Waterholes are a limited resource linked to the conservation of biodiversity in desert ecosystems, are often used by a community-wide group of species, and are a point source for large animal aggregations (Trash et al., Reference Trash, Theron and Bothma1995; Valeix et al., Reference Valeix, Chamaille-Jammes and Fritz2007). Water, other than being an essential resource, provides benefits to ungulates such as reducing thermal stress and assisting in the digestion of drier plants (Ghobrial, Reference Ghobrial1974). Some desert wildlife requires standing water for survival, whereas other species do not drink regularly but will opportunistically use waterholes if available. In the deserts of North Africa and the Middle East, waterholes are a rare resource.
The establishment of feral wildlife populations often has negative consequences for native wildlife. For example, feral animals compete with native wildlife (Madhusudan, Reference Madhusudan2004), modify habitat through grazing (Zalba & Cozzani, Reference Zalba and Cozzani2004), act as a vector for disease transmission (Morgan et al., Reference Morgan, Lundervold, Medley, Shaikenov, Torgerson and Milner-Gulland2006) and prey on native wildlife (Fordham et al., Reference Fordham, Georges, Corey and Brook2006). In arid systems the establishment of feral wildlife populations may be dependent on the availability of waterholes.
Nubian ibex Capra nubiana, categorized as Vulnerable on the IUCN Red List (IUCN, 2008), is one of the few species in the genus Capra to inhabit arid lands. Although Nubian ibex are well adapted to the arid mountains of North Africa and the Middle East, water is a limiting factor for the species (Habibi, Reference Habibi1994). Nubian ibex, unlike many desert antelope species, must drink regularly, especially in the summer (Habibi, Reference Habibi1994; Wakefield et al., Reference Wakefield, Attum, Robinson and Sandoka2008). Waterholes, however, are often subject to disturbance that could potentially interfere with ibex waterhole use. For example, ibex could experience interference competition from livestock, humans and feral herbivores that could diminish opportunities to drink. Waterholes can also be potentially dangerous as predators may be familiar with the location of the waterhole and of prey. This is especially true in arid deserts of the Middle East and North Africa, where human hunters often hide and ambush ungulates (Habibi, Reference Habibi1994).
Given the rarity of natural waterholes in deserts and their importance to Nubian ibex, understanding factors affecting waterhole use has management and conservation implications. We therefore examined the influence of landscape characteristics and anthropogenic factors on the presence of Nubian ibex at waterholes.
The study areas are in northern Elba Protectorate (36,000 km2) and Wadi Gemal Protected Area (7,000 km2) in Egypt's Eastern Desert (Fig. 1). These areas are characterized by a hyperarid climate with hot rainless summers and mild winters. Precipitation falls mainly in the autumn and winter months but is not annual (Baha El Din, pers. comm.), and is often localized in the form of short, heavy rains that may result in flash flooding. The monthly mean temperature is 24–38°C in the summer and 12–26°C in the winter (Baha El Din, pers. comm.).
