Gestational diabetes mellitus (GDM) is a condition in which glucose intolerance occurs in pregnant women without previously diagnosed diabetes mellitus. GDM is usually recognised in the second half of pregnancy(Reference Huhn, Rossi and Hoesli1). Based on the previous data, an increased risk of adverse perinatal consequences and a wide range of unfavourable long-term outcomes for mother and child are attributed to this condition(Reference Plows, Stanley and Baker2). Strong evidence suggests a link between peripheral insulin resistance, changes of inflammatory cytokines and biomarkers of oxidative stress in the pathogenesis of GDM(Reference Moyce and Dolinsky3). Previous studies also suggested that both pre-existing maternal obesity and gestational diabetes are associated with decreased expression of transcription factors involved in carbohydrate and lipid metabolism such as PPAR, sterol regulatory element-binding protein 1c and increased gene expression levels of adipokines, TNF-α, IL-1β and leptin in adipose tissue(Reference Lappas4). It has been shown that dietary interventions in GDM women led to the reduced need for medication treatment, improved glycaemia and related parameters(Reference Yamamoto, Kellett and Balsells5).
Based on the available evidence, changes in long-chain PUFA, mainly n-6 and n-3 fatty acids, are associated with GDM and pregnancies complicated by pre-eclampsia and intra-uterine growth restriction(Reference Wadhwani, Patil and Joshi6). A meta-analysis by Saccone et al.(Reference Saccone, Saccone and Berghella7) indicated that n-3 fatty acid supplementation in pregnant women decreased C-reactive protein (CRP) levels and improved some pregnancy outcomes such as newborn’s hyperbilirubinemia and hospitalisation rate. Zhong & Wang(Reference Zhong and Wang8) have recently demonstrated that in a meta-analysis, n-3 fatty acid supplementation in women with GDM significantly reduced fasting plasma glucose (FPG), homoeostasis model of assessment-insulin resistance and high-sensitive CRP (hs-CRP) levels but did not affect gestational age, the rate of preterm delivery and macrosomia, newborn’s weight and 5-min Apgar score. The results of a meta-analysis suggested that n-3 fatty acid supplementation in patients with type 2 diabetes mellitus led to a significant improvement in HbA1c levels, serum lipids and inflammatory markers(Reference O’Mahoney, Matu and Price9). On the other hand, Jovanovski et al.(Reference Jovanovski, Li and Thanh Ho10) indicated that supplementation with α-linolenic acid (ALA) which is a precursor of PUFA present in plant oil had a neutral effect on parameters of glucose homoeostasis. Our previous study indicated that a 6-week supplementation with 1000 mg fish oil enhanced gene expression of the PPAR-γ, LDL receptor (LDLR), TNF-α and IL-6(Reference Jamilian, Samimi and Mirhosseini11). Other studies investigated the effects of n-3 fatty acid supplementation on gene expression levels in different metabolic conditions. For example, ALA treatment of human renal cell carcinoma led to the up-regulation of PPAR-γ and reduced gene expression of cyclo-oxygenase-2(Reference Yang, Yuan and Liu12). Moreover, both flaxseed oil and fish oil have been reported to improve plasma TAG and PPAR-α gene expression and down-regulate the mRNA transcription of sterol regulatory element-binding protein-1 and inflammatory genes, including TNF-α and IL-6 in streptozotocin-induced diabetic rats(Reference Devarshi, Jangale and Ghule13). However, flaxseed oil administration in sheep infected with Fasciola hepatica did not influence neither production nor gene expression of inflammatory cytokines(Reference Martinez-Perez, Robles-Perez and Benavides14).
Flaxseed oil is rich in ALA which is the precursor of long-chain n-3 fatty acids – PUFA: EPA and DHA(Reference Brenna, Salem and Sinclair15). Evidence suggests that the beneficial effects of flaxseed oil supplementation on metabolic profiles are achieved by the modulation of increased β-oxidation of fatty acids, reduced lipogenesis, enhanced immune function and antioxidant activity(Reference Backes, Anzalone and Hilleman16). So far, the effect of flaxseed supplementation on metabolic and genetic profiles in GDM patients remains unknown. Therefore, the aim of the present study is to evaluate the effect of a 6-week supplementation with 2 g/d flaxseed oil on glycaemic control, lipid profile, parameters of inflammation and oxidative stress and gene expression related to metabolic profiles.
