Monochorionic (MC) twins are recognized to be at increased risk for ‘primary’ structural heart disease even in the absence of TTTS; figures quoted are for between 4–11% for at least one of a twin pair, even in the absence of TTTS; this increased risk is for all cardiac lesions, including for laterality defects that are otherwise considered to be rare. Structural cardiac lesions identified in these monozygotic twins are usually discordant even though the twins are considered to be genetically identical (AlRais et al., Reference AlRais, Feldstein, Srivastava, Gosnell and Moon-Grady2011; Weber & Sebire, Reference Weber and Sebire2010); mechanisms for discordancy are explained in terms of post-zygotic events (Edlow et al., Reference Edlow, Reiss, Benson, Gerrol and Wilkins-Haug2011; Silva et al., Reference Silva, Martins, Matias and Blickstein2011).
TTTS occurs in 10–15% of MC/DA twins and, in its more advanced form and without treatment, is associated with a very high morbidity and mortality for one or both twins. Altered hemodynamics associated with TTTS are manifested in the heart; functional cardiovascular changes, although subtle, are present from very early in the disease process (Baschat et al., Reference Baschat, Chmait, Deprest, Gratacos, Hecher, Kontopoulos and Ville2011) and may be demonstrated in the earliest stages of TTTS (Michelfelder et al., Reference Michelfelder, Gottliebson, Border, Kinsel, Polzin, Livingston and Crombleholme2007), even before the diagnosis of TTTS is made (Zanardini et al., Reference Zanardini, Prefumo, Fichera, Botteri and Frusca2014). It is suggested that increased nuchal translucency, with or without abnormal ductus venosus waveforms in early gestation, is a reflection of early hemodynamic changes (Matias et al., Reference Matias, Montenegro, Loureiro, Cunha, Duarte, Freitas and Severo2010). Prompt diagnosis of chorionicity is essential in order to ensure appropriate monitoring during pregnancy; interestingly, failure to identify monochorionicity may still occur in up to 33% of twin pregnancies and, although accuracy is increasing, there is still room for improvement (Baud et al., Reference Baud, Windrim, Van Mieghem, Keunen, Seaward and Ryan2014).
Evolution of functional heart disease is partly explained as a consequence of unequal circulating volumes, causing both systolic and diastolic dysfunction, particularly in the recipient twin. These functional changes may further lead to the development of ‘acquired’ structural heart disease, again particularly, but maybe not exclusively, in the recipient twin. The cardiovascular complications of TTTS are an important factor contributing to the morbidity and mortality in MC twins.
The physiology of MC/DA twins is effectively that of a single placenta in two separate sacs; within the placenta there are anastomoses, arteriovenous (AV), arterioarterial (AA), and venovenous (VV); as a result of these anastomoses, inter-twin transfusion virtually always occurs. AV vascular anastomoses along the chorionic plate allow unidirectional flow from one twin to the other, with the potential to create an imbalance of flow through this high resistance capillary bed. In 85–90% of MC twins this is a balanced phenomenon due to the presence of protective superficial bidirectional anastomoses in the form of low resistance AAs that can compensate for any imbalance resulting from the unidirectional flow in the AV anastomoses (Lewi et al., Reference Lewi, Deprest and Hecher2013). The significance of the VV anastomoses is less well understood; they are relatively uncommon, present only in 20% of MC placentas, also with bidirectional flow; they are found more frequently in TTTS placentas and are therefore considered to play a role in the development of this process (Zhao et al., Reference Zhao, Cohen, Middeldorp, Klumper, Haak, Oepkes and Lopriore2014), especially in the absence of AA anastomoses; they are not detectable during antenatal ultrasound examination and so their clinical impact is hard to monitor (Zhao et al., Reference Zhao, Cambiaso, Otano, Lewi, Deprest, Sun and Lopriore2015); in effect, the MC placenta consists of three parts, two of which belong to each twin individually and a third part which is shared and supplied by the AV anastomoses (Lewi et al., Reference Lewi, Deprest and Hecher2013).
