five morphological types of small xiphidiocercariae (see fig. 6 for schematic illustrations).
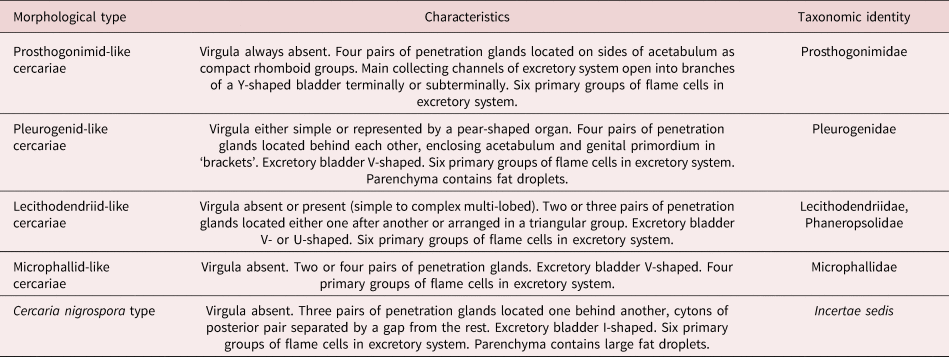
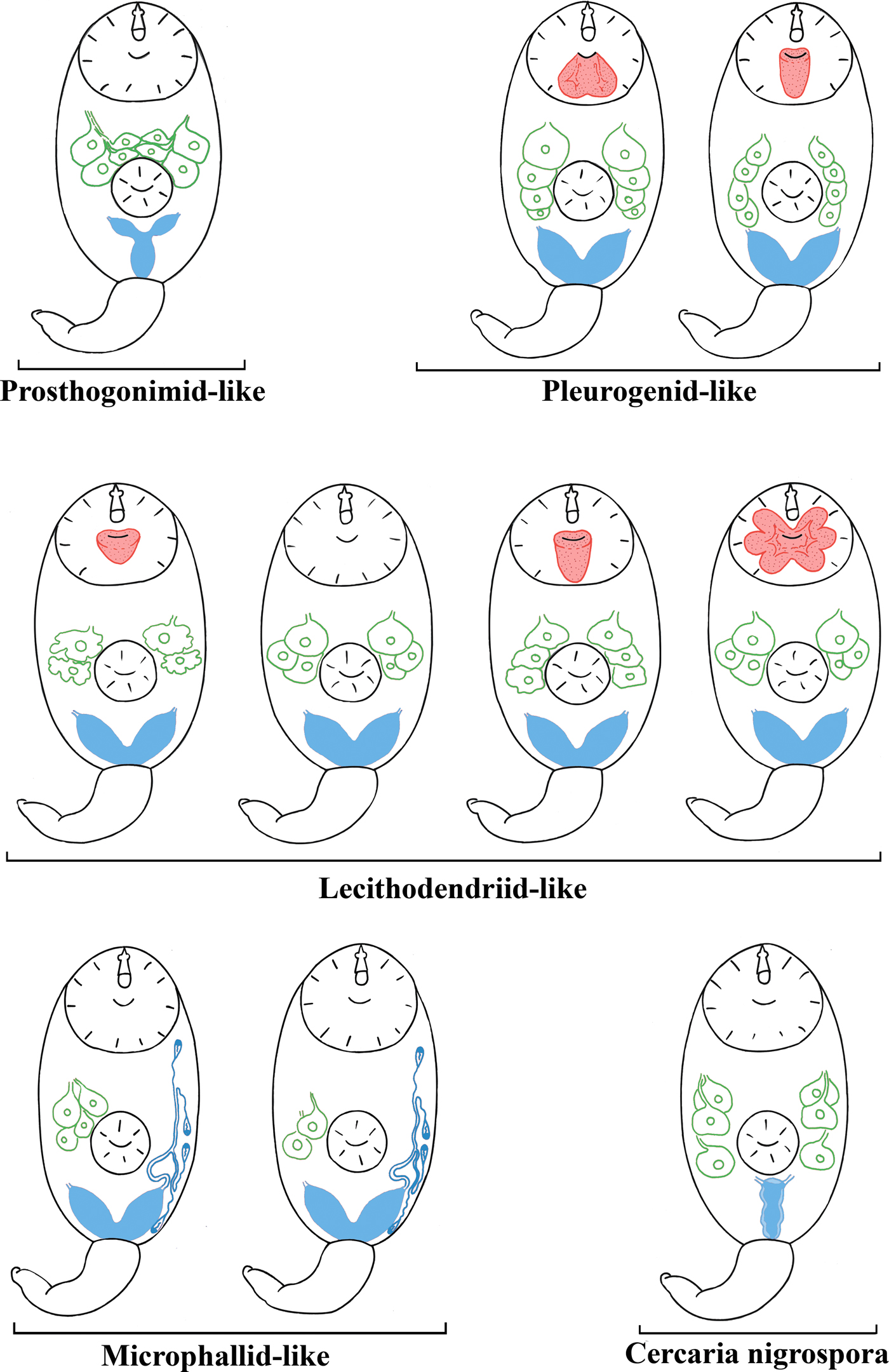
Fig. 6. A scheme of morphological types of cercariae mentioned in the present study. The names of morphological types are given in quotes as the taxa they belong to (if known). Features important for taxonomic identification are given in red (virgula), green (penetration glands) and blue (excretory bladder and primary groups of flame cells in microphallid-like cercariae). Image is not to scale.
Discussion
In this paper, we obtained morphological and molecular data on several species of virgulate and microcotylous xiphidiocercariae. Combining these data with information from the literature, we identified five morphological types of cercariae from these groups.
The general topology of the phylogenetic tree of these cercariae (fig. 1) differs from that of earlier trees (Tkach et al., Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003; Kanarek et al., Reference Kanarek, Zaleśny, Sitko and Tkach2014, Reference Kanarek, Zaleśny, Sitko and Tkach2017). According to the recent molecular phylogenetic data (Pérez-Ponce de Leόn & Hernández-Mena, Reference Pérez-Ponce de Leόn and Hernández-Mena2019), Prosthogonimidae, Pleurogenidae and Microphallidae are sister taxa with low Bayesian supports. Such phylogenetic relationships between families are not supported by our data because of the mutual position of the Microphallidae and the Lecitodendriidae clades, and the basal position of the Prosthogonimidae in relation to the other taxa (fig. 1b). A similar tree topology has