Methods
Trial design and participants
This research, registered in the Iranian website for clinical trials (http://www.irct.ir; IRCT20170513033941N42), was a randomised, double-blind, placebo-controlled clinical trial. Eligible subjects were aged 18–40 years (24–28 weeks of gestation) who were diagnosed with GDM through a ‘one-step’ diagnosis (FPG ≥ 5·1 mmol/l, 1-h oral glucose test tolerance ≥ 10 mmol/l and 2-h OGTT ≥ 8·5 mmol/l) based on the American Diabetes Association guidelines(17). The exclusion criteria were women with eclampsia, pre-eclampsia, smokers, patients with thyroid disorders, kidney or liver diseases at enrolment and those starting with insulin therapy during intervention. The present study was performed in fifty-one women with GDM at 24–28 weeks’ gestation referred to the Kosar Clinic in Arak, Iran, between July 2018 and February 2019. The number of patients needed for the study was calculated based upon previously performed power analysis. The study was performed in accordance with the Declaration of Helsinki, and informed consent was obtained from all participants. The study got the approval of the ethics committee of Arak University of Medical Sciences.
Study design
Randomisation was performed to assign participants to two groups matched for BMI and age. Each woman was randomly assigned to one of the two groups to intake either 2 × 1000 mg/d n-3 fatty acids from flaxseed oil containing 400 mg ALA in each capsule (n 30) or placebo (n 30) for 6 weeks. We used the dose of 2000 mg n-3 fatty acids based on a previous study in women with polycystic ovary syndrome(Reference Oner and Muderris18). n-3 Fatty acid supplements and placebo (sunflower oil) were produced by Barij Essence Company. Patients were asked not to change their routine physical activity or usual dietary pattern throughout the study and not to take any anti-inflammatory and antioxidant medications or supplements that might affect their nutritional status during the 6-week intervention. Consumption of n-3 fatty acid supplements and placebo throughout the study was checked by asking subjects to return the medication containers. Furthermore, a short message was sent to the cell phones of all patients every day to remind participants to use the supplements. A 3-d food record and physical activity records were completed by all participants. The individual’s dietary intake was then calculated and calculated as average at weeks 0, 3 and 6 using Nutritionist IV software (First Databank) modified for Iranian foods. Physical activity was described as metabolic equivalents in h/d. To determine the metabolic equivalents for each participant, we multiplied the times (in h/d) reported for each physical activity by its related metabolic equivalents coefficient using standard tables(Reference Ainsworth, Haskell and Whitt19).
Assessment of anthropometric measures
A trained staff at the clinic took anthropometric parameters at the beginning of the study and 6 weeks after the intervention. Body weight was measured after an overnight fast using the same digital scale (Seca).
Assessment of outcomes
We considered gene expression of PPAR-γ as the primary outcome and other metabolic and genetic profiles as secondary outcomes. Fasting blood samples (20 ml) were collected at baseline and 6 weeks after the intervention at Arak reference laboratory. Then, the samples were stored at −80°C before analysis. Serum insulin levels were assessed by the use of a chimerical ELISA kit (DiaMetra) with inter- and intra-assay CV below 5 %. Homoeostasis model of assessment-insulin resistance and the quantitative insulin sensitivity check index were determined using the standard formula(Reference Pisprasert, Ingram and Lopez-Davila20). Enzymatic kits of Pars Azmun were used to evaluate FPG and serum lipids with inter- and intra-assay CV below 5 %. Serum levels of hs-CRP were determined by a commercial ELISA kit (LDN) with inter- and intra-assay CV below 7 %. Plasma total nitrite was determined using the Griess method(Reference Tatsch, Bochi and Pereira Rda21), total antioxidant capacity using the method of ferric-reducing antioxidant power developed by Benzie & Strain(Reference Benzie and Strain22), GSH using the method of Beutler & Gelbart(Reference Beutler and Gelbart23) and malondialdehyde (MDA) concentrations were determined by the thiobarbituric acid reactive substances spectrophotometric test(Reference Janero24) with inter- and intra-assay CV below 5 %.
