Introduction
Sperm motility is a critical factor in the male reproductive system. Low sperm motility and viability, a measurement of sperm’s capacity for fertilization, are indicative of poor semen quality, which is recognized as a major contributing factor to male infertility (Brugh and Lipshultz, Reference Brugh and Lipshultz2004). Male factor infertility can be treated with assisted reproductive technologies (ARTs), such as intrauterine insemination (IUI) and in vitro fertilization (IVF) with or without intracytoplasmic sperm injection. Gradient separation (Percoll) and swim-up techniques are commonly used to process sperm specimens in IUI and in vitro fertilization (IVF; Akerlöf et al., Reference Akerlöf, Fredricson, Gustafsson, Lundin, Lunell, Nylund, Rosenborg and Pousette1987). The use of a washing medium and centrifugation steps in these processes can result in low motile sperm recovery. In addition to the percentage of total motility, the type of sperm movement is also documented to influence fertilizing capacity (Auger et al., Reference Auger, Serres, Wolf and Jouannet1994).
Physiologically, reactive oxygen species (ROS) are produced in human semen and sperm during spermatogenesis, mitochondrial activity, and capacitation (Agarwal et al., Reference Agarwal, Virk, Ong and du Plessis2014). However, excessive ROS production can disrupt the balance between oxidants and antioxidants in semen, leading to oxidative damage in various macromolecules within spermatozoa such as polyunsaturated acids in the plasma membrane, nucleic acids in DNA structure, and mitochondrial function (Aitken, Reference Aitken2017). This damage contributes to sperm dysfunction related to membrane integrity, resulting in impaired motility, viability, acrosome reaction, sperm–oocyte fusion, and DNA integrity, leading to difficulties in fertilization, implantation, and the maintenance of pregnancy (Alahmar, Reference Alahmar2019).
It is known that neurotransmitters, which are responsible for carrying electrical signals between nerve cells, significantly affect the male reproductive systems. According to studies, varying degrees of secretion of neurotransmitters, especially serotonin, result in significant changes in the mating rate of males. Serotonin (5-HT or 5-hydroxytryptamine hydrochloride) is a neurotransmitter that is produced by the hydroxylation and decarboxylation of tryptophan in the body and is essential for almost all living organisms (Hull et al., Reference Hull, Muschamp and Sato2004; Hull and Dominguez, Reference Hull and Dominguez2007). Meizel and Turner observed that serotonin induced an acrosomal reaction (AR) in capacitated sperm, implying the presence of a 5-HT-mediated receptor effect in the hamster sperm (Meizel and Turner, Reference Meizel and Turner1983). When mammalian spermatozoa exhibit AR in their heads and hyperactivity in their flagella, they are ready for fertilization (Austin, Reference Austin1977). For the sperm and egg plasma membranes to fuse, the acrosome must undergo an exocytotic event known as the AR to reach the zona pellucida (ZP) of the egg (Yudin et al., Reference Yudin, Gottlieb and Meizel1988; Fujinoki, Reference Fujinoki2009). The force for spermatozoa to penetrate the ZP is created by the sperm flagellum’s hyperactivation movement (Fujinoki et al., Reference Fujinoki, Ohtake and Okuno2001; Suarez and Ho, Reference Suarez and Ho2003). Overall, numerous studies so far have shown that serotonin can trigger the acrosome reaction (Austin, Reference Austin1977; Meizel and Turner, Reference Meizel and Turner1982, Reference Meizel and Turner1983; Yudin et al., Reference Yudin, Gottlieb and Meizel1988; Fujinoki et al., Reference Fujinoki, Ohtake and Okuno2001; Suarez and Ho, Reference Suarez and Ho2003; Fujinoki, Reference Fujinoki2009).
Serotonin regulates libido and sexual behaviour at the reproductive level in mammals through a hormonal mechanism; it is therefore crucial for reproductive organs. Serotonin plays reproductive roles by contracting the vas deferens, controlling cAMP, blood flow in the testicles, and producing testosterone (Parisi et al., Reference Parisi, de Prisco, Capasso and del Prete1984). Serotonin promotes motility and raises the rate of spermatozoa fertilization in invertebrates. This enhanced motility caused by serotonin could be due to flagella activation via cAMP-dependent dynein phosphorylation (Bandivdekar et al., Reference Bandivdekar, Segal and Koide1992; Stephens and Prior, Reference Stephens and Prior1992). One study showed that serotonergic neurons control copulation behaviour in Drosophila melanogaster (Yilmazer et al., Reference Yilmazer, Koganezawa, Sato, Xu and Yamamoto2016).