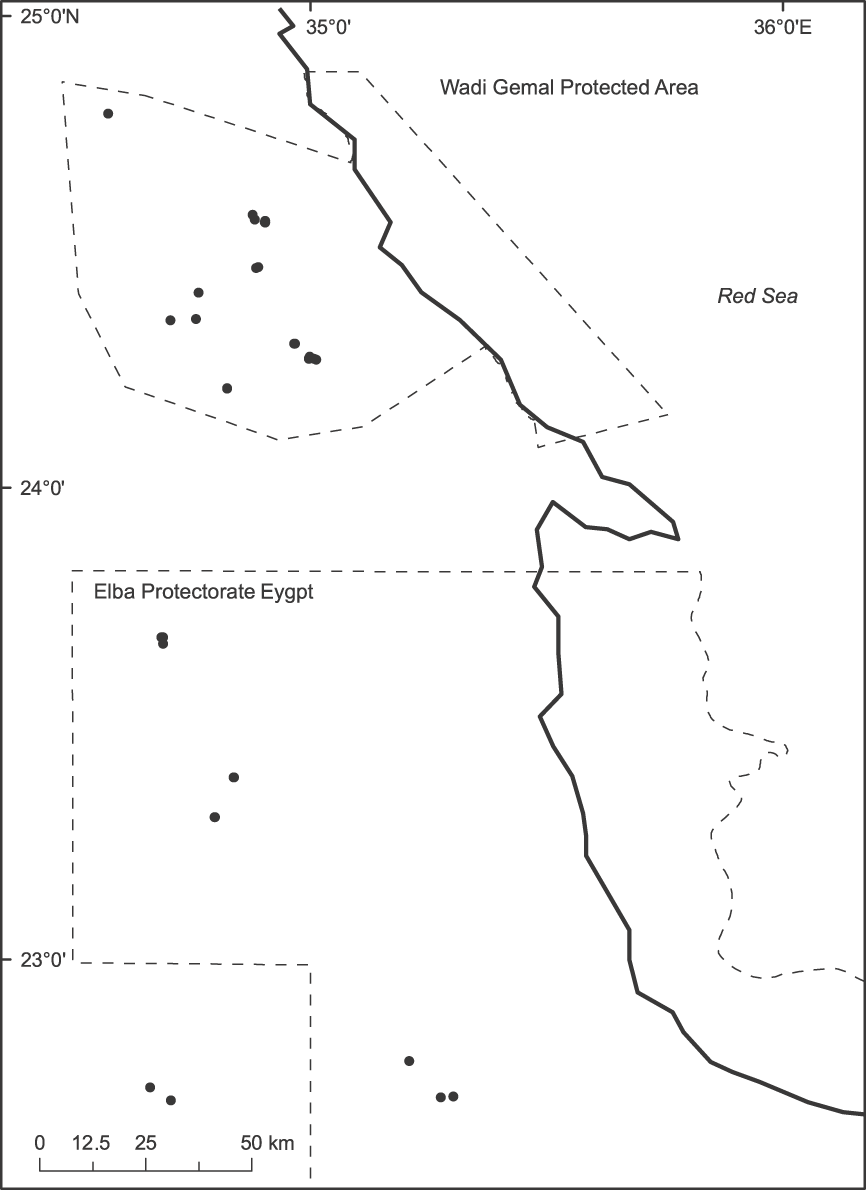
Fig. 1 The locations of surveyed waterholes (black circles). Because of the waterhole's clumped and patchy distribution and the map's large scale, not all the 48 waterholes are visible.
We sampled a total of 48 waterholes during 2–17 May 2007. The waterholes consisted of springs or water accumulations in rock basins in igneous rock mountains and valleys that drain into the Red Sea. The locations of waterholes were obtained from protected area staff and from the local community. Local people indicated that all the waterholes surveyed are in areas inhabited by ibex. The waterholes have a patchy and clustered distribution, with some valleys having multiple waterholes (Fig. 1).
We used four-wheel drive vehicles to reach the general vicinity of waterholes and hiked the remaining distance. For each waterhole we recorded the date and time of visitation, geographical coordinates and altitude with a global positioning system (GPS), and maximum water depth and width with a measuring tape. The valley width in which a waterhole was located was measured by walking the width of the valley and calculating the distance between the valley walls with a GPS. Waterhole distance to the nearest known neighbouring waterhole was calculated post hoc using the geographical information system software ArcMap v. 9.0 (ESRI, Redlands, USA).