recently been found only in the ML analysis (Pérez-Ponce de Leόn & Hernández-Mena, Reference Pérez-Ponce de Leόn and Hernández-Mena2019). The position of Pleurogenidae is unstable when Bayesian and ML analyses are used (fig. 1a, b). Due to such contradictions, phylogenetic relationships within the Microphalloidea are unclear at the family level. Molecular phylogenetic data on trematodes are usually supported by the morphology of adults. Morphological data on xiphidiocercariae clarify the phylogenetic relationships within Microphalloidea and confirm the newly obtained phylogenetic tree.
All xiphidiocercariae are characterized by a heterochronous development (Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). Heterochrony is especially pronounced during the development of the excretory system, penetration and mucoid glands and reservoirs for accumulation of mucin such as caudal pockets and virgulae. Five cercarial morphotypes, which correspond to certain clades within the Microphalloidea, clearly differ as to the level of heterochrony. Grouping based on this character is more natural than the separation of xiphidiocercariae into only two groups, Cercariae virgulae and Cercariae microcotylae (table 2, fig. 6).
An underdevelopment or a total reduction of the ventral sucker is considered as an important morphological character of microcotylous cercariae (e.g. cercariae of Microphallus similis (Jägerskiöld, 1900) Nichol, 1906, which belong to Cercaria ubiquita Lebour, 1907 subtype). However, the tendency towards a reduced ventral sucker is observed not only in Microphallidae but also in Lecithodendriidae (e.g. C. vorskla IV, fig. 2a).