In 10–15% of MC twins there is unequal volume distribution, maybe due to fewer protective AA anastomoses (de Villiers et al., Reference De Villiers, Slaghekke, Middeldorp, Walther, Oepkes and Lopriore2012). This imbalance is the basis for TTTS, perhaps more accurately described as oligopolyhydramnios sequence (TOPS), as usually there is no significant difference in hemoglobin concentration (Saunders et al., Reference Saunders, Snijders and Nicolaides1991). This unequal volume distribution not only leads to altered hemodynamics for both twins but also, as a consequence of the shared and mixed circulation, the wellbeing of one twin critically depends on that of the other (Lewi et al., Reference Lewi, Deprest and Hecher2013), raising specific issues in pregnancy management.
The etiology of TTTS is unknown, although it only affects MC twins; it usually presents between 15 and 25 weeks, although it can be seen earlier or later, sometimes with an acute onset. It is essentially hemodynamic, affecting both twins, with activation of additional hormonal factors; it is described both as a progressive disease and also as potentially devastating but with remarkable powers for recovery following treatment (Moon-Grady, Reference Moon-Grady2014). Progression is unpredictable, thus pregnancies likely to need treatment may be hard to identify. It is acknowledged that clear guidelines for treatment are essential, especially as treatment, although improving, is still considered to be relatively high risk (Diehl et al., Reference Diehl, Diemert and Hecher2014).
The hemodynamics in TTTS are those of chronic imbalance of circulating volumes with hypervolemia in the recipient and hypovolemia in the donor. The consequent pathophysiology of TTTS is determined by the physiological responses to chronic volume inequality and it is these that can cause both functional and acquired structural cardiac anomalies, particularly in the recipient twin; meanwhile donor twins are rarely affected by significant functional anomalies (Michelfelder et al., Reference Michelfelder, Gottliebson, Border, Kinsel, Polzin, Livingston and Crombleholme2007; Moon-Grady, Reference Moon-Grady2014). Most of the functional changes are considered to be reversible with successful in utero treatment (Moon-Grady, Reference Moon-Grady2014), but there is still much to be understood on this subject.
For the recipient, there is increased preload (volume) with an absolute increase in circulating volume compared to that of the donor, with significantly higher cardiac output as measured by umbilical venous volumetric flow (Yamamoto et al., Reference Yamamoto, Nasr, Ortqvist, Bernard, Takahashi and Ville2007); hypervolaemia leads to the release of natriuretic peptides, atrial natriuretic protein (ANP) and brain natriuretic protein (BNP), presumed to be stimulated by myocardial stretch. Both ANP and BNP are biomarkers associated with heart failure; elevated levels are found in the amniotic fluid of the recipient, suggesting the presence of myocardial damage (Habli et al., Reference Habli, Cnota, Michelfelder, Salisbury, Schnell, Polzin and Crombleholme2010; Van Mieghem et al., Reference Van Mieghem, Done, Gucciardo, Klaritsch, Allegaert, Van Bree and Deprest2010), and the levels of these peptides appear to increase with progressive TTTS stages (Habli et al., Reference Habli, Cnota, Michelfelder, Salisbury, Schnell, Polzin and Crombleholme2010). Increased levels of cardiac troponin are also seen in the recipient, adding further support to the theory of myocardial damage in the recipient (Van Mieghem et al., Reference Van Mieghem, Done, Gucciardo, Klaritsch, Allegaert, Van Bree and Deprest2010); a consequence of this hormonal stimulation is a diuresis, producing polyuria and polyhydramnios.
The recipient is also subjected to an increased afterload in the form of increased resistance, as demonstrated by a 1.6–2.8-fold increase in the velocity of tricuspid regurgitation compared to the expected value, reflecting high right heart pressure in the presence of fetal hypertension (Mahieu-Caputo et al., Reference Mahieu-Caputo, Salomon, Le Bidois, Fermont, Brunhes, Jouvet and Dommergues2003). Recipient hypertension is attributed to the presence of various vasoactive mediators, particularly endothelin, but also the consequence of inappropriate renin–angiotensin stimulation. Endothelin is a powerful vasoconstrictor causing hypertension and is assumed to contribute to the development of biventricular hypertrophy both directly, as a result of the increased pressure, and indirectly, by stimulating cardiac myocytes involved in cardiac remodeling. The source of endothelin is probably partly from the placenta and partly from the donor via the vascular communications. The renin-angiotensin system (RAS) is ‘downregulated’ in the recipient's kidneys, but work has shown that the levels are as high as in the donor (Mahieu-Caputo et al., Reference Mahieu-Caputo, Meulemans, Martinovic, Gubler, Delezoide, Muller and Dommergues2005); it is assumed that this is mainly due to transfer from the donor, although there is evidence of increased renin production in the recipient's placental territory (Galea et al., Reference Galea, Barigye, Wee, Jain, Sullivan and Fisk2008). It is relevant that, as a consequence of the placental anastomoses, one twin is exposed to the endocrine environment of the other. All these factors explain the development of significant myocardial ventricular hypertrophy, out of proportion to simple volume overload (Mercanti et al., Reference Mercanti, Boivin, Wo, Vlieghe, Le Ray, Audibert and Nuyt2011). A consequence of this progressive cardiomyopathy is an increase in overall heart size, a reduction in myocardial compliance, atrioventricular valvar regurgitation, and abnormal venous Dopplers (Stirnemann et al., Reference Stirnemann, Mougeot, Proulx, Nasr, Essaoui, Fouron and Ville2010).
