Introduction
The Great Basin of the western United States preserves numerous Cambrian (Stage 3 to Guzhangian) Burgess Shale-type (BST) deposits (Robison et al., Reference Robison, Babcock and Gunther2015; Foster and Gaines, Reference Foster, Gaines, Comer, Inkenbrandt, Krahulec and Pinnell2016; Lieberman et al., Reference Lieberman, Kurkewicz, Shinogle, Kimmig and MacGabhann2017; Kimmig, Reference Kimmig, Elias and Alderton2021). The best known examples are the Pioche Formation of Nevada (Lieberman, Reference Lieberman2003; Kimmig et al., Reference Kimmig, Meyer and Lieberman2019a), the Spence Shale Lagerstätte (Spence Shale from here on) of Utah and Idaho (Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b; Whitaker and Kimmig, Reference Whitaker and Kimmig2020), the Drum Mountains and House Range Wheeler Formation Lagerstätten (Robison et al., Reference Robison, Babcock and Gunther2015; Lerosey-Aubril et al., Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernández2020a), the Marjum Formation (Robison et al., Reference Robison, Babcock and Gunther2015; Leibach et al., Reference Leibach, Lerosey-Aubril, Whitaker, Schiffbauer and Kimmig2021; Pates et al., Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a), and the Weeks Formation (Lerosey-Aubril et al., Reference Lerosey-Aubril, Gaines, Hegna, Ortega-Hernández, Van Roy, Kier and Bonino2018).
The Cambrian (Miaolingian, Wuliuan) Spence Shale of northeastern Utah and southeastern Idaho occupies a distinctive position among the Lagerstätten of the Great Basin, because it preserves a range of environments from shallow-water carbonates to deep-shelf shales. Although this by itself is not unique, the fact that biomineralized and soft-bodied organisms are found in all of these environments, and the biota differs between localities (Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b; Whitaker et al., Reference Whitaker, Schiffbauer, Briggs, Leibach and Kimmig2022) makes the Spence Shale a deposit of utmost importance to understanding Cambrian communities and biodiversity. Whereas the depositional environment varies within the Spence Shale, all known exposures are dominated by panarthropods (Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b; Whitaker and Kimmig, Reference Whitaker and Kimmig2020). This is not surprising, because panarthropods usually dominate Cambrian Lagerstätten (Robison et al., Reference Robison, Babcock and Gunther2015; Paterson et al., Reference Paterson, García-Bellido, Jago, Gehling, Lee and Edgecombe2016; Hou et al., Reference Hou, Siveter, Siveter, Aldridge, Cong, Gabbott, Ma, Purnell and Williams2017; Nanglu et al., Reference Nanglu, Caron and Gaines2020; Yang et al., Reference Yang, Kimmig, Zhai, Liu, Kimmig and Peng2021).
Here we describe 21 new specimens of exceptionally preserved panarthropods from six localities of the Spence Shale, including a new hurdiid carapace element; the first specimens of Naraoia? Walcott, Reference Walcott1912, Thelxiope cf. T. palaeothalassia Simonetta and Delle Cave, Reference Simonetta and Delle Cave1975, Perspicaris? dilatus Robison and Richards, Reference Robison and Richards1981, Branchiocaris pretiosa Resser, Reference Resser1929, and Tuzoia guntheri Robison and Richards, Reference Robison and Richards1981; the first bradoriids; as well as new specimens of Dioxycaris argenta Walcott, Reference Walcott1886 from the deposit. In addition, Naraoia? sp. indet. represents the first occurrence of a soft-bodied taxon from the Two-Mile Canyon locality; Dioxycaris argenta and the bradoriids are the first soft-bodied panarthropods from the Spence Shale type-locality of Spence Gulch; Branchiocaris pretiosa is the first soft-bodied panarthropod from the Smithfield Creek locality; and the bradoriid from High Creek represents the first soft-bodied panarthropod from this location. These specimens not only extend the geographic and stratigraphic range of these taxa, but also add to the already diverse panarthropod fauna of the Spence Shale, suggesting that there might be more species to be found in currently understudied Spence Shale localities.
Geological setting
There are several Lagerstätte intervals that preserve soft tissues within the Spence Shale Member of the Langston Formation. The deposit is regionally extensive, with outcrops in southeastern Idaho and northeastern Utah (Fig. 1). The Spence Shale Member ranges in age from Mexicella mexicana to Glossopleura walcotti biozones (Cambrian, Miaolingian, Wuliuan, 507.5–506 Myr) (Liddell et al., Reference Liddell, Wright and Brett1997; Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b), with all soft-bodied fossils to date coming from the Glossopleura walcotti Biozone. It was deposited on a slope on the passive western margin of Laurentia, and outcrops record an overall increase in depth from Utah to Idaho. The Spence Shale Member ranges from ~9 m at Blacksmith Fork to ~120 m at Oneida Narrows, conformably overlies the Naomi Peak Limestone Member of the Langston Formation and, is in turn, conformably overlain by the High Creek Limestone member of the Langston Formation.
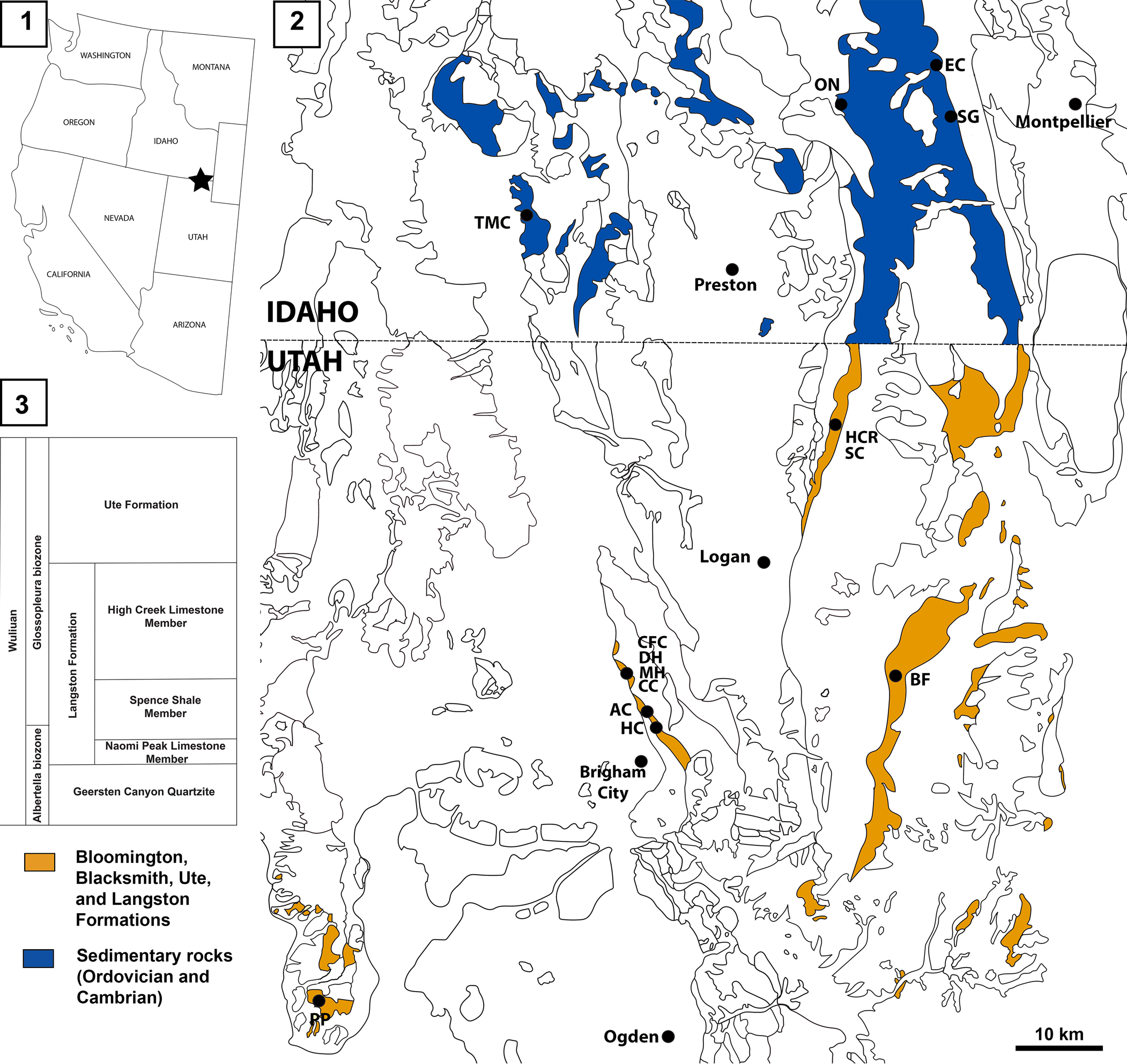
Figure 1. Locations of the Spence Shale Lagerstätte: (1) map of the western USA showing the location of the Spence Shale; (2) geological map (based on the USGS state maps for Google Earth Pro) of northern Utah and southern Idaho showing the principal localities within the Spence Shale; (3) simplified stratigraphy of the Langston Formation. AC, Antimony Canyon; BF, Blacksmith Fork; CC, Cataract Canyon; CFC, Calls Fort Canyon; DH, Donation Canyon; EC, Emigration Canyon; HC, Hansen Canyon; HCR, High Creek; MH, Miners Hollow; ON, Oneida Narrows; PP, Promontory Point; SC, Smithfield Creek; SG, Spence Gulch; TMC, Two Mile Canyon.