According to previous studies, dietary selenium can increase sperm quality in infertile individuals (Keskes-Ammar et al., Reference Keskes-Ammar, Feki-Chakroun, Rebai, Sahnoun, Ghozzi, Hammami, Zghal, Fki, Damak and Bahloul2003; Moslemi and Tavanbakhsh, Reference Moslemi and Tavanbakhsh2011). Also in vitro selenium supplementation is shown to improve sperm motility and viability (Ghafarizadeh et al., Reference Ghafarizadeh, Vaezi, Shariatzadeh and Malekirad2018). Reactive oxygen species are reduced by zinc antioxidant effects (Bray and Bettger, Reference Bray and Bettger1990; Narasimhaiah et al., Reference Narasimhaiah, Arunachalam, Sellappan, Mayasula, Guvvala, Ghosh, Chandra, Ghosh and Kumar2018). High ROS concentrations can impair sperm function (Sikka, Reference Sikka2001). Sperm quality, including motility, morphology, concentration, and acrosome response in the serum and seminal plasma, may be influenced by vitamin D (Aquila et al., Reference Aquila, Guido, Middea, Perrotta, Bruno, Pellegrino and Andò2009; Blomberg Jensen et al., Reference Blomberg Jensen, Bjerrum, Jessen, Nielsen, Joensen, Olesen, Petersen, Juul, Dissing and Jørgensen2011; Ramlau-Hansen et al., Reference Ramlau-Hansen, Moeller, Bonde, Olsen and Thulstrup2011; Jueraitetibaike et al., Reference Jueraitetibaike, Ding, Wang, Peng, Jing, Chen, Ge, Qiu and Yao2019). Vitamin E may lessen the effects of oxidative stress, enhancing the viability of sperm for in vitro fertilization (Suleiman et al., Reference Suleiman, Ali, Zaki, El-Malik and Nasr1996; Greco et al., Reference Greco, Iacobelli, Rienzi, Ubaldi, Ferrero and Tesarik2005).
Some studies have shown the individually significant effect of serotonin, selenium, zinc, and vitamins D and E supplementation on sperm motility. Therefore, we decided to evaluate the combination of these supplementation effects on human sperm motility and ROS levels in vitro conditions. The aim of this study was to evidence the synergistic incidence of antioxidants and serotonin on the motility and ROS production of spermatozoa. The combined effect of antioxidants and serotonin is stronger than the effect alone.
Materials and methods
Sample collection
In this study, 150 normozoospermic men were included and their semen samples were analyzed according to the World Health Organization (2010) guidelines (World Health Organization, 2010) for the effectiveness of serotonin, selenium, zinc, and vitamins D and E on sperm motility. After 3–5 days of sexual abstinence, samples were collected via masturbation. Ejaculates were incubated for 30 min at 37°C for liquefaction. After that, a Makler counting chamber and 10 µl aliquot of semen were used to assess basal sperm characteristics including concentration and motility. All volunteers read and signed an informed consent form before agreeing to take part in this study, which was approved by the Bezmialem Vakif University’s Ethical Committee (Ethical Approval Number 15/166).
Sperm preparation
The three-layer density gradient technique was used for sperm preparation (Allamaneni et al., Reference Allamaneni, Agarwal, Rama, Ranganathan and Sharma2005). The first step was to place 1 ml of 90% gradient medium at the bottom of a centrifuge tube, followed by 1 ml of 70% gradient medium and, finally, 1 ml of 50% gradient medium, to construct a gradient column. Then, 2 ml semen was added on top of the 50% layer, and the mixture was centrifuged at 300 rpm for 20 min. After centrifugation, the pellet was resuspended and washed with 3 ml of sperm-wash medium and then centrifuged for 10 min at 500 rpm. The supernatant was once more discarded, and the pellet was resuspended to the final volume in 0.55 ml of sperm-wash medium. Prepared sperm were then added and incubated in equal concentrations in different test groups as well as in the control group. For the control group, semen was incubated with HEPES-containing Modified Human Tubal Fluid (HTF) medium.