An extremely juvenilized excretory system is a common feature of microphallid-like and lecithodendriid-like cercariae, which all have small V- or U-shaped excretory bladders. The excretory formula in cercariae of Microphallidae is secondarily simplified, with only four primary flame-cell groups being present, while the other larvae (Lecithodendriidae, Pleurogenidae, etc.) have six primary flame-cell groups (Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). Pleurogenid-like cercariae also have V-shaped excretory bladders and six primary flame-cell groups. Prosthogonimid-like cercariae have Y-shaped excretory bladders, which also occurs in the families Plagiorchiidae and Telorchiidae (Grabda-Kazubska, Reference Grabda-Kazubska1971). The morphological type of C. nigrospora has a very unusual I-shaped excretory bladder (figs 5 and 6). A similar bladder has been described in Cercaria rhionica II Olenev & Dobrovolskij, 1987 incertae sedis (Manafov, Reference Manafov2010). This feature sharply distinguishes C. nigrospora and C. rhionica II from other morphological types. However, the entire diversity of cercariae is unknown. An assessment of the taxonomic identity of the larvae based solely on the excretory bladder shape, without molecular phylogenetic analysis, can be lead to incorrect results and should be used with caution.
Oligomerization (reduction of the number) and differentiation (the presence of various types of secretory material) of penetration glands often characterize transition from evolutionarily primitive forms to advanced ones (Ginetsinskaya, Reference Ginetsinskaya1968; Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). For instance, these processes are observed during the transition from the relatively primitive Prosthogonimidae and Pleurogenidae clades (figs 3, 4 and 6) to C. nigrospora and Lecithodendriidae (figs 2, 5 and 6). However, microphallids have four or two pairs of penetration glands, while cercariae from the groups basal to Microphallidae have only three pairs (fig. 6). This feature probably reflects a high specialization of the Microphallidae.
There are both simple non-lobed and complex multi-lobed virgulae in pleurogenid-like and lecithodendriid-like cercariae (table 2, figs 2, 3 and 6). None of the prosthogonimid and microphallid cercariae have any special mucoid accumulation structures such as caudal pockets and virgulae.
Different types of the virgula are unevenly distributed among Microphalloidea. For example, some of their cercariae possess special structures for the accumulation of mucoid secretions (C. vorskla IV, present study). These features are useful in taxonomic identification. Thus, cercaria with four pairs of penetration glands and a simple non-lobed virgula or a bilobed virgula should be classified as pleurogenid-like (table 2, figs 3 and 6). Cercariae with two or three pairs of penetration glands and a simple or a large lobed virgula should be classified as lecithodendriid-like (table 2, figs 2 and 6). Microphallid-like cercariae are easily distinguishable from prosthogonimid-like ones by the lesser number of primary flame-cell groups (table 2, fig. 6).
The presence of the virgula organ is the synapomorphy of the Lecithodendriidae and the Pleurogenidae. Based on this, morphologically similar cercariae have been previously combined into the ‘lecithodendriid-like’ group of digeneans (Kudlai et al., Reference Kudlai, Cutmore and Cribb2015a, Reference Kudlai, Stunzenas and Tkachb). However, a detailed description of the virgula alongside with the morphology of the penetration glands and the excretory system makes it possible to assign cercariae to one of the five morphological types described in the present paper (fig. 6). Since there are many species of Microphalloidea with undescribed cercariae, it is quite possible that new morphological types would be discovered among them.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X19000853.
Acknowledgements
All the data were obtained using the equipment of the Research Park of St. Petersburg State University (Centre for Molecular and Cell Technologies), research project number 109-9140. We thank Sergey G. Sokolov, Konstantin V. Khalturin and Ilya I. Gordeev for their help in the preparation of this publication.
Financial support
The research was financially supported by the Russian Foundation for Basic Research (project number 18-34-00632).
Conflicts of interest
None.