For the donor there is an absolute reduction in the circulating volume leading to chronic under perfusion, resulting in oliguria and oligohydramnios; hypoperfusion stimulates upregulation of the RAS to try to maintain perfusion, resulting in increased vascular resistance with smooth muscle hypertrophy. The donor's circulation is underfilled but hyperdynamic; increased vascular resistance, including in the placenta, may lead to intrauterine growth restriction (IUGR), cerebral redistribution and abnormal arterial Doppler assessment. Studies suggest that usually the heart of the donor is functionally normal and that any diastolic dysfunction is assumed to be due to the effects of severe IUGR (Van Mieghem et al., Reference Van Mieghem, Done, Gucciardo, Klaritsch, Allegaert, Van Bree and Deprest2010).
Historically, and currently, TTTS has been classified according to the Quintero as summarized in Table 1.
TABLE 1 Quintero Staging of TTTS is the Established Framework for Staging TTTS

AEDF; absent end diastolic flow; REDF: reversed end diastolic flow; DV: ductus venosus.
The presentation of TTTS can be mixed and thus not fit clearly into this staging system; there may be abnormal Doppler assessments while the donor still has a demonstrable bladder, and it is widely acknowledged that progression is notoriously difficult to predict (Stirnemann et al., Reference Stirnemann, Mougeot, Proulx, Nasr, Essaoui, Fouron and Ville2010; Ville, Reference Ville2007). A limitation of the Quintero staging is that it does not reflect the whole situation; in particular, it only partly quantifies cardiac involvement. Stages l and ll are a measure of renal perfusion, and stages lll and lV are a consequence of cardiovascular compromise. It is argued that since TTTS is essentially a cardiovascular disorder, there is a role for defining cardiac dysfunction, thus quantifying cardiac involvement and severity of disease; in doing so, the aim is to improve prediction of progression and thus identification of pregnancies that might benefit from treatment. If used in conjunction with Quintero staging, this is a tool to refine the staging process, thus contributing to pregnancy management (Habli et al., Reference Habli, Michelfelder, Livingston, Harmon, Lim, Polzin and Crombleholme2008; Michelfelder et al., Reference Michelfelder, Gottliebson, Border, Kinsel, Polzin, Livingston and Crombleholme2007). However, this is a subject for debate, particularly between fetomaternal specialists and cardiologists (Quintero, Reference Quintero2010; Rychic et al., Reference Rychic, Tian, Bebbington, Moldenhauer, Khalek and Johnson2010; Stirnemann et al., Reference Stirnemann, Mougeot, Proulx, Nasr, Essaoui, Fouron and Ville2010; Ville, Reference Ville2007).
Diastolic dysfunction has an important influence on the preservation of the fetal circulation; in the recipient twin, diastolic dysfunction precedes systolic dysfunction, with the right ventricle involved before the left (Rychic et al., Reference Rychik, Tian, Bebbington, Xu, McCann, Mann and Johnson2007); this process is considered by some to be specific to the hemodynamics of TTTS, thus distinguishing it from other complications of MC/DA twins, including selective growth restriction (Moon-Grady et al., Reference Moon-Grady, Rand, Lemley, Gosnell, Hornberger and Lee2011). The E/A ratio refers to the ratio of the two peaks observed with pulsed wave Doppler across the atrioventricular valves; the E wave is the early passive diastolic filling, dependent on ventricular wall relaxation, and in the normal fetal heart is smaller than the later A wave of the active phase of diastolic filling. Changes in the E/A ratio reflect ventricular diastolic dysfunction, and the thickened myocardium with reduced compliance causes monophasic filling with the loss of the normal E/A ratio. The Myocardial Performance Index (MPI, Figure 1) also known as the TEI index, is a measure of global systolic and diastolic myocardial function and increases with myocardial dysfunction; although the donor may develop right ventricular diastolic impairment as a result of increased placental resistance, this is less quantifiable in terms of scoring.