Sperm incubation with enriched medium
To evaluate the effectiveness of the supplements, serotonin, selenium, zinc, and vitamins D and E were added to HEPES-containing Modified HTF medium. The different doses of each supplements used are as follows: 25, 50, 100, 200, and 300 µM serotonin (5-HT, Santa Cruz-298707), 1, 2, and 4 µg/ml selenium (sodium selenite, Santa Cruz-253595A), 1, 10, and 20 µg/ml zinc (MERCK-108883), 50, 100, and 150 nM vitamin D 1α,25-dihydroxyvitamin D3 (1,25D, Calbiochem-Milliopore-5.097210001), and 1, 2 and 3 mmol vitamin E (α-tocopherol, Sigma-Aldrich-258024). For each supplement group, 30 samples obtained from normozoospermic men were used. Three distinct combinations were formulated using an HTF-based sperm-washing medium enriched with HEPES buffer, guided by initial findings and varying concentrations of serotonin, vitamins, and minerals. These combinations are referred to as Mix 1, Mix 2, and Mix 3. Mix 1 consists of 100 µM serotonin, 2 µg/ml selenium, 10 µg/ml zinc, 100 nM vitamin D, and 2 mmol vitamin E. Mix 2 consists of 50 µM serotonin, 1 µg/ml selenium, 5 µg/ml zinc, 50 nM vitamin D, and 1 mmol vitamin E. Mix 3 consists of 25 µM serotonin, 1 µg/ml selenium, 1 µg/ml zinc, 50 nM vitamin D, and 1 mmol vitamin E. To determine the best combination, 30 normozoospermic men’s samples were used in sperm motility and ROS experiments. The sperm motility of the prepared spermatozoa was then assessed after 75 min of incubation in each of these media at 37°C with 5% CO2.
Assessment of sperm motility
After the incubation period, 10 μl of sperm suspension from each experimental group was applied to a Makler counting chamber (Sefi Medical Industries, Haifa, Israel), and motility parameters were assessed. A minimum of five microscopic fields was examined to determine the percentage of sperm motility, involving at least 200 spermatozoa for each sample. Following the criteria outlined by WHO in 2010, sperm motility was categorized into three groups: progressive motile (Group A, representing sperm that swim primarily in a straight line or large circles), non-progressive motile (Group B, encompassing all other patterns of active tail movements without progression, including swimming in small circles), and immotile (Group C, indicating sperm cells with no observable movement). Total motility denotes the overall number of sperm cells in motion.
Determination of ROS levels in human spermatozoa
Within the scope of the ROS experiment, 2′,7′-dichlorofluorescin diacetate H2DCF-DA (H2-DCF, DCF; cat. no. D399, Invitrogen) fluorescent dye was used to detect intracellular H2O2 level in the spermatozoa by flow cytometry (CytoFLEX, Beckman Coulter Life Sciences, CA, USA).
Briefly, sperm samples were aliquoted into negative control and test groups and analyzed by direct flow cytometry without adding any dye to the control group (25 × 105 ml/spermatozoa). In each group, DCFDA (final concentration of 10 μM) was added to the sperm suspension (25 × 105 /ml spermatozoa). The mixture was incubated at 25°C for 30 min in the dark, and the labelled spermatozoa were analyzed by flow cytometry. In comparison with the untreated group, the ROS levels in sperm cells were measured as a percentage of fluorescence intensity (Guthrie amd Welch, 2006).
Statistical analysis
Three repeated-measures analysis of variance (ANOVA) was used to assess the effect of medium on sperm motility using the IBM SPSS Statistics 25 software package. Statistical significance was determined using one-way ANOVA (Tukey’s post hoc) and paired t-test. A P < 0.05 value was considered statistically significant. The data were presented as mean ± standard deviation.
Results
Effect of 5-HT supplementation on sperm motility
After a 75-min incubation period, sperm motility was significantly increased in solution with 100 and 200 µM serotonin. The average total motility was found to increase from 71.36% (in the control group), to 73.90% in 25 µM, 74.89% in 50 µM, 77.16% in 100 µM, 79.44% in 200 µM and 71.95% in 300 µM serotonin-containing solution. Similarly, the average progressive motility increased from 51.37%, to 54.58% in 25 µM, 56.90% in 50 µM, 58.68% in 100 µM, 60.10% in 200 µM and 52.05% in 300 µM serotonin-containing solution (Figure 1).