The specimens described herein come from six localities: Antimony Canyon and Miners Hollow in the Wellsville Mountains, north of Brigham City, Utah; High Creek and Smithfield Creek, in the Bear River Range, north of Logan, Utah; the type locality of the Spence Shale, Spence Gulch, in the Bear River Range, near Montpelier, Idaho; and Two-Mile Canyon, the westernmost Spence Shale locality, near Malad City, Idaho (Fig. 1). They are preserved in carbonate-rich siliciclastic mudstones in all localities except Two-Mile Canyon, where the Naraoia? sp. indet. specimen is preserved in siliciclastic shale.
Materials and methods
The specimens collected since 2016 were collected under U.S. Department of Agriculture Forest Service permits to JK and LJK. All specimens collected prior to 2016 were collected by avocational paleontologists and donated to the relevant repositories by those individuals. All specimens were collected with hand tools.
The specimens were photographed using a Nikon D5500 digital single-lens reflex camera with a Nikon 40 mm DX Micro-Nikkor Lens at the MCZ, a Canon EOS 60D digital single-lens reflex camera with a 60 mm EF-S macro lens at the YPM, a Canon EOS 7D equipped with a Canon macro lens at the IMNH, and a Canon EOS 5D Mark II digital single-lens reflex camera equipped with a Canon 50 mm macrolens at the KUMIP. Pictures of IMNH and KUMIP specimens were taken with specimens submerged in alcohol. Pictures of MCZ and YPM specimens were taken with cross-polarized lights. Close-ups of KUMIP 314201 were taken with a Nikon SMZ-1500 stereoscope with a DS-Ri1 camera and Nikon NIS Elements v. 2 and 3 software. The contrast, color, and brightness of images were adjusted using Adobe Photoshop ver. 24. Fossil images were kept in grayscale for improved contrast and visibility of structures. Line drawings were created in Adobe Illustrator ver. 27.4.1.
Ratios of endite length:podomere height for radiodont appendages were made using ImageJ (Schneider et al., Reference Schneider, Rasband and Eliceiri2012) from specimens of Hurdia and Buccaspinea spp. published in the literature (full list of sources and figure numbers in Table 2). Only the proximalmost two endites and associated podomeres were measured for each specimen. These ratios were visualized as a jitter plot using the ggplot2 package (Wickham, Reference Wickham2016) in R (R Core Team, 2022).
Table 1. Radiodont specimen fragments reported from the Spence Shale, Langston Formation (Cambrian: Wuliuan), Utah, USA. Specimens described in this contribution in bold font.

Table 2. Specimens, references, figure numbers, and generic assignments for ratios of endite length to podomere height visualized as jitter plot in Figure 4. Only proximal two endites were measured for each specimen. * = same specimen measured multiple times because it was figured in multiple studies. Specimen numbers reproduced as written in original publications.
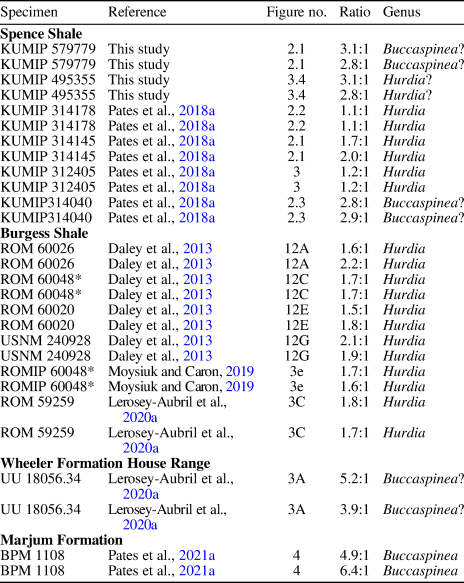

Figure 2. Isolated frontal appendages of Buccaspinea? from the Spence Shale Member, Langston Formation (Cambrian: Wuliuan), Utah, USA: (1, 2) KUMIP 579779 (Wellsville Mountains), collected by the Gunther family, and explanatory drawing; (3, 4) KUMIP 495356 (Miners Hollow), collected by Paul Jamison, and explanatory drawing. aux, auxiliary spines; ds, dorsal spines; en#, endite; pd#, podomere; ts, terminal spine.
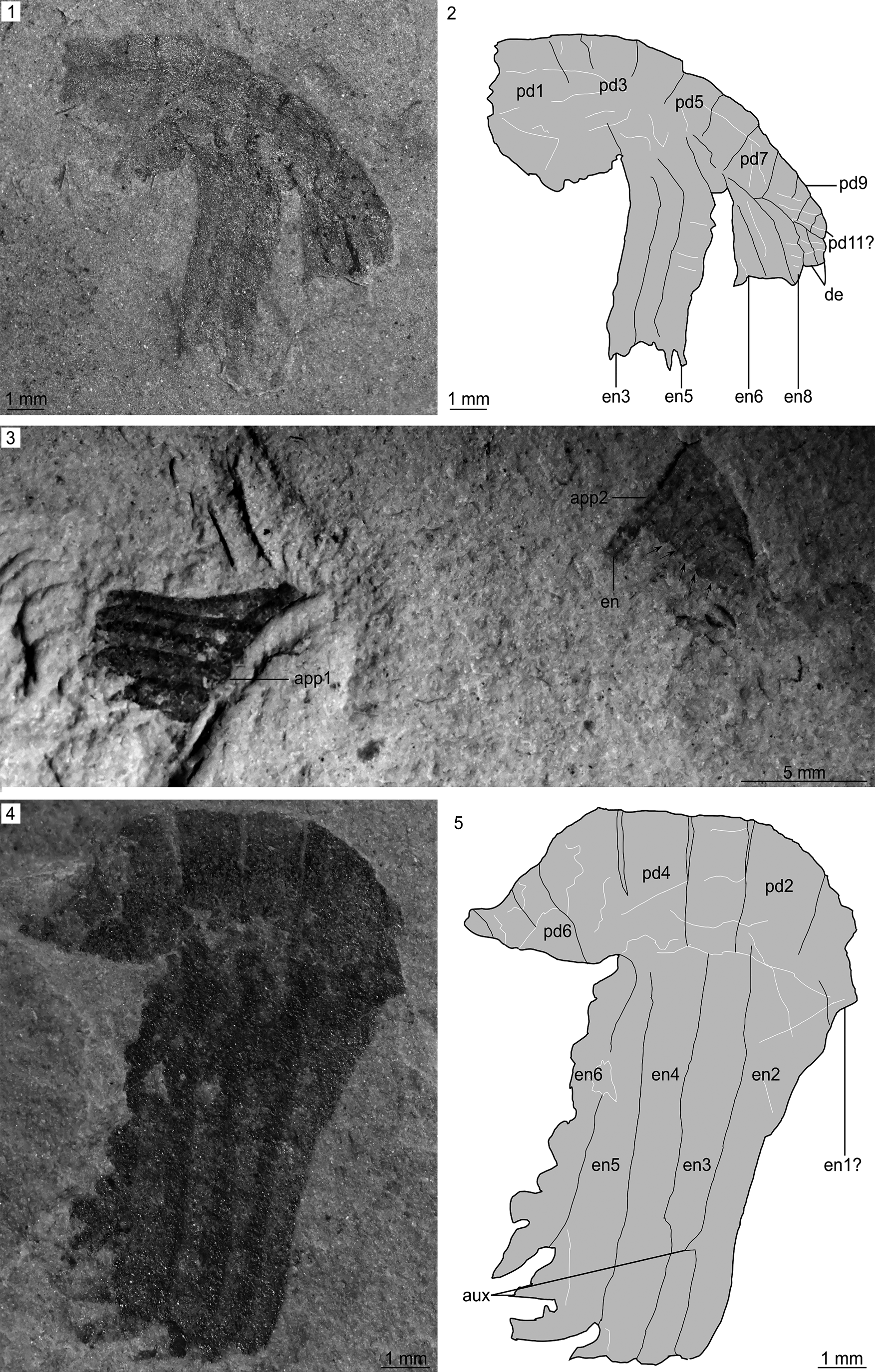
Figure 3. Isolated frontal appendages of Hurdia? collected by Paul Jamison from the Miners Hollow locality, Spence Shale, Langston Formation (Cambrian: Wuliuan), Utah, USA: (1, 2) KUMIP 491056 and explanatory drawing; (3) Overview of slab KUMIP 495355 (counterpart); (4, 5) close-up of well-preserved Hurdia? sp. indet. appendage on slab KUMIP 495355 (part) and explanatory drawing. app#, appendage; aux, auxiliary spine; de, reduced distal endite; en#, endite; pd#, podomere.