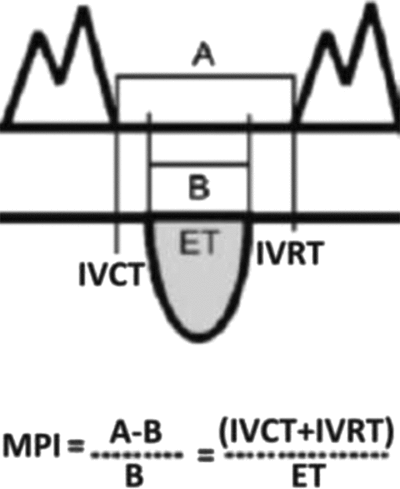
FIGURE 1 The Myocardial Performance Index (MPI) is a Doppler-derived measure of systolic and diastolic function; it reflects global myocardial performance by assessing the duration of flow through the atrioventricular and outflow tracts and can be used for serial evaluations. MPI rises as myocardial function decreases. Note: A = time between atrioventricular (AV) valve closing and AV valve opening. B = ejection time (ET). IVCT = isovolumic contraction time. IVRT = isovolumic relaxation time.
The ‘benefit’ of these changes is that they can be used to produce a cardiovascular profile score with the purpose of quantifying the degree of cardiac involvement. There are various methods in use — an example is the CHOP score (Rychik et al., Reference Rychik, Tian, Bebbington, Xu, McCann, Mann and Johnson2007), which aims systematically and objectively to calculate hemodynamic changes, particularly in the recipient twin. The higher the score, the greater the cardiac involvement (see Table 2), assumed to reflect increased severity of the TTTS process (see Table 3). It is, however, acknowledged that these assessments are difficult, time consuming and operator dependent, and reproducibility is not assured (Gapp-Born et al., Reference Gapp-Born, Sananes, Weingertner, Guerra, Kohler, Fritz and Favre2014)
TABLE 2 Quantifiable Functional Changes in the Recipient Twin
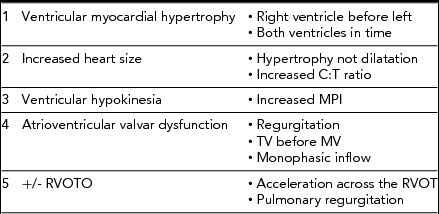
C:T ratio = cardiothoracic; TV = tricuspid valve; MV = mitral valve; RVOT = Right ventricular outflow tract.
TABLE 3 Parameters Used to Create the CHOP Cardiovascular Score for Quantifying TTTS
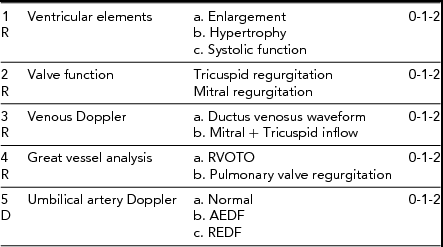
TR = tricuspid regurgitation, MR = mitral regurgitation, RVOTO = right ventricular outflow tract obstruction, AEDF = absent end diastolic flow, REDF = reversed end diastolic flow. R = recipient, D = donor. Note: Very little contribution by the donor to this score.
It is also important to acknowledge that Doppler assessments may be invalidated in the presence of structural heart disease, known to be increased in MC twins even in the absence of TTTS (AIRais et al., Reference AlRais, Feldstein, Srivastava, Gosnell and Moon-Grady2011). An abnormal ductus venosus waveform is seen with structural right heart lesions in mid gestation, whatever the cause (Berg et al., Reference Berg, Kremer, Geipel, Kohl, Germer and Gembruch2006).