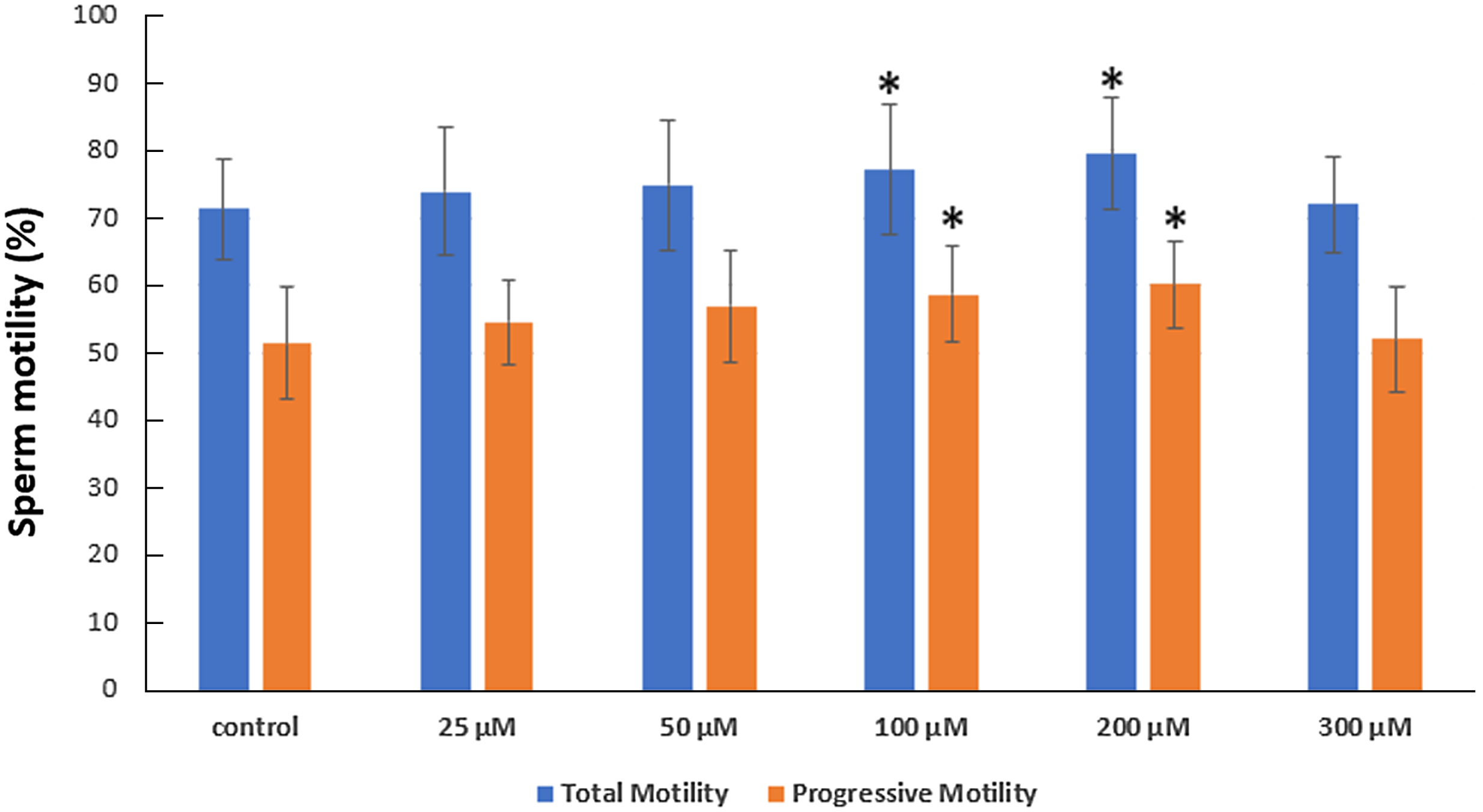
Figure 1. The impact of serotonin addition on sperm motility was investigated after 75 min of incubation. Mean standard deviation (SD). *P < 0.05 indicates a significant deviation from the control group.
Effect of selenium supplementation on sperm motility
After 75 min of incubation, sperm motility was significantly increased in solution with 2 µg/ml selenium. The average of total motility was found to increase from 63.95% (in the control group) to 67.88% in 1 µg/ml, 73.61% in 2 µg/ml, and 68.60% in 4 µg/ml of selenium-supplemented solution. The average progressive motility was found to increase from 41.27% to 51.18% in 1 µg/ml, 55.58% in 2 µg/ml, and 50.36% in 4 µg/ml of selenium-supplemented solution (Figure 2).

Figure 2. Following the 75 min of incubation, the effect of selenium addition on sperm motility was investigated. Mean ± standard deviation (SD). *P < 0.05 significant difference compared with the control group.
Effect of zinc supplementation on sperm motility
The incubation of spermatozoa for 75 min in zinc-supplemented medium created an increase in the total motility from 64.30% (in the control group) to 67.55% in 1 µg/ml, 72.71% in 10 µg/ml and 68.87% in 20 µg/ml zinc-supplemented medium. The average progressive motility was found to increase from 37.99 to 44.14% in 1 µg/ml, 50.64% in 10 µg/ml and 40.65% in 20 µg/ml zinc-supplemented medium (Figure 3). Sperm motility was significantly increased in solution with 10 µg/ml zinc.