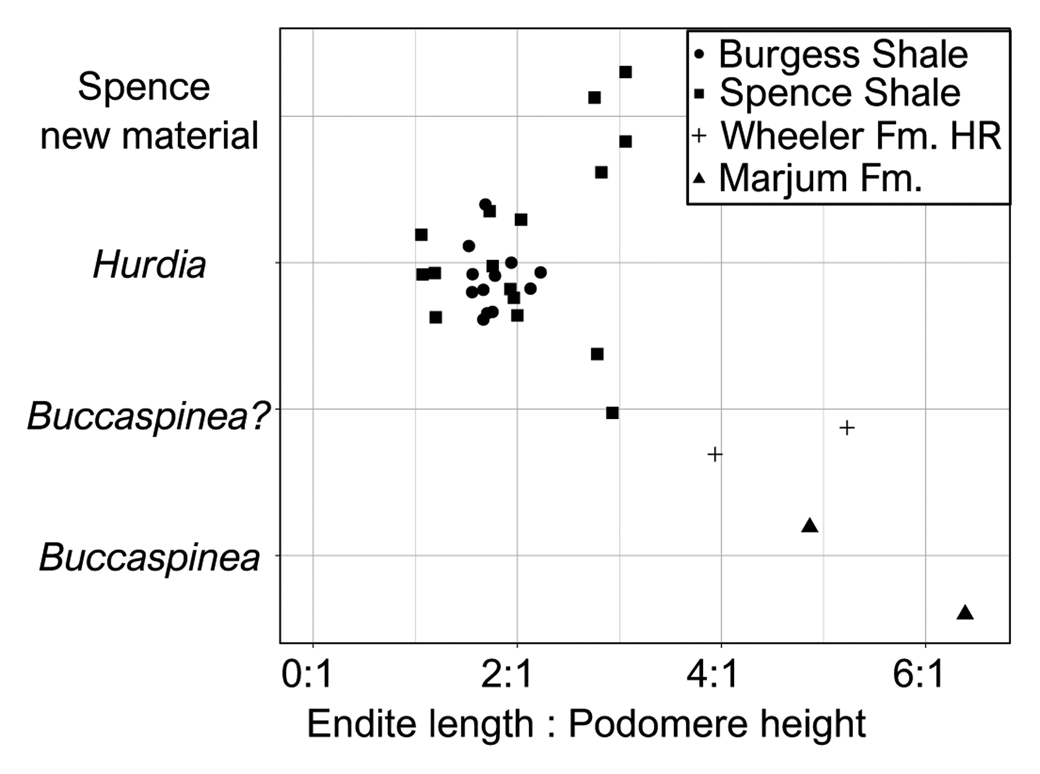
Figure 4. Jitter plot showing ratio of endite length to podomere height for published Buccaspinea and Hurdia appendages, alongside appendages recently tentatively reassigned to Buccaspinea? by Pates et al. (Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a), and those described in this contribution. Proximal two endites and associated podomeres measured for each specimen. Numeric values for ratios and sources for published images are provided in Table 2.

Figure 5. Partial radiodont frontal appendage tentatively assigned to Hurdiidae (KUMIP 490912) collected by Paul Jamison from Miners Hollow locality, Spence Shale, Langston Formation (Cambrian: Wuliuan), Utah, USA: (1, 2) specimen in lateral view and explanatory drawing. ds, dorsal spine; en#, endite; pd#, podomere.
Classification and terminology herein followed Kimmig and Pratt (Reference Kimmig and Pratt2015), Lerosey-Aubril and Pates (Reference Lerosey-Aubril and Pates2018), Lerosey-Aubril et al. (Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernández2020a, Reference Lerosey-Aubril, Skabelund and Ortega-Hernándezb), Pates et al. (Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a), Wu et al. (Reference Wu, Fu, Ma, Lin, Sun and Zhang2021a, Reference Wu, Ma, Lin, Sun, Zhang and Fub), and Zhu et al. (Reference Zhu, Lerosey-Aubril and Ortega-Hernández2021).
Repositories and institutional abbreviations
BPM, Back to the Past Museum, Quintana Roo, Mexico; IMNH, Idaho Museum of Natural History, Pocatello, Idaho, USA; KUMIP, Division of Invertebrate Paleontology, Biodiversity Institute, University of Kansas, Lawrence, USA; MCZ IP, Invertebrate Paleontology, Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA; ROM and ROMIP, Royal Ontario Museum, Toronto, Ontario, Canada; USNM, National Museum of Natural History [United States National Museum], Washington, DC, USA; UU, Department of Geology & Geophysics of the University of Utah, Salt Lake City, USA; YPM, Yale Peabody Museum, Yale University, New Haven, Connecticut, USA.
Systematic paleontology
Superphylum Panarthropoda Nielsen, Reference Nielsen1995
Order Radiodonta Collins, Reference Collins1996
Family Hurdiidae Lerosey-Aubril and Pates, Reference Lerosey-Aubril and Pates2018
Included genera
Aegirocassis Van Roy, Daley, and Briggs, Reference Van Roy, Daley and Briggs2015; Buccaspinea Pates et al., Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a; Cambroraster Moysiuk and Caron, Reference Moysiuk and Caron2019; Cordaticaris Sun, Zeng, and Zhao, Reference Sun, Zeng and Zhao2020; Hurdia Walcott, Reference Walcott1912; Pahvantia Robison and Richards, Reference Robison and Richards1981; Peytoia Walcott, Reference Walcott1911; Stanleycaris Pates et al., Reference Pates, Daley and Ortega-Hernández2018b; Titanokorys Caron and Moysiuk, Reference Caron and Moysiuk2021; and Ursulinacaris Pates et al., Reference Pates, Daley and Butterfield2019. Questionably Schinderhannes Kühl, Briggs, and Rust, Reference Kühl, Briggs and Rust2009.
Occurrence of new material
KUMIP 495355 and 495356 originate from the Lower Cycle 6 of the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Miners Hollow locality (GPS: 41.602, -112.033), Wellsville Mountains, Box Elder County, Utah. KUMIP 491056 originates from Cycle 6, ~47.2 m from the base of the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Miners Hollow locality (GPS: 41.602, -112.033), Wellsville Mountains, Box Elder County, Utah. KUMIP 579779 originates from an unknown locality of the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Wellsville Mountains, Box Elder County, Utah.
Description of new material
KUMIP 579779 (Fig. 2.1, 2.2) is an isolated frontal appendage that measures ~13 mm along the dorsal margin. Eight well-defined podomeres with tall rectangular outlines (Fig. 2.2, pd2–pd9) are preserved. The appendage is bent, with the five podomeres of the distal region orientated near-parallel to the endites borne by the podomeres of the intermediate region. Evidence for an additional ninth podomere is provided by the endites. Four endites, approximately three times as long as the height of the podomere to which they attach, are preserved (Fig. 2.2, en1–en4). The distal three clearly attach to a podomere, but the podomere that should bear en1 is missing. Endites are curved, with their tips approximately perpendicular to the bases, and bear robust triangular auxiliary spines 2–3 mm in length (Fig. 2.2, aux). A short (submillimeter), straight terminal spine is present (Fig. 2.2, ts).
KUMIP 495356 (Fig. 2.3, 2.4) is a more poorly preserved partial frontal appendage. At least four, possibly five, endites are present (Fig. 2.4, en1–en5?) proximal to poorly preserved organic material that might represent distal podomeres orientated ventrally as in KUMIP 579779 (Fig. 2.4, pd?). Endites are incomplete at their tips, but display a prominent curve, and bear robust triangular auxiliary spines ~1 mm in length (Fig. 2.4, aux).
KUMIP 491056 (Fig. 3.1, 3.2) is an isolated frontal appendage preserved laterally compressed that comprises at least 10, likely 11, podomeres and measures ~11 mm along the dorsal margin. Podomeres have tall rectangular outlines and decrease in size distally. Six plate-like endites, borne by intermediate-region podomeres (pd3–pd8), can be observed. The proximal three endites (Fig. 3.2, en3–en5) measure ~6 mm along the long axis, approximately twice the length of the height of the podomere to which they attach. These endites display a slight curve, orientated convex forward. The distal three endites in the intermediate region (Fig. 3.2, en6–en8) are incomplete, but run parallel to the proximal part of en3–en6. Two reduced endites are observed on the distal podomeres (Fig. 3.2, de). No dorsal spines or terminal spines were observed.
KUMIP 495355 (Fig. 3.3–3.5) is an incomplete isolated frontal appendage preserved laterally compressed (Fig. 3.3, app1), in close association (~10 mm) with additional organic material, potentially a second poorly-preserved partial appendage in oblique ventral view (Fig. 3.3, app2). The well-preserved appendage (Fig. 3.3, app1) measures ~9 mm along the dorsal margin, and comprises at least six, possibly up to eight, tall rectangular podomeres (Fig. 3.5, pd). Five endites approximately three times as long as the height of the podomere to which they attach are preserved (Fig. 3.5, en2–en6). The proximal four endites (Fig. 3.5, en2–en5) measure ~8 mm along the long axis, the distalmost endite (Fig. 3.5, en6) measures ~6 mm. A possible broken endite is associated with pd1 (Fig. 3.5, en1?). Complete endites are straight and bear small (submillimeter) auxiliary spines, with a larger ~1 mm auxiliary spine orientated distally at their tips. The dorsal surface and distal part of the appendages are incomplete, precluding the observation of dorsal or terminal spines. The associated organic material tentatively interpreted as a partial poorly preserved appendage (Fig. 3.3, app2) measures ~7mm along its dorsal margin. A number of linear divisions observed are interpreted as podomere boundaries. A ventral protrusion extends from the most proximal of these, interpreted as an incomplete endite (Fig. 3.3, en), but at least five further divisions can be observed (Fig. 3.3, arrows). The proximal and distal portions of this appendage are not preserved, and it is associated with additional unidentified organic remains.
New material
KUMIP 491056, 495355, 495356, and 579779, frontal appendages preserved as lateral compressions.