Several studies have shown that changes in cardiac function can be demonstrated even before the development of TTTS, as judged by the presence of oligo/polyhydramnios. Stage l TTTS represents a heterogeneous group in whom 70–80%, will remain stable or even regress spontaneously (Diehl et al., Reference Diehl, Diemert and Hecher2014; O'Donoghue et al., Reference O'Donoghue, Cartwright, Galea and Fisk2007; Ville, Reference Ville2007); it has been observed that even early in stage l, up to 55% of fetuses have a degree of myocardial dysfunction, as demonstrated by an increased MPI (Stirneman et al., Reference Stirnemann, Mougeot, Proulx, Nasr, Essaoui, Fouron and Ville2010). Maybe serial cardiovascular assessments enable differentiation within this group to distinguish those in whom TTTS is less likely to progress, thereby avoiding unnecessary treatment with its associated risks (Ville, Reference Ville2007). By stage ll, the incidence of recipient cardiomyopathy has been found to be as high as 65% (Habli et al., Reference Habli, Michelfelder, Cnota, Wall, Polzin, Lewis and Crombleholme2012).
There is significant variation in different units around the world in terms of treatment protocols and developing international guidelines with the aim of optimizing outcome is in progress (Diehl et al., Reference Diehl, Diemert and Hecher2014). Cardiac assessment has the potential to help define specific criteria for laser therapy. However, it is acknowledged that as cardiovascular changes precede Quintero staging, there is a tendency to upgrade the severity of the disease, which may lead to earlier intervention as well as potentially producing falsely reassuring figures for success of treatment; but, as deterioration of the hemodynamics can be rapid, any help in identifying this earlier is desirable (Bebbington, Reference Bebbington2010).
In summary, quantitative description of cardiac function is a challenge (Crispi & Gratacos, Reference Crispi and Gratacos2012) and still controversial; different methods are described but all may be uncomfortable for the patient as well as for the clinician. The presence of polyhydramnios can lead to maternal discomfort, and both polyhydramnios and oligohydramnios can make accurate assessment technically difficult and reproducibility unreliable. Optimal methods for assessment remain subject to debate and further studies are in progress; a recently published paper from the Cincinnati group suggests that MPI is the method of choice (Villa et al., Reference Villa, Habli, Votava-Smith, Cnota, Lim, Divanovic and Michelfelder2014). Quantified cardiovascular changes are a useful adjunct to Quintero staging in guiding management, but in busy clinical practice, the reality may be a compromise. Measurement of cardiac size, myocardial dimensions, subjective assessment of function, quantification of AV valve regurgitation, E/A ratios and evidence of outflow tract obstruction are all achievable and reproducible and can be monitored serially to assess progression or otherwise of the TTTS process.
There is interest in the measurement of biomarkers, particularly ANP and BNP, reflecting the degree of cardiac involvement or failure, with the advantage of reducing inter-observer variability (Merz & Gembruch, Reference Merz and Gembruch2014; Miura et al., Reference Miura, Higashijima, Miura, Mishima, Yamasaki, Abe and Masuzaki2014); evidence suggests that when used in conjunction with assessment of cardiac dysfunction, quantification of amniotic fluid markers may identify fetuses particularly at risk of post-procedure demise (Van Mieghem et al., Reference Van Mieghem, Done, Gucciardo, Klaritsch, Allegaert, Van Bree and Deprest2010).
Treatment for TTTS is covered by other contributors, but historically, amnioreduction was the treatment of choice. Although this gave symptomatic relief and the potential to reopen anastomoses (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004), it often required multiple procedures and, vitally, did not treat the cardiovascular pathology, which therefore progressed (Barrea et al., Reference Barrea, Alkazaleh, Ryan, McCrindle, Roberts, Bigras and Hornberger2005). In contrast, laser treatment, involving coagulation of the placental anastomoses, addresses the pathophysiology by disconnecting the two circulations and is therefore considered by many to cure TTTS, whatever the stage. Effectively converting the MC pregnancy to a dichorionic pregnancy, it prevents further inter-twin flow, ends the volume imbalance, and removes unwelcome vasoactive mediators from the other twin.