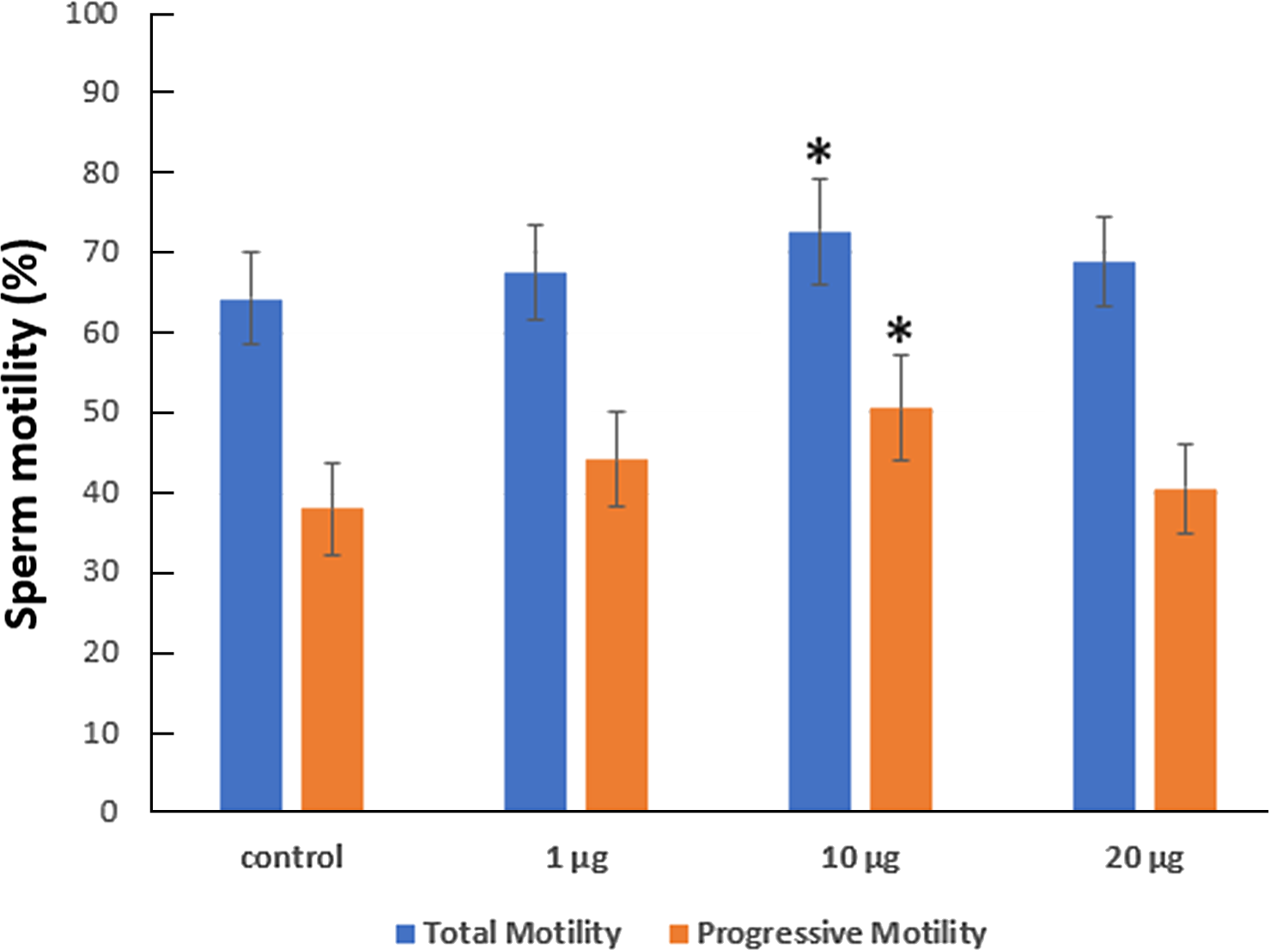
Figure 3. The function of zinc supplementation on sperm motility was investigated after 75 min of incubation. Mean standard deviation (SD). *P < 0.05 indicates a significant deviation from the control group.