Isolation of lymphocyte, RNA extraction and complementary DNA synthesis
Lymphocytes were isolated using 50 % Percoll solution (Sigma-Aldrich) gradient by centrifugation for 20 min and 3000 rpm at 4°C.(Reference Gmelig-Meyling and Waldmann25) Total RNA was extracted based on acid guanidinium–phenol–chloroform procedure using an RNX™-plus reagent (Cinnacolon) according to the manufacturer’s instructions. RNA was treated with DNAase I (Fermentas) for elimination of any genomic DNA contamination. A total of 3 mg of RNA was used for complementary DNA synthesis with random hexamer and oligo (dT) 18 primers through RevertAid™ Reverse Transcriptase (Fermantase) in 20 μl reaction mixture(Reference Gmelig-Meyling and Waldmann25).
Real-time PCR analysis
Appropriate primers for PPAR-γ, LDLR, IL-1, IL-8, TNF-α, transforming growth factor β, vascular endothelial growth factor (VEGF) and glyceraldehyde 3-phosphate dehydrogenase were designed (Table 1). Quantitative real-time PCR was performed by the LightCycler® 96 sequence detection systems (Roche Diagnostics) using 4 μl of 5 × EVA GREEN I master mix (Salise Biodyne), 10 ng complementary DNA, 200 nm of each forward and reverse primers in a volume of 20 μl.
Table 1. Specific primers used for real-time quantitative PCR

GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F, forward; R, reverse; LDLR, LDL receptor; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
Sample size
In the present study, we used a randomised clinical trial sample size calculation formula where type one (α) and type two errors (β) were 0·05 and 0·20 (power = 80 %), respectively. Based upon our previous trial(Reference Nasri, Hantoushzadeh and Aghadavod26), we used 0·25 as the sd and 0·20 as the change in mean (d) of PPAR-γ as a primary outcome. Based on the power analysis, we needed twenty-five subjects in each group. After allowing for five dropouts in each group, the final sample size was thirty subjects in each group.
Randomisation
Randomisation was performed using computer-generated random numbers. Randomisation and allocation were hidden from the researchers and pregnant women until the final analyses were completed. The randomised allocation sequence, enrolling participants and allocating them to intervention were carried out by a trained midwife at the clinic.
Statistical methods
The Kolmogorov–Smirnov test was done to determine the normality of data. To detect the differences in anthropometric parameters, dietary intakes and gene expression related to insulin, lipids and inflammation markers between groups, we used the independent-samples t test. Multiple linear regression models were used to evaluate treatment effects on variables after adjusting for confounding variables, including baseline values of biochemical variables. We have used linear regression models with follow-up values of outcomes as the response (dependent) variable and treatment group, baseline values of the outcomes, and other potential confounders including age and BMI at baseline as explanatory (independent) variables. The effect sizes were presented as the mean differences with 95 % CI. P-values <0·05 were considered statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 18 (SPSS Inc.).
Results
Among individuals in the n-3 fatty acids group, four persons due to personal reasons were excluded (Fig. 1). In the placebo group, five participants were also excluded due to personal reasons. Fifty-one participants completed the trial. The compliance rate in our study was high; participants reported that more than 90 % of both n-3 fatty acids and placebo capsules were taken during the trial. No side effects were reported following the intake of n-3 fatty acids in patients with GDM throughout the study.