Remarks
These appendages can be confidently assigned to the family Hurdiidae within Radiodonta, because they are composed of podomeres bearing unpaired plate-like endites with endites along a single margin only. Although both appendages are incomplete, the separation of the distal articulated region of the appendage into two distinct regions—the intermediate one with plate-like endites and distal one with either reduced or no endites—provides further support for this assignment. Within Hurdiidae, these appendages bear most similarities to genera Buccaspinea and Hurdia. Distinguishing between these genera based only on frontal appendages is not straightforward. Both genera possess elongate endites that can bear robust and elongate auxiliary spines. Endites in Buccaspinea are far longer than the height of the podomere to which they attach (up to six times; Pates et al., Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a; Fig. 4), and auxiliary spines are very robust in this genus. Endite length to podomere height ratios in Hurdia are generally between 1:1 and 2:1 (Fig. 4), and auxiliary spines can be elongate and hooked (e.g., Moysiuk and Caron, Reference Moysiuk and Caron2019), but are generally less robust than in Buccaspinea. Reduced endites in the distal region have been observed in both Buccaspinea and Hurdia (e.g., Pates et al., Reference Pates, Daley and Butterfield2019, Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a) but are not known in all Hurdia appendages (e.g., Daley et al., Reference Daley, Budd and Caron2013). The extent of curvature and relative width of endites observed in hurdiid fossilized appendages can depend on orientation of preservation relative to bedding (Pates et al., Reference Pates, Daley and Lieberman2018a) and/or relative mesial and distal curvature (Moysiuk and Caron, Reference Moysiuk and Caron2019).
Considering all of these details together, KUMIP 579779 and 495356 are considered more likely to be Buccaspinea and KUMIP 491056 and 495355 more likely Hurdia, although these assignments are only tentative, as outlined below.
Evidence in support of identifying the two appendages as possible Buccaspinea (Fig. 2) comes from the relative length of their endites to podomeres, and the robust nature of the auxiliary spines. The relative length of endites to podomeres in KUMIP 579779 (~3:1) exceeds those of figured Hurdia specimens from the Burgess Shale and Spence Shale (Fig. 4) and is comparable to that of KUMIP 314040 (Pates et al., Reference Pates, Daley and Lieberman2018, fig. 2.3) that was recently tentatively reassigned to Buccaspinea (Pates et al., Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a). The lack of clear podomeres in KUMIP 495356 precludes measurement of this ratio for this specimen. Both specimens display robust auxiliary spines that overlap the endite immediately distal to the one to which they are attached, just as in the holotype (Pates et al., Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a) and other Spence Shale specimens tentatively reassigned to the genus: ROM 59634 (Daley et al., Reference Daley, Budd and Caron2013, fig. 24C), KUMIP 314040 (Pates et al., Reference Pates, Daley and Lieberman2018a, fig. 2.3, 2.4), and UU 18056.34 (Lerosey-Aubril et al., Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernández2020a). Hurdia appendages typically display less-elongate auxiliary spines that only partly overlap the endite anterior to the one to which they are attached (e.g., Daley et al., Reference Daley, Budd and Caron2013, fig. 12, fig. 24A, B, D), although this could in part be due to the distal tips of endites not being completely prepared out the matrix (compare Moysiuk and Caron, Reference Moysiuk and Caron2019, fig. 3e to Daley et al., Reference Daley, Budd and Caron2013, fig. 12 C). Hurdia auxiliary spines also tend to be less robust than those of Buccaspinea. However, these two appendages (KUMIP 579779 and 495356; Fig. 2) cannot be confidently assigned to Buccaspinea because they could be considered Hurdia appendages with particularly elongate endites and robust auxiliary spines, because these features do vary within the genus. Furthermore, the lack of reduced endites in the distal region (four are observed in the type specimen of Buccaspinea; Pates et al., Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a) also precludes confident assignment to the genus. Reduced distal endites are known from a single Spence Shale Hurdia specimen, ROM 59651 (Daley et al., Reference Daley, Budd and Caron2013, fig. 24D), and numerous Burgess Shale specimens (e.g., Daley et al., Reference Daley, Budd and Caron2013, fig. 12), although other published examples lack evidence for distal endites.
Evidence in support of identifying KUMIP 491056 and 495355 as Hudia, rather than Buccaspinea, comes from the robustness of the auxiliary spines, as well as comparison to other specimens previously assigned to Hurdia. Endites of KUMIP 495355 (Fig. 3.4) are more elongate relative to podomere height than published Burgess Shale and Spence Shale specimens (Fig. 4), comparable to what was measured for KUMIP 579779 (Fig. 2). Those of KUMIP 491056 are incomplete, precluding measurement of a ratio, however what is preserved is < 2:1, the usual range for Hurdia appendages. Both specimens lack prominent auxiliary spines, although this could be due to these spines being overlain by the endites, due to the orientation of preservation. These two specimens appear to display different extents of curvature. The convex forward curvature of endites observed for KUMIP 491056 is also known from KUMIP 314145, a previously described Hurdia appendage from the Spence Shale (Pates et al., Reference Pates, Daley and Lieberman2018a, fig. 2.1). KUMIP 495355 endites can be determined to be mostly straight and lack a prominent mesial or distal curvature, because the spines at the tips of the endites farthest from the ventral margin of the podomeres can be observed (on endites 5, 6, and possibly 4), and the visible podomere boundaries provides evidence of lateral (rather than frontal) compression. If endites had a prominent mesial curve, then the tips of the endites would not be visible (as they are not in KUMIP 491056). In addition, breaks or deformities from compression would be visible, or the curvature would be visible in the specimen. The straight endites of KUMIP 495355 (Fig. 3.3, app1) are best compared to KUMIP 314042 and 312405 (Pates et al., Reference Pates, Daley and Lieberman2018a, figs. 2.5, 3). In addition to relatively straight endites, both of these previously described specimens display a distalmost plate-like endite that is shorter than the others—a feature also observed in KUMIP 495355 (Fig. 3.3, app1) and other Spence Shale Hurdia appendages (Daley et al., Reference Daley, Budd and Caron2013, fig. 24A, B, D; Pates et al., Reference Pates, Daley and Lieberman2018a, fig. 2.1, 2.2). The oral cone adjacent to the pair of appendages in KUMIP 312405 bears internal tooth rows, confirming the identity of these appendages as Hurdia (Daley and Bergström, Reference Daley and Bergström2012; Pates et al., Reference Pates, Daley and Lieberman2018a, fig. 3). Thus, despite the relatively elongate nature of the endites in this specimen, assignment to Hurdia is preferred, albeit only very tentatively.
The affinities of the possible second appendage on slab KUMIP 495355 (Fig. 3.3, app2) are less clear. If the single endite in this possible appendage is interpreted as the distalmost of the plate-like endites of a hurdiid appendage, a total of six podomeres lacking endites would be present distal to the series of podomeres bearing plate-like endites. Notably, KUMIP 314178 (Pates et al., Reference Pates, Daley and Lieberman2018a, fig. 2.2) also displays a similar array of five very tall rectangular podomeres distal to the podomeres bearing plate-like endites, which are similar to those of KUMIP 495355 (Fig. 3.3, app1), as noted above. Thus KUMIP 495355 (Fig. 3.3, app2) is tentatively identified as the pair of KUMIP 495355 (Fig. 3.3, app1).
Family indet.
Gen. et sp. indet.
Figure 5
Occurrence
KUMIP 490912 originates from the Lower Cycle 6, ~45.7 m from the base of the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Miners Hollow locality (GPS: 41.602, -112.033), Wellsville Mountains, Box Elder County, Utah.
Description
KUMIP 490912 (Fig. 5) is a partial isolated appendage that measures ~40 mm along the dorsal margin. Evidence of at least 11 podomeres can be tentatively identified. Only a small amount of material remains of pd1, which is separated from pd2 by a curved boundary (Fig. 5, pd1, pd2). Podomeres display a tall rectangular outline in both intermediate and distal regions of the appendage (proximal region most likely not preserved). The middle part of the appendage is not preserved, and the distal region is curved ventrally, approximately perpendicular to the intermediate region. The ventral margin is not well preserved, and only the base of a single endite can be tentatively identified (Fig. 5, en6?). The dorsal margin is well preserved proximally but not distally. No dorsal spines are present on pd2–pd4, and the dorsal margin is not preserved between pd5–pd8. Small protrusions from the dorsal surface can be seen on pd9 and pd10, which might be dorsal spines (Fig. 5, ds?), however these features cannot be confidently distinguished from the incomplete dorsal margin and indents resulting from boundaries between podomeres.
Material
KUMIP 490912, a partial frontal appendage preserved as a lateral compression.
Remarks
This specimen can be identified as a partial arthropodized appendage, because it is sclerotized and composed of articulating segments (Ma et al., Reference Ma, Edgecombe, Legg and Hou2014). The curvature, podomere shape, and podomere number are all compatible with a radiodont affinity. Because the appendage is incomplete, and only the base of endites in the proximal region are preserved, assignment to the family level is difficult, with possible arguments favoring an amplectobeluid or hurdiid affinity. The comparatively low number of podomeres (11, assuming that there are no additional proximal podomeres missing) and the distal region approximately perpendicular to the proximal region are both characters observed in hurdiid radiodonts. This includes both Buccaspinea and Hurdia, the hurdiid radiodonts known from other material from Miners Hollow in the Spence Shale (described above), although the amplectobeluid Lyrarapax (only currently known from the Stage 3 Chengjiang in China) also bears only 11 podomeres in its distal articulated region (Cong et al., Reference Cong, Ma, Hou, Edgecombe and Strausfeld2014, Reference Cong, Daley, Edgecombe, Hou and Chen2016; Liu et al., Reference Liu, Lerosey-Aubril, Steiner, Dunlop, Shu and Paterson2018). The putative dorsal spines lack the size and recurved nature common for distal podomeres of amplectobeluid and anomalocaridid radiodonts (e.g., Amplectobelua stephenensis Daley and Budd, Reference Daley and Budd2010, Anomalocaris canadensis Whiteaves, Reference Whiteaves1892; see Daley and Budd, Reference Daley and Budd2010; Daley and Edgecombe, Reference Daley and Edgecombe2014), however this could be accounted for by their poor preservation. The strong curvature or the appendage distally is also observed in both amplectobeluid and hurdiid appendages. Features that would allow more confidence in assigning the isolated appendage specimen to Hurdiidae (e.g., plate-like spines, presence of auxiliary spines on one margin of endites only) or Amplectobeluidae (e.g., hypertrophied proximal endite, spiniform endites with few or no auxiliary spines) are not preserved, and so this specimen is left in open nomenclature and not identified to the family level.