Effect of vitamin D supplementation on sperm motility
At the end of the 75-min incubation period the average total motility decreased from 76.28% to 63.40% in 150 nM vitamin D solution, while it increased to 77.82% in 50 nM and 82.58% in 100 nM vitamin D-containing solution. The average progressive motility decreased from 56.93% to 47.76% in 150 nM vitamin D solution, but increased to 58.14% in 50 nM and 65.89% in 100 nM vitamin D-containing solution (Figure 4). Sperm motility was significantly increased in solution with 100 nM vitamin D.

Figure 4. After 75 min of incubation, the effect of Vitamin D supplementation on sperm motility was studied. Mean ± standard deviation (SD). *P < 0.05 significant difference compared with the control group.
Sperm motility of vitamin E
According to the control group, the average total motility decreased from 74.77% to 55.84% in 3 mmol vitamin E solution, whereas it increased to 75.15% in 1 mmol and 77.78% in 2 mmol vitamin E-containing solution. The average progressive motility was found to decrease from 55.21% to 46.04% in 3 mmol vitamin E solution, but increased to 55.95% in 1 mmol and 64.07% in 2 mmol vitamin E-containing solution (Figure 5). Sperm motility was significantly increased in solution with 2 mmol vitamin E.
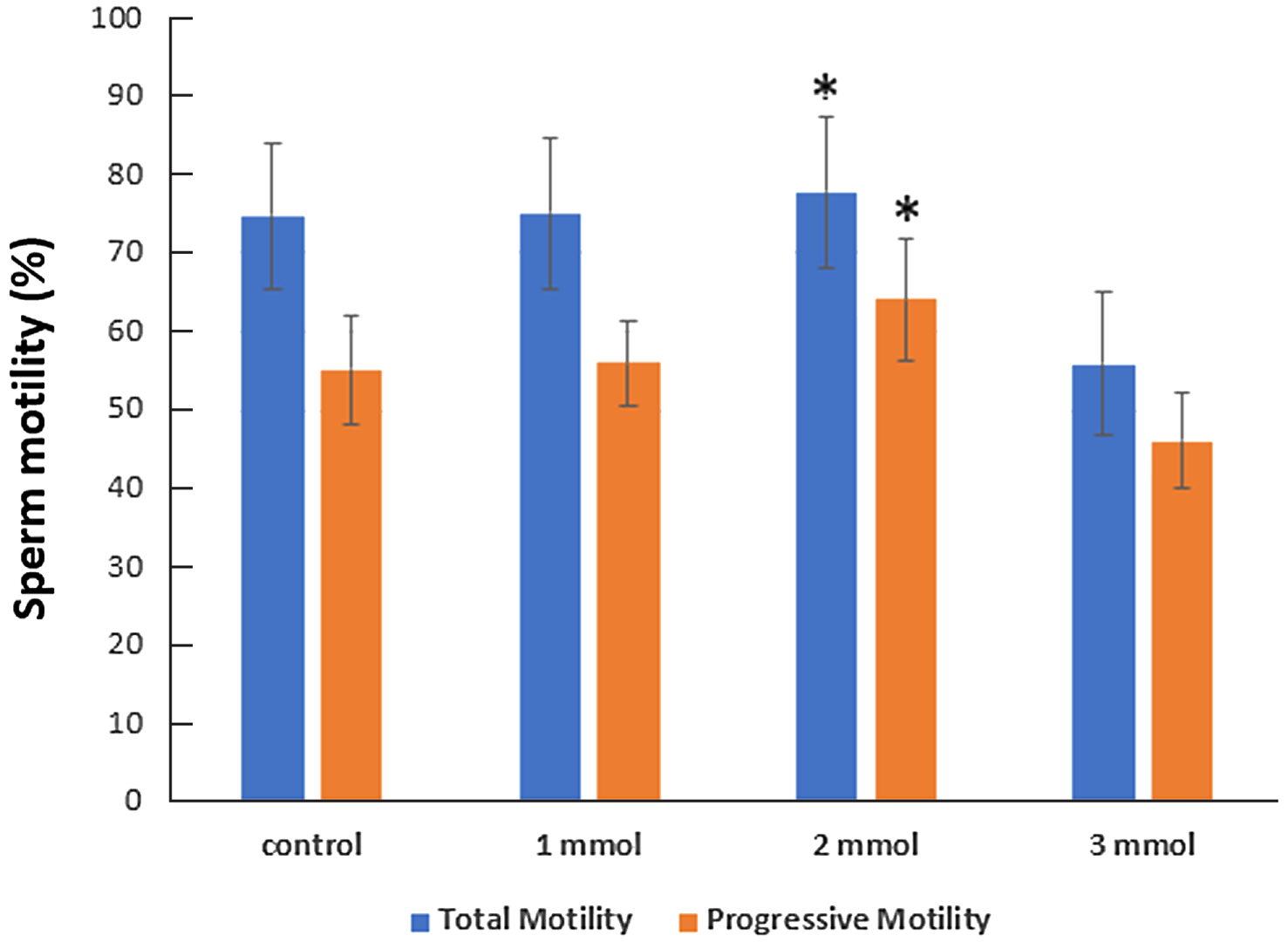
Figure 5. Vitamin E affecting supplementation on sperm motility was studied. Mean ± standard deviation (SD). *P < 0.05 significant difference compared with the control group.