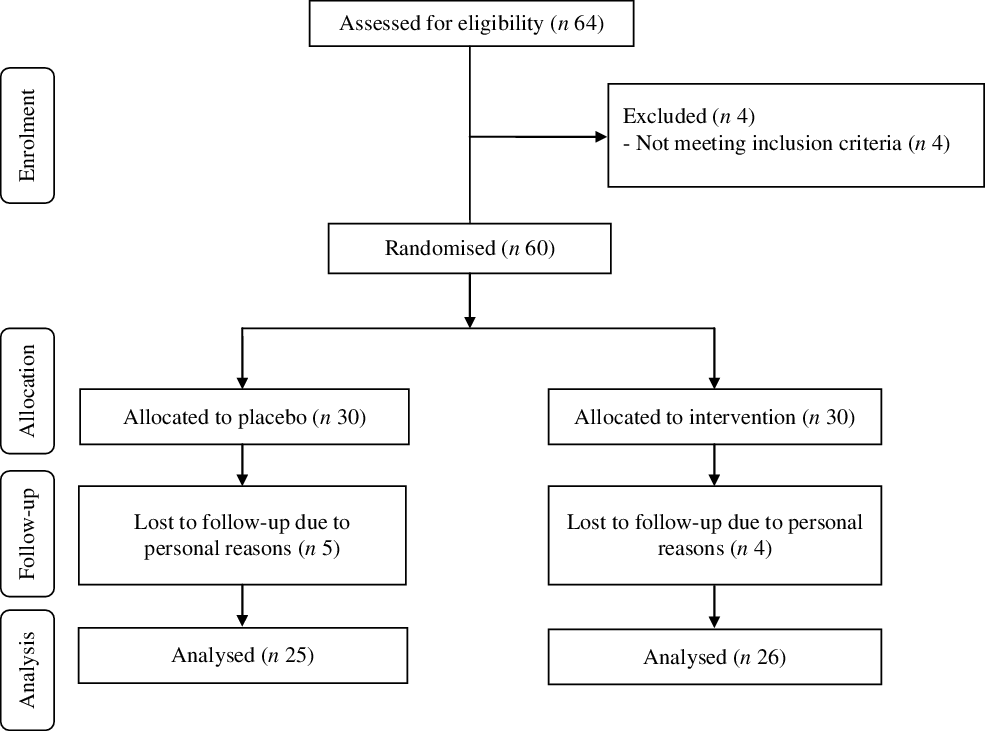
Fig. 1. Summary of patient flow diagram.
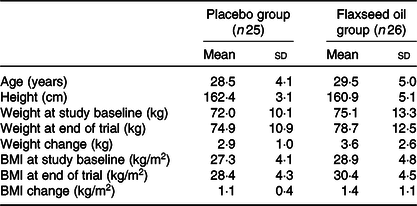
Mean age, height, weight and BMI at the beginning of the study and mean weight and BMI after intervention were not statistically different between the two groups (Table 2).
Table 2. General characteristics of study participants
(Mean values and standard deviations)
Based on the 3-d dietary records obtained during the trial, there were no significant changes in dietary macro- and micronutrient intakes (data not shown). Moreover, there was no significant change in the mean n-3 dietary intake at baseline (1·11 (sd 0·20) for the n-3 group v. 1·04 (sd 0·17) g/d for the placebo group, P = 0·18).
n-3 Fatty acid intake up-regulated PPAR-γ (P < 0·001) and LDLR (P = 0·004) and down-regulated gene expression of IL-1 (P = 0·002) and TNF-α (P = 0·001) in peripheral blood mononuclear cells of subjects with GDM (Figs. 2 and 3). n-3 Fatty acid supplementation did not affect transforming growth factor β and VEGF expression.

Fig. 2. Fold change in gene expression levels of PPAR-γ and LDL receptor (LDLR) in women with gestational diabetes mellitus who received probiotic supplements and placebo. Values are means, with standard deviations represented by vertical bars. P values were obtained from independent t tests. ![]() , Placebo;
, Placebo; ![]() , n-3.
, n-3.