Laser treatment has a profound effect on fetal hemodynamics; for the recipient, the cardiac status worsens during the procedure, but several studies demonstrate that there is improved function over the following few days (Papanna et al., Reference Papanna, Mann, Molina, Johnson and Moise2011). The donor experiences an acute relative volume overload manifested as an increase in cardiac size (Sueters et al., Reference Sueters, Middeldorp, Vandenbussche, Teunissen, Lopriore, Kanhai and Oepkes2008), abnormal ductus venosus flow, sometimes transient hydrops (Gratacos et al., Reference Gratacos, Van Schoubroeck, Carreras, Devlieger, Roma, Cabero and Deprest2002) and with reappearance of end diastolic flow in the umbilical artery (Van Mieghen et al., Reference Van Mieghem, Klaritsch, Done, Gucciardo, Lewi, Verhaeghe and Deprest2009). There is encouraging evidence that using selective laser techniques, occluding donor to recipient communications before those of the recipient to donor, might facilitate achieving hemodynamic equilibrium by allowing transient net transfusion to the volume depleted donor (Chmait et al., Reference Chmait, Kontopoulos and Quintero2014). The effect of treatment is regression of the cardiovascular pathology and improved myocardial performance (Habli et al., Reference Habli, Michelfelder, Livingston, Harmon, Lim, Polzin and Crombleholme2008); recovery may take longer in more severely affected pregnancies (Van Mieghem et al., Reference Van Mieghem, Martin, Weber, Barrea, Windrim, Hornberger and Ryan2013), but this is not the case in all series. It is possible that it is the duration rather than severity that is more relevant in terms of recovery (Van Mieghem et al., Reference Van Mieghem, Martin, Weber, Barrea, Windrim, Hornberger and Ryan2013). Cardiac function alone does not predict outcome (Eixarch et al., Reference Eixarch, Valsky, Deprest, Baschat, Lewi, Ortiz and Gratacos2013) and much of the evidence is still conflicting and confusing, in spite of an exponential number of publications on the subject. The expectation following treatment, even for severe TTTS, is for improvement with likely resolution in utero; thus, selective fetal reduction is rarely indicated (Van Mieghen et al., Reference Van den Boom, Battin and Hornung2010); an earlier study concluded that survival, particularly for the recipient, is likely to be compromised if treatment is delayed (Crombleholme et al., Reference Crombleholme, Shera, Lee, Johnson, D'Alton, Porter and Young2007); thus, early diagnosis, potentially facilitated by including assessment of cardiac function, is to be recommended.
Acquired structural cardiac anomalies are recognized in a proportion of treated pregnancies, presumed to be a consequence of the altered hemodynamics. They may require longer-term follow-up and even postnatal treatment; for the recipient, this is usually in the form of right ventricular outflow tract (RVOT) anomalies (Michelfelder et al., Reference Michelfelder, Tan, Cnota, Divanovic, Statile, Lim and Crombleholme2015); for the donor there is a suggestion of an increased incidence of coarctation of the aorta (van den Boom et al., Reference Van den Boom, Battin and Hornung2010), although this is less well documented.
RVOT anomalies in the recipient may be in the form of valvar dysplasia, stenosis, regurgitation, or functional atresia; early involvement of the RVOT may be demonstrated by observing that the pulmonary valve annulus is equal in size to or smaller than that of the aortic valve, and this can progress to produce significant valvar pathology. It is not clear whether RVOT anomalies mirror the severity of the TTTS process, or whether the anomalies are simply due to altered hemodynamics or are a consequence of the vasoactive mediators. Little is known about the immediate and longer term impact on the tricuspid valve.
The prevalence of RVOT anomalies varies in different series but may be seen in some form in up to 20% of MC/DA twins, but only in recipient twins; between 9 and 12.5% had persistent anomalies requiring postnatal treatment, in spite of otherwise successful treatment for the TTTS. In a recent study (Michelfelder et al., Reference Michelfelder, Tan, Cnota, Divanovic, Statile, Lim and Crombleholme2015), approximately 9% of recipient twins had RVOT anomalies with pulmonary atresia seen more frequently than pulmonary stenosis, and although there was rapid improvement in utero in many, presumed to be due to improved RV function, up to 40% had persistent RVOT anomalies. This study also suggested that survival of those with RVOT involvement was lower than in those without and that the majority of cases with RVOT involvement were in Quintero stage lll or lV. Other studies have shown that pulmonary valve pathology is not necessarily associated with a worse outcome (Moon-Grady et al., Reference Moon-Grady, Rand, Lemley, Gosnell, Hornberger and Lee2011). It is recognized that these acquired structural lesions can progress even when function has improved, perhaps due to endothelial damage leading to the development of a dysplastic pulmonary valve (Michelfelder et al., Reference Michelfelder, Tan, Cnota, Divanovic, Statile, Lim and Crombleholme2015).