Effect of different mixtures of supplementations on sperm motility
Three different solutions were prepared with an HTF-based sperm-washing medium containing 10 ml of HEPES buffer and different concentrations of each of serotonin, vitamins, and minerals in each. After 75 min of incubation, while the average total motility was 71.96% in the control group (without supplement), it was found to increase to 82.85% in Mix 1, 73.20% in Mix 2 and 72.89% in Mix 3. The average progressive motility was found to increase from 41.94% to 54.52% in Mix 1, 49.60% in Mix 2 and 43.72% in Mix 3 (Figure 6). Finally, it was found that the mixture with the highest serotonin concentration (Mix 1) was superior to the others in terms of enhancing sperm motility.

Figure 6. After 75 min of incubation, the effect of solution that mixtures in different ratios on sperm motility was studied. Mean ± standard deviation (SD). *P < 0.05 significant difference compared with the control group.
Effect of different mixtures of supplementations on ROS production
A ROS experiment was performed using flow cytometry after 75 min of incubation. Sperm motility was highest in the Mix 1 group, and ROS production was also found to be lowest in Mix 1. After 75 min of the incubation, while the average of ROS level was 8.97% in the control group (without supplement), it was decreased to 4.23% in the first, 7.62% in the second and 8.12% in the third mixture (Figure 7).

Figure 7. After 75 min of incubation, the effect of supplement mixtures in different ratios on ROS levels was studied. (a) Forward scatter dot plot used to select the respective population. (b) Histograms displaying fluorescence of fluorescein isothiocyanate (FITC) channel, used to measure fluorescence intensity of 2′,7′-dichlorofluorescin diacetate H2DCF-DA that is proportional to ROS production. Representative example of one sample ROS experiment. (c) Comparison of the average of ROS production after 75 min incubation of three mixtures. Mean ± standard deviation (SD). *P < 0.05 significant difference compared with the control group.
Discussion
Male fertility problems are becoming more prevalent around the world, as evidenced by a decrease in sperm concentration and spermatozoa motility (Levine et al., Reference Levine, Jørgensen, Martino-Andrade, Mendiola, Weksler-Derri, Mindlis, Pinotti and Swan2017). Recent studies have shown that nutrients, vitamins, and minerals have essential roles in sperm motility and quality (Keskes-Ammar et al., Reference Keskes-Ammar, Feki-Chakroun, Rebai, Sahnoun, Ghozzi, Hammami, Zghal, Fki, Damak and Bahloul2003; Aquila et al., Reference Aquila, Guido, Perrotta, Tripepi, Nastro and Andò2008, Reference Aquila, Guido, Middea, Perrotta, Bruno, Pellegrino and Andò2009; Ghafarizadeh et al., Reference Ghafarizadeh, Vaezi, Shariatzadeh and Malekirad2018). The main ingredient of our mixture is serotonin, which is crucial for the growth and metabolism of spermatozoa while promoting sperm motility (Jimeńez-Trejo et al., Reference Jimeńez-Trejo, Tapia-Rodríguez, Cerboń, Kuhn, Manjarrez-Gutiérrez, Mendoza-Rodríguez and Picazo2012). Furthermore, the serotonergic system plays multiple roles in the testes during spermatogenesis, insemination, the mating phase and the sperm preparation processes that occur in the caput epididymis (Jiménez-Trejo et al., Reference Jiménez-Trejo, Coronado-Mares, Boeta, González-Santoyo, Vigueras-Villaseñor, Arriaga-Canon, Herrera and Tapia-Rodríguez2018). The 5-HT present in seminal fluid or uterine interacts with 5-HT receptors and 5-HT transporters on the surface of sperm to affect sperm physiology (Mann et al., Reference Mann, Seamark and Sharman1961).