Notably all radiodont appendages that are known from the Spence Shale have been assigned to Hurdiidae. A single body specimen from which frontal appendages are unknown was assigned to Anomalocaris Whiteaves, Reference Whiteaves1892 (Briggs et al., Reference Briggs, Lieberman, Hendricks, Halgedahl and Jarrard2008, fig. 1), however following the vast number of new radiodont taxa described since then, the exact taxonomic placement of this specimen within Radiodonta is unclear. Given that amplectobeluids and anomalocaridids are known from both older and younger deposits in the Great Basin (e.g., Lerosey-Aubril et al., Reference Lerosey-Aubril, Hegna, Babcock, Bonino and Kier2014, Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernández2020a; Pates et al., Reference Pates, Daley, Edgecombe, Cong and Lieberman2021b), it might be expected that representatives of these families will be discovered in the future, and are lacking due to their rarity in Laurentian deposits of Wuliuan age and younger, rather than truly absent.
Order Radiodonta? Collins, Reference Collins1996
Figure 6
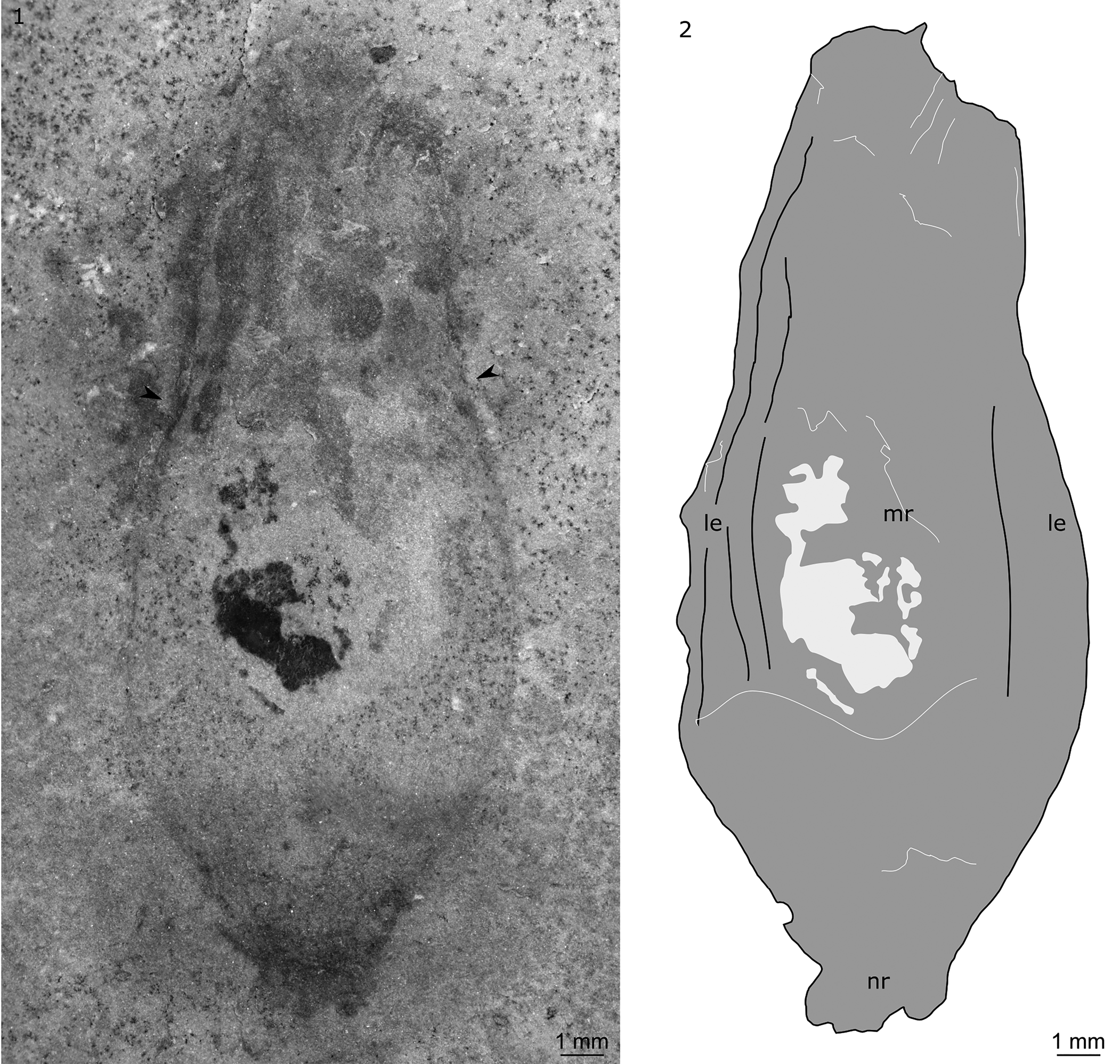
Figure 6. Radiodonta? (KUMIP 491057) collected by the Gunther family from the Antimony Canyon locality, Spence Shale, Langston Formation (Cambrian: Wuliuan), Utah, USA: (1, 2) possible central carapace element of a hurdiid radiodont in dorsal view and explanatory drawing. le, lateral extension; mr, main region; nr, nuchal region. Arrows indicate change in slope of outer margin from concave to convex.
Occurrence
KUMIP 491057 originates from the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Antimony Canyon locality (GPS: 41.563, -112.018), Wellsville Mountains, Box Elder County, Utah.
Description
KUMIP 491057 is a euarthropod carapace with a vase-shaped outline (Fig. 6). It measures ~23 mm along its long axis, and ~9 mm at the widest point perpendicular to the long axis (in dorsal view). The posterior part of the nuchal region and anterior part of the carapace are not preserved. The nuchal region is ~3 mm wide. The carapace element then broadens to its widest point at approximately one-third of the distance from the nuchal region to the anteriormost preserved point. The carapace then narrows anteriorly, with the outline changing from concave to convex at approximately two-thirds of the distance from the nuchal region to the anteriormost preserved point (Fig. 6, arrows). Relief is highest in the main region (Fig. 6, mr), with slender lateral extensions (Fig. 6, le) delineated by faint lines parallel to the long axis. On the left side of the element, these lines are associated with near-parallel compression wrinkles.
Material
KUMIP 491057, a possible central carapace element preserved compressed dorsoventrally.
Remarks
The specimen is likely a radiodont, and the vase-shaped outline and apparent relief allows this specimen to be tentatively identified as a hurdiid central carapace element. This specimen displays features comparable to a number of different hurdiid radiodonts, however the differences indicate that if a hurdiid affinity is confirmed, it most likely represents a new genus and species or belongs to a hurdiid for which the carapace is currently unknown, e.g., Buccaspinea.
The identification of a nuchal region (known in hurdiids, e.g., Cordaticaris and Pahvantia; Lerosey-Aubril and Pates, Reference Lerosey-Aubril and Pates2018; Sun et al., Reference Sun, Zeng and Zhao2020) allows the anteroposterior axis of the carapace to be identified. The widest part of the carapace is closest to the posterior, as in all hurdiid central carapace elements. The slender outline of KUMIP 491057 is similar to that known in Aegirocassis, Hurdia victoria Walcott, Reference Walcott1912, and Pahvantia, and contrasts with the broad central elements of Cambroraster, Cordaticaris, and H. triangulata Walcott, Reference Walcott1912. However, the specific shape of the outline differs. A nuchal region (identified in KUMIP 491057, present in Pahvantia) is unknown in Aegirocassis and H. victoria. However, although the presence of lateral extensions separated from the main region by faint lines is also known in Pahvantia (Lerosey-Aubril and Pates, Reference Lerosey-Aubril and Pates2018), KUMIP 491057 lacks the prominent ocular notches and associated spines described for Pahvantia (Lerosey-Aubril and Pates, Reference Lerosey-Aubril and Pates2018; Lerosey-Aubril et al., Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernández2020a; Pates et al., Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a). A change in outline from convex to concave is also known in Cordaticaris and Hurdia (Daley et al., Reference Daley, Budd and Caron2013; Sun et al., Reference Sun, Zeng and Zhao2020). However, in these two genera, this change in slope occurs very close to the distal tip of the carapace, not one-third of the distance from the tip as in KUMIP 491057.