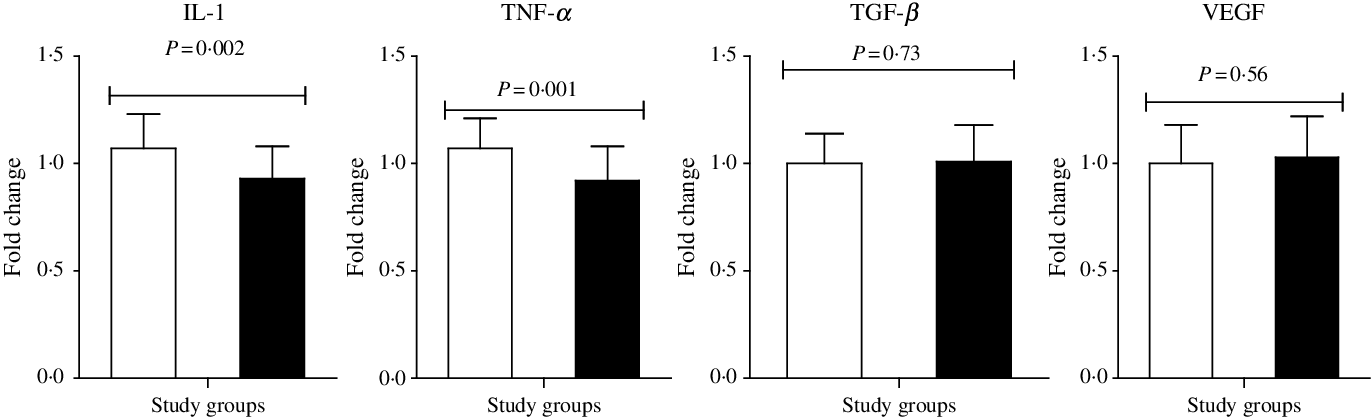
Fig. 3. Change in gene expression levels of IL-1, TNF-α, transforming growth factor β (TGF-β) and vascular endothelial growth factor (VEGF) in women with gestational diabetes mellitus who received probiotic supplements and placebo. Values are means, with standard deviations represented by vertical bars. P values were obtained from independent t tests. ![]() , Placebo;
, Placebo; ![]() , n-3.
, n-3.
After the 6-week intervention, n-3 fatty acid supplementation reduced FPG (β −0·26 mmol/l; 95 % CI −0·42, −0·11; P = 0·001), insulin levels (β −15·56 pmol/l; 95 % CI −24·4, −6·75; P = 0·001) and homoeostasis model of assessment-insulin resistance (β −0·63; 95 % CI −0·92, −0·33; P < 0·001) and increased quantitative insulin sensitivity check index (β 0·01; 95 % CI 0·003, 0·01; P = 0·005) when compared with placebo (Table 3). n-3 Fatty acid supplementation was associated with a decrease in TAG (β −0·46 mmol/l; 95 % CI −0·69, −0·22; P < 0·001), VLDL-cholesterol (β −0·21 mmol/l; 95 % CI −0·32, −0·10; P < 0·001), total cholesterol (β −0·58 mmol/l; 95 % CI −1·04, −0·12; P = 0·01) and total cholesterol:HDL-cholesterol ratio (β −0·58; 95 % CI −1·06, −0·11; P = 0·01) when compared with placebo. Finally, n-3 fatty acid administration caused a significant reduction in hs-CRP (β −1·27 mg/l; 95 % CI −2·17, −0·38; P = 0·006) and MDA (β −0·47 µmol/l; 95 % CI −0·69, −0·25; P < 0·001) and a significant elevation in total nitrite (β 5·42 µmol/l; 95 % CI 3·84, 7·00; P < 0·001) and GSH levels (β 116·55 µmol/l; 95 % CI 34·36, 198·74; P = 0·006) when compared with placebo. In addition, when we adjusted the analysis for baseline variables, age, baseline BMI and mean n-3 intake at baseline study and findings did not alter (data not shown).
Table 3. Metabolic profiles, biomarkers of inflammation and oxidative stress at baseline and after the 6-week intervention in patients with gestational diabetes mellitus who received either flaxseed oil supplements or placebo
(Mean values and standard deviations; β-coefficients and 95 % confidence intervals)

FPG, fasting plasma glucose; HOMA-IR, homoeostasis model of assessment-insulin resistance; QUICKI, quantitative insulin sensitivity check index; hs-CRP, high-sensitivity C-reactive protein; TAC, total antioxidant capacity; MDA, malondialdehyde.