In this study, it was found that after 75 min of incubation, samples treated with 200 μM serotonin, 2 μg/ml selenium, 10 μg/ml zinc, 100 nM vitamin D, and 2 mmol vitamin E solution had significantly increased progressive motility compared with the control group (P < 0.05). These results indicated that in vitro serotonin and antioxidant supplementation in the sperm-washing/incubation medium could provide and create protective and beneficial effects on sperm motility according to our findings. The findings of this study also support previous research findings that antioxidant supplementation improves sperm motility in in vitro medium (Amenta et al., Reference Amenta, Vega, Ricci and Collier1992; Banihani et al., Reference Banihani, Sharma, Bayachou, Sabanegh and Agarwal2012; Yun et al., Reference Yun, Gong, Song and Lee2013). The absence of double or triple combinations of these ingredients in literature encouraged us to develop combinations with different dosages. According to our studies, sperm motility was highest in the Mix 1 group due to a positive correlation between sperm motility and serotonin content in Mix 1.
The overproduction of ROS can result in oxidative stress, leading to damage to proteins, DNA, and the plasma membrane of human spermatozoa (Bui et al., Reference Bui, Sharma, Henkel and Agarwal2018). In our experiments, we observed a reduction in ROS production after 75 min incubation with serotonin and antioxidant supplementation. With an increase in the serotonin content in Mix 1, there was a corresponding decrease in ROS production. While ROS production plays a crucial role in sperm capacitation (O’Flaherty and Matsushita-Fournier, Reference O’Flaherty and Matsushita-Fournier2017), excessive ROS levels can adversely affect sperm functions (Sikka, Reference Sikka2001). Studies have also shown previously that in vitro selenium supplementation protects sperm plasma membrane from oxidant damage (Ghafarizadeh et al., Reference Ghafarizadeh, Vaezi, Shariatzadeh and Malekirad2018), and zinc exhibits antioxidant properties and has the potential to reduce ROS levels (Bray and Bettger, Reference Bray and Bettger1990). Vitamin E also serves as a protective agent against ROS-induced damage to spermatozoa (Verma and Kanwar, Reference Verma and Kanwar1999).
In this study, the supplements used in the evaluated mixture solutions are selenium, zinc, vitamin D, and vitamin E. Another crucial element is selenium (Se), which is required for male fertility maintenance. Selenium deficiency in the diet impairs spermatogenesis and increases oxidative stress. Normal spermatogenesis requires selenium. Here, low selenium levels in the seminal are associated with barren sperm quality and a higher risk of male infertility. Sperm quantity, motility, regular morphology, and vitality are all markedly correlated with seminal plasma selenium levels (Morbat et al., Reference Morbat, Hadi and Hadri2018). However, Zn deficiency appears to have a greater influence on sperm movement. Some researchers have found a positive correlation between seminal fluid Zn and active sperm motility and an inverse correlation between immotile sperm motility nevertheless, sperm motility appears to be more affected by Zn deficiency. According to some studies, semen Zn has a positive correlation with active sperm motility and an inverse correlation with immotile sperm motility (Yamaguchi et al., Reference Yamaguchi, Miura, Kikuchi, Celino, Agusa, Tanabe and Miura2009). All these findings indicate that seminal plasma Zn levels are crucial for spermatogenesis, sperm maturation, sperm parameter quality, and spermatozoa’s capacity to fertilize. Sperm motility is regulated by vitamin D in seminal plasma. When vitamin D is in its active form, it might improve sperm motility by activating the sperm mitochondrial respiratory chain and increasing intracellular calcium ion concentration via the cAMP/PKA pathway (Jueraitetibaike et al., Reference Jueraitetibaike, Ding, Wang, Peng, Jing, Chen, Ge, Qiu and Yao2019). Vitamin E can help spermatozoa survive and make better sperm viability and motility. Vitamin E supplementation at sperm diluent may improve sperm quality by lowering lipid peroxidation of the sperm plasma membrane.
To sum up, our results show that serotonin, selenium, zinc, vitamin D, and vitamin E can be added to the commercial medium to improve sperm preparation for ARTs. These nutrients may also help to protect sperm quality during preparation and sampling. Further investigation is required to determine the effect of serotonin and antioxidant supplementation on fertility and embryo development in these patients during IVF or intracytoplasmic sperm injection (ICSI) procedures. Our findings will be applied to the development of a new, more effective sperm-wash medium containing serotonin and antioxidants.
Acknowledgements
This study was financially supported by the Turkish Scientific and Technological Research Council (TUBITAK), Project Number: 2170504. Yasemin Yilmazer is a co-inventor on the patent ‘Composition intended to increase the velocity of sperms containing serotonin as an active compound’ PCT/TR2019/050796.
Competing interests
The authors deny any conflict of interest.