For the donor, there is a less well documented suggestion that the risk for developing coarctation of the aorta is increased (van den Boom et al., Reference Van den Boom, Battin and Hornung2010), although the effect of treatment is unclear, and whether this is an example of ‘primary’ structural CHD in MC twins or a consequence of hypovolemia is not yet established (Pruetz et al., Reference Pruetz, Slansky, Detterich, Korst, Llanes and Chmait2011)
Mechanisms for these specific anomalies are still speculative but are summarized in Table 4 and demonstrate the ‘flow-grow’ phenomenon for the growth of cardiac structures in utero. Identification of those likely to need postnatal treatment remains an important challenge (Michelfelder et al., Reference Michelfelder, Tan, Cnota, Divanovic, Statile, Lim and Crombleholme2015), and further work is needed to predict those that will have significant lesions after birth. In a recent study (Stagnati et al., Reference Stagnati, Chalouhi, Essaoui, Guiseppi, Stirnemann, Le Bidois and Ville2015), accurate identification of recipient twins likely to develop RVOT anomalies was not achieved, but two factors appeared to predict a need for postnatal treatment: a shorter interval between onset of TTTS and the diagnosis of PS was associated with a higher chance of needing postnatal treatment, maybe due to poor adaptation to an acute onset of TTTS; antenatal severity was also a risk factor for requiring postnatal treatment. However, other studies observed that even functional pulmonary atresia usually recovers following successful laser treatment (Moon-Grady et al., Reference Moon-Grady, Rand, Lemley, Gosnell, Hornberger and Lee2011).
TABLE 4 Possible Mechanisms for the Development of Acquired Structural Heart Disease
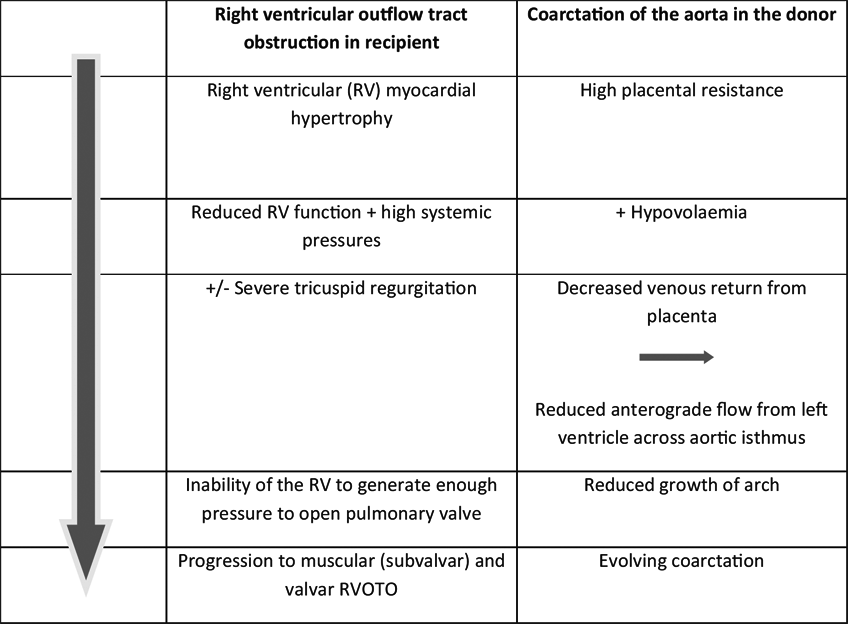
Coarctation of the aorta, more difficult to diagnose in the fetus whatever the cause, emphasizes the importance of careful postnatal cardiac assessment, including echocardiography, in survivors of TTTS (Van Miegen et al., Reference Van Mieghem, Done, Gucciardo, Klaritsch, Allegaert, Van Bree and Deprest2010).
Postnatal cardiovascular sequelae (Table 5) are becoming more important as so many more twins are surviving, partly due to improved laser techniques and experience and partly due to improved early neonatal care (Akkermans et al., Reference Akkermans, Peeters, Klumper, Lopriore, Middeldorp and Oepkes2015). As discussed, the incidence of ‘primary’ structural heart disease is increased in MC twins, even without evidence of TTTS, and it has been reported that donor CHD is less frequently detected antenatally compared to that in recipient twins — 17% compared to 43% (Pruetz et al., Reference Pruetz, Slansky, Detterich, Korst, Llanes and Chmait2011). The risk factors and incidence for developing persistent pulmonary hypertension of the newborn in the recipient remains unclear, but the risk may be increased if there is a decline in cardiac function following treatment (Delsing et al., Reference Delsing, Lopriore, Blom, Te Pas, Vandenbussche and Walther2007; Takahashi et al., Reference Takahashi, Takahashi, Tsukamoto, Ito, Nakamura, Hayashi and Sago2012). It is also relevant that if treatment for TTTS is successful earlier in the pregnancy, it may be difficult to identify which twin is which neonatally.