Although the morphological features above support a hurdiid affinity for this euarthropod carapace element, a positive assignment to Hurdiidae, or indeed Radiodonta, requires the recognition of associated radiodont elements, e.g., frontal appendages, oral cones, or other body parts. Caution is warranted because previous euarthropod carapaces that have been assigned to Hurdiidae based on the morphology of carapace elements alone (e.g., Zeng et al., Reference Zeng, Zhao, Yin and Zhu2018) have subsequently been reassigned following the recognition of tergites (Cong et al., Reference Cong, Edgecombe, Daley, Guo, Pates and Hou2018)—a feature that precludes a radiodont affinity.
Phylum Euarthropoda Lankester, Reference Lankester1904
Subphylum Chelicerata? Heymons, Reference Heymons1901
Order Mollisoniida Lerosey-Aubril et al., Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernández2020
Family Mollisoniidae Lerosey-Aubril et al., Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernández2020
Genus Thelxiope Simonetta and Delle Cave, Reference Simonetta and Delle Cave1975
Type species
Thelxiope palaeothalassia Simonetta and Delle Cave, Reference Simonetta and Delle Cave1975.
Other species
Thelxiope holmani Lerosey-Aubril et al., Reference Lerosey-Aubril, Skabelund and Ortega-Hernández2020b; Thelxiope spinosa Conway Morris and Robison, Reference Conway Morris and Robison1988; Thelxiope sp. nov. A (Van Roy et al., Reference Van Roy, Orr, Botting, Muir, Vinther, Lefebvre, El Hariri and Briggs2010; Lerosey-Aubril et al., Reference Lerosey-Aubril, Skabelund and Ortega-Hernández2020b).
Diagnosis
Mollisoniids with well-developed sagittal spines on cephalic shield (one), thorax (one per tergite), and pygidium (three); length of each thoracic spine at least 1.5 times length (sag.) of corresponding tergite. (Lerosey-Aubril et al., Reference Lerosey-Aubril, Skabelund and Ortega-Hernández2020b)
Occurrence
Laurentia, middle Cambrian, Miaolingian, Wuliuan and Drumian; possibly Tremadocian strata of the Fezouata Shale in the Ternata Plain, southeastern Morocco.
Thelxiope cf. T. palaeothalassia Simonetta and Delle Cave, Reference Simonetta and Delle Cave1975
Figure 7
- cf. Reference Simonetta and Delle Cave1975
Thelxiope palaeothalassia Simonetta and Delle Cave, p. 28, pl. 3, fig. 1, pl. 12, figs. 3–5.
- cf. Reference Lerosey-Aubril, Skabelund and Ortega-Hernández2020b
Thelxiope palaeothalassia; Lerosey-Aubril et al., p. 6, figs. 2, 3.

Figure 7. Thelxiope cf. T. palaeothalassia Simonetta and Delle Cave, Reference Simonetta and Delle Cave1975 (KUMIP 314201), collected by Phil Reese from the Miners Hollow locality, Spence Shale Member, Langston Formation (Cambrian: Wuliuan), Utah, USA: (1, 2) general view and explanatory drawing. cs, cephalic shield; css, cephalic sagittal spine; ms#, marginal spine; pss#, pygidial sagittal spine; py, pygidium; T#, thoracic tergite; tss#, thoracic sagittal spine.
Diagnosis
Species of Thelxiope characterized by relatively short cephalic, thoracic, and pygidial sagittal spines, except for posteriormost pygidial spine hypertrophied and exceeding main body length. (Lerosey-Aubril et al., Reference Lerosey-Aubril, Skabelund and Ortega-Hernández2020b)
Occurrence
KUMIP 314201 originates from approximately two-thirds from the top of the Spence Shale, Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Miners Hollow locality (GPS: 41.602, -112.033), Wellsville Mountains, Box Elder County, Utah.
Description
Most of cephalic shield missing; only posteriormost part preserved. Posterior margin straight with short cephalic sagittal spine extending dorsally (Fig. 7.1).
Thorax 10 mm long and up to 7 mm wide, composed of seven tergites (Fig. 7, T1–T7), each ~7 mm wide and 3 mm long. Anterior part of each tergite partially concealed by tergite in front of it and spineless. Posterior part preserving short, thick-based, dorsally projecting sagittal spine.
Center part of pygidium not preserved. Pygidium 2 mm long and 3 mm wide, not including posterior spine, appearing to be composed of three nonarticulated tergites similar in morphology to those of thorax based on presence of three pygidial sagittal spines. Two anterior pygidial sagittal spines extending dorsally, slenderer and shorter than thoracic ones, whereas third spine hypertrophied, straight, and projecting posteriorly. Posterior spine approximately same length (12 mm) as thorax and pygidium combined (12 mm), appearing to be mostly complete; particularly thick proximally, but gently narrowing distally until reaching approximate half of its proximal diameter at distal tip.
Material
KUMIP 314201, an almost complete specimen in lateral view.
Remarks
Thelxiope cf. T. palaeothalassia from the Spence Shale has a thoracic tergite arrangement, and thoracic sagittal spines, resembling the holotype from the Burgess Shale, although the specimen is not as well preserved as the Burgess Shale specimens, and is missing most of the cephalon. The hypertrophied, straight, and posteriorly projecting third pygidial spine is also only known from Thelxiope palaeothalassia. This is the first and currently only occurrence of Thelxiope palaeothalassia outside of the Burgess Shale, although it still suggests that Thelxiope palaeothalassia was restricted to the Wuliuan of Laurentia.
Order Hymenocarina Clarke, Reference Clarke and Zittel1900
Family Perspicaridae Briggs, Reference Briggs1978
Genus Perspicaris Briggs, Reference Briggs1977
Type species
Canadaspis dictynna Simonetta and Delle Cave, Reference Simonetta and Delle Cave1975, by original designation.
Other species
Perspicaris recondita Briggs, Reference Briggs1977; Perspicaris? dilatus Robison and Richards, Reference Robison and Richards1981; Perspicaris? ellipsopelta Robison and Richards, Reference Robison and Richards1981.
Diagnosis
Carapace with hinge line, valves suboval, tapering anteriorly, rostral plate absent. Pendunculate eyes large, borne on elongate projection of cephalon. Abdominal somites lacking appendages, telson not posteriorly produced, caudal furca spinose. (Briggs, Reference Briggs1977)
Occurrence
Laurentia, middle Cambrian, Miaolingian, Wuliuan, and Drumian.
Perspicaris? dilatus Robison and Richards, Reference Robison and Richards1981
Figure 8.1, 8.2
- Reference Robison and Richards1981
Perspicaris? dilatus Robison and Richards, p. 4, pl. 1, fig. 4, pl. 2, figs. 5–7.
- Reference Lieberman2003
?Perspicaris dilatus; Lieberman, p. 677, fig. 1.2.
- Reference Kimmig and Pratt2015
Perspicaris? dilatus; Kimmig and Pratt, p. 55, figs. 6.1–6.9, 7.1–7.6, 9.4, 9.6, 11.3, 11.4, 11.6.
- Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernández2020a
Perspicaris? dilatus; Lerosey-Aubril et al., p. 22, fig. 12.

Figure 8. Bivalved panarthropods from the Spence Shale Member, Langston Formation (Cambrian: Wuliuan), Utah, USA: (1, 2) Perspicaris? dilatus Robison and Richards, Reference Robison and Richards1981 (YPM IP 530101), Antimony Canyon, collected by Lloyd Gunther, and explanatory drawing; (3) Branchiocaris petriosa Resser, Reference Resser1929 (KUMIP 314058), Smithfield Creek locality, collected by Reboul; (4) Branchiocaris petriosa (MCZ IP 164071, part), Antimony Canyon, collected by Lloyd Gunther.
Holotype
Right valve (KUMIP 135128) from the Wheeler Formation, House Range, Utah, USA (Robison and Richards, Reference Robison and Richards1981, pl. 1, fig. 4).
Diagnosis
Perspicaris? with valve subovate in outline, posterior process larger than anterior process. (Kimmig and Pratt, Reference Kimmig and Pratt2015)
Occurrence
YPM IP 530101 originates from the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, southern side of Antimony Canyon locality, approxmately half-way up slope between stream and ridge (GPS: 41.561, -112.006), Wellsville Mountains, Box Elder County, Utah.
Description
Valve subovate in outline; posterior portion moderately wider than anterior, with maximum width approximately two-thirds along length of hinge line from anterior margin. Maximum length 41 mm, maximum width 24 mm. Posterior process larger than anterior process and exhibiting angle of ~100°. Hinge line straight.
Material
YPM IP 530101, one valve in lateral view.
Remarks
Although valves and complete carapaces of Perspicaris? dilatus are relatively common, YPM IP 530101 is the only known specimen to contain soft tissues (black film) possibly representing the animal being protected by said valves. Unfortunately, the preservation does not allow for a conclusive assessment, because no segments or other morphological information are preserved. The specimen is conspecific with Perspicaris? dilatus based on the outline of the valve (Fig. 8.2) with the maximum width being near the posterior end, and the relatively large posterior process. The shape also fits within the variation of the Perspicaris? dilatus valves from the Rockslide Formation (Kimmig and Pratt, Reference Kimmig and Pratt2015). The valve overlies a Marpolia sp. specimen (Fig. 8.1).
Family uncertain
Genus Branchiocaris Briggs, Reference Briggs1976
Type species
Protocaris pretiosa Resser, Reference Resser1929, by original designation.
Diagnosis
As for species.
Occurrence
Laurentia and China, middle Cambrian, Miaolingian, Wuliuan, and Drumian; possibly China, Series 2, Stages 3 and 4.