Ibex were considered recently present at a waterhole if fresh tracks or black/dark brown ibex faecal pellets were found. We also recorded the presence of feral donkeys, camels and goats/sheep using the same criteria. Camels are owned by the local community but range freely to graze and often return to their owners at night. There are feral donkey populations (a result of the release of unwanted animals) and goats and sheep within the two areas but the goats and sheep are owned by people residing in the protected areas and are always accompanied by herders. Herders also accompany goats/sheep and camels to waterholes to collect drinking water for personal use, to make sure livestock do not fall in the waterhole and to pour water into drinking containers for the animals. Herders and livestock typically visit waterholes during the day. We grouped goats/sheep and camels in our analysis because of their association with humans at waterholes. Waterholes were categorized as inhabited by humans if they were within 500 m of any tent or agricultural field. These agricultural fields are relatively small, not being larger than a few hundred metres long or wide.
We ran a series of logistic regressions with every possible combination of the landscape characteristics (altitude, valley width, distance to nearest known waterhole, waterhole maximum depth and diameter) and anthropogenic disturbances (presence of feral donkeys, livestock and human dwellings) to predict Nubian ibex occurrence at waterholes. The model with the lowest Akaike information criterion (AIC) score was chosen as the final model.
We detected ibex at 65% (n = 31) of the waterholes (n = 48). Donkeys, livestock and human dwellings were recorded at 48% (n = 23), 42% (n = 20) and 17% (n = 8) of the waterholes, respectively. Waterholes were located at a mean altitude of 438 ± SE 27 m, in valleys that were 41 ± SE 11 m wide, and a mean distance of 970 + SE 320 m from the nearest known waterhole. Mean water depth was 33 ± SE 11 cm and mean maximum diameter 170 ± SE 27 cm.
The model with the lowest AIC score (16.61) contained the predictors donkeys and human dwellings, which correctly predicted 77.1% of ibex occurrence at waterholes (χ 2 = 21.74, df = 2, P < 0.0001). Ibex were significantly more likely to use waterholes (Fig. 2) that did not have human dwellings (Wald = 5.76, B = 3.08 ± SE 1.28, P = 0.016) and that did not have any recent visits by feral donkeys (Wald = 8.04, B = 2.46 ± SE 0.87, P = 0.005). Despite ibex being less likely to occur at waterholes used by livestock (Fig. 2) this effect was not significant, and the livestock variable was not part of the final model.
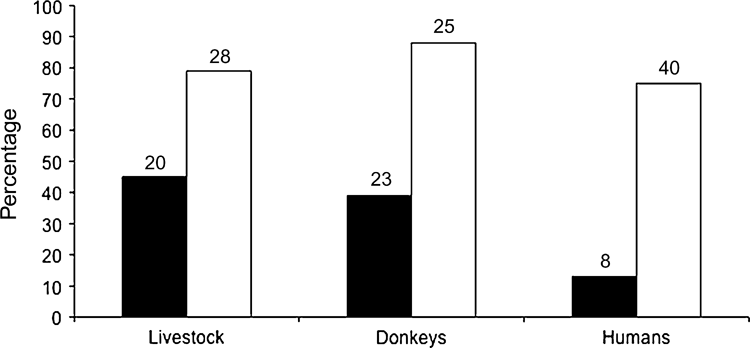
Fig. 2 Percentage of waterholes used by Nubian ibex Capra nubiana with (black bars) and without (white bars) disturbance by livestock, donkeys and humans. The number of waterholes in each case is indicated above the bar.
Our results suggest that anthropogenic factors could play a larger role in waterhole use than landscape characteristics. Ibex appear to use any waterhole opportunistically regardless of isolation, size, maximum water depth and the width of the valley in which the waterhole is located. This is unsurprising, given the rarity of waterholes, but anthropogenic factors may be limiting ibex from using all available waterholes.
Of all the introduced herbivores, feral donkeys are the most likely to compete with ibex for limited resources (Attum, Reference Attum2007). The larger donkeys may be competitively interfering and discouraging the smaller ibex from using the waterholes. It was clearly apparent that waterholes used by feral donkeys were associated with large accumulations of donkey faeces that degraded water quality, causing an odour and discolouration of the water.