TABLE 5 Summary of Cardiovascular Considerations for Twins Surviving TTTS
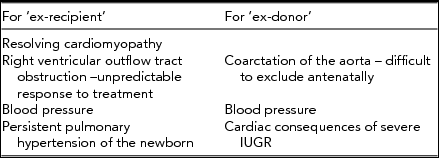
There is a logic for measuring blood pressure postnatally in both the donor and the recipient twin; growth restriction, myocardial dysfunction, chronic renal under perfusion, alteration in vascular stiffness, and prematurity are all risk factors for hypertension. Evidence for longer-term follow-up, in accordance with the Barker hypothesis (Sueters et al., Reference Sueters, Middeldorp, Vandenbussche, Teunissen, Lopriore, Kanhai and Oepkes2008) is interesting and requires more work; both twins are subjected to risk factors for lasting cardiovascular changes, which could predispose to hypertension.
A recent study found that both systolic and diastolic blood pressures were still increased at the age of 2 years, with no difference as to whether they were the donor or recipient (Pruetz et al., Reference Pruetz, Schrager, Wang, Llanes, Chmait and Vanderbilt2015). By the age of 10 years, the message is more encouraging and two recent studies have shown no significant myocardial dysfunction in either twin, even when there had been severe myocardial dysfunction in utero (Gardiner et al., Reference Gardiner, Matsui, Roughton, Greenwald, Diemert, Taylor and Hecher2014; Herberg et al., Reference Herberg, Bolay, Graeve, Hecher, Bartmann and Breuer2014), thus demonstrating the remarkably successful mechanisms for myocardial preservation, with or without remodeling, even after severe TTTS (Herberg et al., Reference Herberg, Gross, Bartmann, Banek, Hecher and Breuer2006). Longer-term consequences of smooth muscle hypertrophy and increased vascular stiffness, particularly relevant to the donor, remains unknown.
In conclusion, it appears that assessment of the cardiovascular manifestations of TTTS can have a role in the management of TTTS by providing a useful and hopefully increasingly reliable tool to allow earlier diagnosis while providing a method for refining staging and guiding treatment. It also has a role in quantifying the success of treatment, monitoring recovery as well as the need for appropriate peri- and postnatal surveillance. As TTTS is essentially a cardiovascular disorder, it is appropriate to attempt to quantify cardiovascular involvement, there is a need for as accurate classification of the clinical picture as is possible as this is essential for clinicians both for managing the pregnancy and for counseling parents on treatment options and possible outcomes.
Recovery of functional heart disease is now assumed and appears to have an excellent prognosis, even in the most severe cases (Baschat et al., Reference Baschat, Chmait, Deprest, Gratacos, Hecher, Kontopoulos and Ville2011) in both the short term and also, importantly, in the longer term (Halvorsen et al., Reference Halvorsen, Bilock, Pilo, Sonesson and Norman2009). Identification of those needing longer-term monitoring is important and as yet inadequately defined. Whether cardiovascular reprogramming takes place despite apparent recovery in utero remains to be seen (Crispi et al., Reference Crispi, Figueras, Cruz-Lemini, Bartrons, Bijnens and Gratacos2012; Diehl et al., Reference Diehl, Diemert and Hecher2014).
Many of the studies are conflicting in terms of their findings and conclusions, further emphasizing the complexity of the nature of the cardiac involvement; there is still much to be understood on the subject (Benoit & Baschat, Reference Benoit and Baschat2014; Lewi et al., Reference Lewi, Deprest and Hecher2013; Moon-Grady, Reference Moon-Grady2014). The impact of compromised cardiac function in terms of cerebral perfusion and longer-term neurological development is relevant and also requires further investigation. Now that perinatal survival for TTTS is improved, understanding the longer-term sequelae of TTTS has never been more important.