Branchiocaris pretiosa Resser, Reference Resser1929
Figure 8.3, 8.4
- Reference Resser1929
Protocaris pretiosa Resser, p. 6, pl. 4, figs. 1, 2.
- Reference Shimer and Shrock1944
Protocaris pretiosa; Shimer and Shrock, p. 655, pl. 277, figs. 3, 4.
- Reference Rolfe and Moore1969
Protocaris pretiosa; Rolfe, p. 331, fig. 154.6.
- Reference Briggs1976
Branchiocaris pretiosa; Briggs, p. 6, fig. 2.4–2.7, pl. 1, figs. 5, 6; pls. 2–5; pl. 6, fig. l.
- Reference Briggs and Robison1984
Branchiocaris pretiosa; Briggs and Robison, p. 6, figs. 5–9.
- Reference Aria and Caron2017
Branchiocaris pretiosa; Aria and Caron, p. 90, fig. 2a, e, i, j, l, n, extended data figs. 6, 7.
- Reference Saleh, Antcliffe, Lefebvre, Pittet, Laibl, Peris, Lustri, Gueriau and Daley2020
Branchiocaris pretiosa; Saleh et al., p. 2, fig. 1b.
Holotype
Nearly complete specimen (USNM 80843) from the Burgess Shale, Stephen Formation, between Wapta Mountain and Mount Field, British Columbia, Canada (Resser, Reference Resser1929, pl. 4, figs. 1, 2).
Diagnosis
Carapace valves subovate, dorsal margin produced anteriorly and posteriorly into short pointed process, border with unevenly spaced, elongate, shallow pits normal to margin. Cephalic region bearing pair of uniramous antennae, anterior to pair of large segmented appendages. Trunk with up to 47 divisions; appendages lamellate, with segmented proximal element, attached to full length of trunk anterior to telson; telson processes short, pointed, bladelike. (Briggs, Reference Briggs1976)
Occurrence
KUMIP 314058 originates from the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Smithfield Creek locality (GPS: 41.874, -111.754), north of Logan, Wasatch Range, Cache County, Utah. MCZ IP 164071 and 164074 originate from the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation Antimony Canyon locality (GPS: 41.561, -112.006), Wellsville Mountains, Box Elder County, Utah.
Description
Valve semicircular in outline; maximum length 25 mm, maximum width 14 mm. Anterior process slightly smaller than posterior process, both relatively small. Anterior muscle scar indistinct (Fig. 8.3, 8.4), located close to anterior margin.
Material
Partial valve in lateral view (KUMIP 314058) and one complete valve in part and counterpart (MCZ IP 164071, 164074).
Remarks
Two valves are placed in Branchiocaris pretiosa because of their semicircular outline and the size and positioning of the anterior and posterior processes similar to previously described specimens of Branchiocaris pretiosa, which occurs in strata of approximately the same age (Briggs, Reference Briggs1976; Briggs and Robison, Reference Briggs and Robison1984). These are the first specimens of Branchiocaris pretiosa from the Spence Shale. Although the specimens do not preserve soft tissues, the valve of Branchiocaris pretiosa is fairly unique in its outline among carapaces of Cambrian arthropods and differs from Perspicaris? dilatus and Dioxycaris argenta in the smaller processes, the anterior process that is slightly smaller than the posterior process, and the semicircular rather than subovate to subelliptical outline of the valve.
Family Tuzoiidae Raymond, Reference Raymond1935
Genus Tuzoia Walcott, Reference Walcott1912
Type species
Tuzoia retifera Walcott, Reference Walcott1912, by original designation.
Other species
Tuzoia burgessensis Resser, Reference Resser1929; Tuzoia canadensis Resser, Reference Resser1929; Tuzoia polleni Resser, Reference Resser1929; Tuzoia manchuriensis Resser and Endo in Endo and Resser, Reference Endo and Resser1937; Tuzoia sinensis Pan, Reference Pan1957; Tuzoia australis Glaessner, Reference Glaessner1979; Tuzoia guntheri Robison and Richards, Reference Robison and Richards1981; Tuzoia bispinosa Yuan and Zhao, Reference Yuan and Zhao1999; Tuzoia tylodesa Luo and Hu in Luo et al., Reference Luo, Fu, Hu, Li, Chen, You and Liu2006; Tuzoia jianheensis Chen and Zhao in Chen et al., Reference Chen, Zhao, Yang and Wen2017; and Tuzoia lazizhaiensis Wen et al., Reference Wen, Babcock, Peng, Liu and Liang2019a. Questionably Tuzoia? parva Walcott, Reference Walcott1912; and Tuzoia? peterseni Robison and Richards, Reference Robison and Richards1981.
Diagnosis
Tuzoiid with ocular segment extending beyond carapace margin, bearing pair of elongated peduncular lobes. Carapace bearing spinose lateral ridge. Midposterior spine present in most species. Additional small marginal spines present in some species, mostly on ventroposterior margin of carapace. Endopod heptopodomerous with elongated basipod: distalmost podomere claw-shaped, second distalmost podomere always bearing series of spines. At least first two pairs of legs bearing spines on each podomere. Body terminating in two pairs of caudal rami, one on top of other, each pair fused into singular truncate paddle. (Izquierdo-López and Caron, Reference Izquierdo-López and Caron2022)
Occurrence
Laurentia, China and South Australia, lower and middle Cambrian, Series 2 and Miaolingian, Stage 3 to Drumian.
Tuzoia retifera Walcott, Reference Walcott1912
Figure 9.1
- Reference Walcott1912
Tuzoia retifera Walcott, p. 187, pl. 33, fig. 2.
- Reference Walcott1929
Tuzoia retifera; Resser, p. 7, pl. 1, fig. 2.
- Reference Simonetta and Delle Cave1975
Tuzoia retifera; Simonetta and Delle Cave, p. 8, pl. 47, fig. 2.
- Reference Robison and Richards1981
Tuzoia retifera; Robison and Richards, p. 14, pl. 8, fig. 3.
- Reference Vannier, Caron, Yuan, Briggs, Collins, Zhao and Zhu2007
Tuzoia retifera; Vannier et al., p. 459, figs. 3–5, 25.1, 26, 27.2, 31.
- Reference Izquierdo-López and Caron2022
Tuzoia retifera; Izquierdo-López and Caron, p. 3, figs. 3, 5.
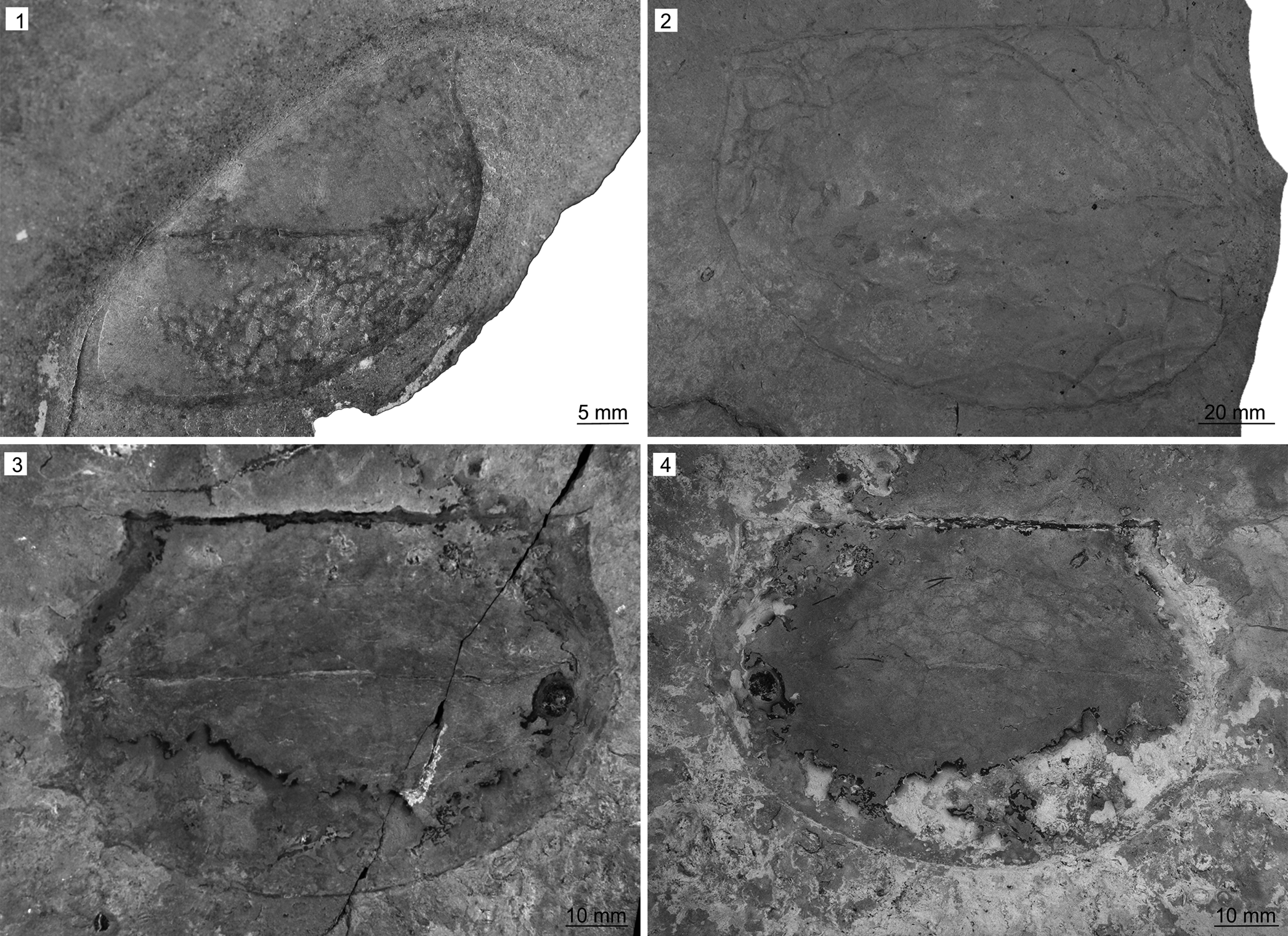
Figure 9. Tuzoia spp. from the Miners Hollow locality, Spence Shale Member, Langston Formation (Cambrian: Wuliuan), Utah, USA: (1) Tuzoia retifera Walcott, Reference Walcott1912 (KUMIP 561726), collected by Paul Jamison; (2) Tuzoia guntheri Robison and Richards, Reference Robison and Richards1981 (KUMIP 314036), valve in lateral view with burrows beneath, collected by Phil Reese; (3, 4) Tuzoia guntheri (KUMIP 561725, part and counterpart), collected by Paul Jamison.