* ‘Outcome measures’ refer to the change in values of measures of interest between baseline and week 6. β (difference in the mean outcome’s measures between treatment groups (flaxseed oil group = 1 and placebo group = 0)).
† Obtained from the multiple regression model (adjusted for baseline values of each biochemical variables).
Discussion
In the present study, we investigated the effects of n-3 fatty acids from flaxseed oil on genetic and metabolic profiles in subjects with GDM. We found that n-3 fatty acid supplementation during 6 weeks to women with GDM had beneficial effects on gene expression related to insulin, lipids, glycaemic control, inflammatory markers and oxidative stress.
Effects on glycaemic control and lipids
GDM is associated with changes, including increased insulin resistance, dyslipidaemia, oxidative stress and inflammatory state(Reference Moyce and Dolinsky3). We observed a significant improvement in PPAR-γ and LDLR mRNA expression as well as parameters of glycaemic control, TAG, VLDL-cholesterol, total cholesterol and total cholesterol:HDL-cholesterol ratio. However, we were unable to find any significant effects on other serum lipids following flaxseed supplementation in women with GDM. There are several studies investigating the effects of n-3 fatty acids on gene expression of proteins involved in carbohydrates and lipid metabolism. Our previous study indicated that 6-week supplementation with 1000 mg/d fish oil enhanced gene expression of the PPAR-γ and LDLR in GDM women(Reference Jamilian, Samimi and Mirhosseini11). The treatment of human renal cell carcinoma with ALA up-regulated the PPAR-γ gene expression(Reference Yang, Yuan and Liu12). Ebrahimi et al.(Reference Ebrahimi, Rajion and Goh27) reported that linseed oil increased gene expression of the PPAR-γ in goats. A meta-analysis by Zhong & Wang(Reference Zhong and Wang8) showed that n-3 fatty acid supplementation in GDM patients significantly reduced FPG and homoeostasis model of assessment-insulin resistance score which is in accordance with our results. n-3 Fatty acid supplementation in type 2 diabetes mellitus patients caused a significant improvement in TAG, VLDL-cholesterol and LDL-cholesterol levels(Reference O’Mahoney, Matu and Price9). In some studies, n-3 fatty acid supplementation in patients with non-alcoholic fatty liver disease(Reference Yan, Guan and Gao28) and polycystic ovary syndrome(Reference Yang, Zeng and Bao29) decreased plasma TAG levels. However, in contrast to our findings, Jovanovski et al.(Reference Jovanovski, Li and Thanh Ho10) indicated that ALA did not affect glycaemic control. In pregnant women, hyperglycaemia and dyslipidaemia are associated with adverse clinical consequences for mother and neonates(Reference Farrar, Simmonds and Bryant30). Correction of glycaemic control and serum lipids in women with GDM is associated with reduced risk for many important adverse pregnancy outcomes such as gestational hypertension, macrosomia, large for gestational age births and shoulder dystocia and may provide long-lasting health benefits(Reference Poolsup, Suksomboon and Amin31). In this context, the activity of PPAR-γ is important because of its regulatory effects on the gene expression of carboxykinase, glucose-6-phosphatase and fatty acid transporter-1, which cause decreased production of NEFA and improved insulin sensitivity(Reference Li, Zhou and Deng32). n-3 Fatty acid supplementation increases β-oxidation of fatty acids, reduces lipogenesis, improves antioxidant functions and facilitates insulin action(Reference Backes, Anzalone and Hilleman16).