Any fitness costs, such as increased thermal stress and interference with the consumption of dry food as a result of ibex not being able to drink from an available waterhole because of the presence of donkeys, are unknown. In areas with greater waterhole availability there may be no such fitness costs. For example, just a few hundred metres away from a waterhole used by feral donkeys ibex presence was often detected at an alternative waterhole, inaccessible to feral donkeys because it was located on top of a cliff or required climbing large boulders. However, any fitness costs incurred by ibex will be much greater if they have to travel long distances to find alternative water sources. Fitness costs will be greater during the summer when ibex need to drink more frequently (Wakefield et al., Reference Wakefield, Attum, Robinson and Sandoka2008).
The absence of a significant relationship between ibex presence and livestock was probably because we used presence/absence data rather than disturbance intensity and ibex visitation rates. The disturbance caused by people bringing livestock to waterholes is probably related to the frequency and duration of waterhole visits. For example, short visits will probably cause a temporary disturbance in which ibex change the timing of their waterhole visits to avoid livestock or humans (Wakefield & Attum, Reference Wakefield and Attum2006; Valeix et al., Reference Valeix, Chamaille-Jammes and Fritz2007). Human occupation in the vicinity of waterholes discourages ibex from regular visits because there are few periods free from disturbances.
Waterhole and ibex conservation will require working with local communities to protect and ensure sustainable use of this vital resource, taking into account the social sensitivities of resource use by local people within protected areas (Grainger, Reference Grainger2003). Local people have an interest in maintaining waterholes for sustainable use. We have begun involving local communities in waterhole conservation, with local community guards patrolling waterholes for poachers and monitoring water availability and quality. Future ibex and waterhole conservation should allow local people access to waterholes for drinking water and to water their livestock but discourage any permanent structures or agricultural fields from being developed within the immediate vicinity. Small-scale agriculture dependent upon pumping water from spring-fed waterholes should be discouraged as this could cause a decline in the water table.
The feral donkey population needs to be removed or minimized by regular culling (Carrion et al., Reference Carrion, Donlan, Campbell, Lavoie and Cruz2007) but not without the consultation of the local community. Although the donkeys are feral, the local community view the feral population as a potential resource: a donkey can be caught and either domesticated or sold. The feral donkey population could be removed by involving the local community in a programme similar to that conducted in St. Katherine's Protectorate, Egypt (Alaa El Deen, pers. comm.). For example, people are given a period in which to claim and conspicuously mark their donkeys, after which members of the local community are paid to remove the remaining feral donkeys.
Local community guards still patrol the waterholes in Wadi Gemal Protected Area and camera traps are now employed at select waterholes to monitor ibex waterhole use.
Acknowledgements
We would like to thank Dr Moustafa Fouda and the Egyptian Environmental Affairs Agency for encouraging us to undertake this work, and Mohammed Abbas, Sherif Baha El Din, John Dorr, Mohammed Gad, John Grainger, Mahmound Hanafy, Waheed Salama and Mustafa Ali for their assistance and logistical support. We are grateful for the field assistance provided by Elkramy Elabassry, Adnan Abdel Hamid, Wissam Farag and the numerous protected area rangers and local community guards. This work was the by-product of IUCN technical assistance and the Egyptian-Italian Environmental Cooperation Programme.
Biographical sketches
Omar Attum has a broad interest in the conservation of North African and Middle Eastern fauna. He currently devotes most of his time to studying conservation issues related to the Egyptian tortoise, dorcas gazelle and Nubian ibex. Sayed K. El Noby’s responsibilities as a biologist for Wadi Gemal Protected Area include carrying out bird surveys on Wadi Gemal Island and overseeing dorcas gazelle and Nubian ibex surveys on the mainland. Ibrahim N. Hassan is responsible for the fauna of Elba Protectorate, with an emphasis on monitoring the status of dorcas gazelle, lappet-faced vulture and Egyptian vulture populations.