Effects on biomarkers of inflammation and oxidative stress
The present study showed that the ingestion of flaxseed oil by women with GDM caused a significant decrease in TNF-α and IL-1 expression. It was also effective in improving hs-CRP, NO, GSH and MDA but did not affect total antioxidant capacity values and gene expression of transforming growth factor β and VEGF. Previously, we have observed that 1000 mg/d fish oil supplementation for 6 weeks decreased gene expression of TNF-α and IL-6 in patients with GDM(Reference Jamilian, Samimi and Mirhosseini11). It has been reported that both flaxseed oil and fish oil supplementation in streptozotocin-induced diabetic rats improved down-regulated mRNA expression of genes for TNF-α and IL-6(Reference Devarshi, Jangale and Ghule13). A 12-week flaxseed oil supplementation at a dosage of 2 g/d in type 2 diabetes mellitus patients with CHD decreased TNF-α and IL-1 expression but did not change transforming growth factor β and VEGF expression(Reference Hashemzadeh, Nasoohi and Raygan33). However, flaxseed oil administration in sheep infected with F. hepatica did not affect production or gene expression of IL-4 and interferon-γ (Reference Martinez-Perez, Robles-Perez and Benavides14). Similar to our findings, Zhao & Wang(Reference Zhao and Wang34) found that n-3 fatty acids applied as a part of parenteral nutrition in postoperative patients with gastrointestinal malignancy decreased CRP and other inflammatory markers. An in vitro study demonstrated that peripheral blood lymphocytes of women with GDM when exposed to n-3 fatty acids caused elevated GSH and decreased MDA cell levels(Reference Djelti, Merzouk and Merzouk35). A 12-week n-3 fatty acid supplementation with 2 g/d flaxseed oil improved hs-CRP and GSH levels in patients with grade 3 diabetic foot ulcer(Reference Soleimani, Hashemdokht and Bahmani36). Our previous study indicated that 1000 mg n-3 fatty acids from flaxseed oil plus 360 mg vitamin E supplementation to women with GDM increased NO levels and decreased MDA concentrations but did not affect hs-CRP and GSH levels(Reference Jamilian, Hashemi Dizaji and Bahmani37). Our findings differ from the results of previous meta-analyses which suggested that supplementation with flaxseed and its derivatives(Reference Ren, Chen and Chen38) and ALA(Reference Su, Liu and Chang39) did not reduce CRP circulating levels. In addition, in contrast to our findings, a 6-week flaxseed oil supplementation at a dosage of 6 g/d did not improve oxidative stress markers in haemodialysis patients(Reference Mirfatahi, Tabibi and Nasrollahi40). The evidence proposed that flaxseed oil supplementation may improve inflammation and oxidative stress via reduction of NF-κB-induced gene expression(Reference Barcelo-Coblijn and Murphy41), modification of mitogen-activated protein kinase and protein kinase B signalling pathways(Reference Chikara, Mamidi and Sreedasyam42) and enhancement of NADPH oxidase activity(Reference Han, Yan and Chen43). In our study, beneficial effects on cardio-metabolic markers may be due to the type of n-3 fatty acids used and baseline levels of biochemical variables. However, data on the effects of n-3 fatty acids from flaxseed oil are limited. As there are multiple metabolic disorders such as insulin resistance, dyslipidaemia, increased inflammatory markers and oxidative stress which occur during pregnancy, especially in women with GDM, n-3 fatty acids may have better effects than in other metabolic diseases. Also, several studies used higher doses than 2 g/d – up to 4 g/d of n-3 fatty acids from fish oil in patients with metabolic disorders(Reference Karakas, Perroud and Kind44–Reference Garcia-Lopez, Villanueva Arriaga and Najera Medina46). Studies with longer duration of the intervention are needed to confirm our findings.
The present study has some limitations. We could not measure fatty acid profiles at baseline and at the end of the trial. However, the total amount of dietary n-3 fatty acid intake was not different between the placebo group and the group taking supplements so that the observed effect was clearly due to the supplement intake and not due to the changes in the diet. Because of funding limitations, we could not assess gene expression related to oxidative stress.
Conclusions
n-3 Fatty acid supplementation during 6 weeks to women with GDM had beneficial effects on gene expression related to insulin, lipids, glycaemic control, inflammatory markers and oxidative stress.
Acknowledgements
The authors would like to thank the staff of Kosar Clinic (Arak, Iran) for their assistance in this project.
The research grant was provided by Research Deputy of Arak University of Medical Sciences.
Z. A.: conception, design, and statistical analysis, drafting of the manuscript and supervising the study. M. J., Z. T., Z. R., I. P., F. N., E. A., E. A., M. T., R. S., M. S. and M. R. M.: data collection and manuscript drafting.
The authors declare no conflicts of interest.