Holotype
Right valve (USNM 57720) from the Burgess Shale, Stephen Formation, between Wapta Mountain and Mount Field, British Columbia, Canada (Walcott, Reference Walcott1912, pl. 33, fig. 2).
Diagnosis
Tuzoia with ovoid amplete to slightly postplete/preplete outline (mean L:H 1.45, N = 47, specimens in lateral aspect clearly showing ventral margin). Carapace length 20–120 mm; mean length ~77 mm (N = 47, SD = 16.5). Dorsal margin straight to slightly convex. Anterior cardinal processes broad-based, very short, not pointed, directed slightly downward. Well-developed anterior notch. Posterior cardinal processes Blunt, very small. Midposterior spine and posteroventral spine present, but very short. Midposterior spine often longer than posteroventral spine. One additional marginal spine between midposterior spine and posteroventral spine present or not. Posterior cardinal angle ~110°. No dorsal spines. Low-relief lateral ridge running almost parallel to dorsal margin (H:A ~2.0) underlined by very narrow frill with very small crenulation. Reticulate pattern dense, uniform, very small along anterior, posterior, and dorsal margins. Juvenile morphology similar to that of adults. (Vannier et al., Reference Vannier, Caron, Yuan, Briggs, Collins, Zhao and Zhu2007)
Occurrence
KUMIP 561726 originates from the Cycle 3 ‘Kootenia Quarry’ of the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Miners Hollow locality (GPS: 41.602, -112.033), Wellsville Mountains, Box Elder County, Utah.
Description of new material
KUMIP 561726 represents a partial single valve in lateral view. Only approximately half of the anterior part of the specimen is exposed and the posterior part is covered. The exposed part of the specimen is 48 mm long and ~ 41 mm in height at its widest point. The anterior cardinal process is exposed and projects 2 mm from the anterior margin at an angle of ~105°. The exterior surface is covered by somewhat irregular reticulate pattern of fine carbonaceous lines surrounding slightly convex interiors. The specimen preserves compaction wrinkles in the center of the valve, suggesting this area was vaulted in life.
New material
KUMIP 561726, partial right valve in lateral view.
Other material
KUMIP 153918, partial left valve (part and counterpart).
Remarks
Tuzoia retifera was previously known from a single specimen (KUMIP 153918) in the Spence Shale. This new specimen might represent a mostly complete valve, but preparation attempts were unsuccessful and, as such, the above description is based on the exposed part of the specimen. The specimen is assigned to Tuzoia retifera based on the exposed outline of the valve, the angle of the anterior cardinal process, and the somewhat irregular reticulate pattern of fine grooves surrounding slightly convex interiors covering the exterior surface of the valve. The pattern on the exterior surface of the valve is reminiscent of the pattern exhibited by Tuzoia guntheri (see Kimmig and Pratt, Reference Kimmig and Pratt2015).
Tuzoia guntheri Robison and Richards, Reference Robison and Richards1981
Figure 9.2–9.4
- Reference Robison and Richards1981
Tuzoia guntheri Robison and Richards, p. 13, pl. 7, figs. 1, 2, pl. 8, figs. 4, 5, pl. 9, fig. 2.
- Reference Robison, Simonetta and Conway Morris1991
Tuzoia guntheri; Robison, p. 84, fig. 7.1.
- Reference Lieberman2003
Tuzoia guntheri; Lieberman, p. 679, fig. 1.3.
- Reference Vannier, Caron, Yuan, Briggs, Collins, Zhao and Zhu2007
Tuzoia guntheri; Vannier et al., p. 462, figs. 16, 25.4, 26, 31.
- Reference Kimmig and Pratt2015
Tuzoia guntheri; Kimmig and Pratt, p. 57, fig. 9.2, 9.3.
- Reference Robison, Babcock and Gunther2015
Tuzoia guntheri; Robison et al., p. 62, fig. 173.
- Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b
Tuzoia sp.; Kimmig et al., p. 614, fig. 4e.
Holotype
Left valve (KUMIP 153917) from the Marjum Formation, House Range, Utah, USA (Robison and Richards, Reference Robison and Richards1981, pl. 7, fig. 2).
Diagnosis
Tuzoia with ovoid, slightly postplete outline (L:H 1.35 in holotype). Length (exclusive of spine) can exceed 80 mm. Dorsal margin straight to slightly convex. Anterior cardinal processes pointing straight forward, overhanging notch. Posterior cardinal processes shorter than anterior cardinal processes, directed upward and backward. At least two dorsal spines (one large and subvertical), both inserted along anterior third of dorsal margin. Posteroventral spine long, slender. Midposterior spine short. Two additional marginal spines present: one, very small, dorsal of midposterior spine; the other, longer, ventral of posteroventral spine. Lateral ridge with low relief, running obliquely (H:A ~3.0 at valve midlength in holotype). Reticulate pattern over entire lateral surface. (Vannier et al., Reference Vannier, Caron, Yuan, Briggs, Collins, Zhao and Zhu2007)
Occurrence
KUMIP 561725 originates from the Cycle 3 ‘Kootenia Quarry’ of the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Miners Hollow locality (GPS: 41.602, -112.033), Wellsville Mountains, Box Elder County, Utah. KUMIP 314036 originates from the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Miners Hollow locality (GPS: 41.602, -112.033), Wellsville Mountains, Box Elder County, Utah.
Description of new material
KUMIP 561725 (Fig. 9.3, 9.4) is 120 mm long and 77 mm wide and preserved as part and counterpart. The valve has four spines on the posterior margin; the most dorsal 5 mm long, the midposterior spine 5 mm long, the posteroventral spine 10 mm long, and the ventral spine 2 mm long; and two dorsal spines (Fig. 9.3), the more anterior of which is 4 mm long, and the more posterior one 2 mm long. The preserved part of the anterior cardinal projects 2 mm from the anterior margin at an angle of ~110°. The posterior cardinal process projects 2 mm from the posterior margin at an angle of ~110°. Dorsal margin straight.
KUMIP 314036 (Fig. 9.4) is 165 mm long and 115 mm wide. None of the spines are preserved, but the outline of the valve corresponds to the outline of Tuzoia guntheri. The anterior cardinal process projects 2 mm from the anterior margin at an angle of ~110°.
Material
Two valves in lateral view (KUMIP 314036, 561725).
Remarks
Tuzoia guntheri has been previously reported from the Cambrian (Wulian to Drumian) of the Great Basin and the Drumian Rockslide Formation of northern Canada (Robison and Richards, Reference Robison and Richards1981; Liebermann, Reference Lieberman2003; Vannier et al., Reference Vannier, Caron, Yuan, Briggs, Collins, Zhao and Zhu2007; Kimmig and Pratt, Reference Kimmig and Pratt2015), but this is its first record in the Spence Shale.
KUMIP 314036 preserves groove-shaped, simple burrows between 1 and 3 mm wide and several centimeters long (Fig. 9.2). Bifurcation is observed in a few instances, but likely due to intersection rather than true branching. They resemble the structures described from below panarthropod valves in other BST deposits (Zhang et al., Reference Zhang, Bergström, Bromley and Hou2007; Mángano, Reference Mángano2011; Mángano et al., Reference Mángano, Bromley, Harper, Nielsen, Smith and Vinther2012; Kimmig and Pratt, Reference Kimmig and Pratt2016) and are closest in shape and size to those described on valves of Perspicaris? dilatus from the Drumian Ravens Throat River Lagerstätte of northern Canada (Kimmig and Pratt, Reference Kimmig and Pratt2016).
Order and Family uncertain
Genus Dioxycaris Gürich, Reference Gürich1929
Type species
Lerpeditia argenta Walcott, Reference Walcott1886.
Diagnosis
As for species.
Occurrence
Laurentia, middle Cambrian, Miaolingian, Wuliuan.
Dioxycaris argenta Walcott, Reference Walcott1886
Figure 10
- Reference Walcott1886
Lerpeditia argenta Walcott, p. 146, pl. 8, fig. 5.
- Reference Gürich1929
Dioxycaris argenta; Gürich, p. 36, text-fig. 1.3.
- Reference Roger and Piveteau1953
Dioxycaris argenta; Roger, p. 311, pl. 1, fig. 4a.
- Reference Brooks and Caster1956
Dioxycaris argenta; Brooks and Caster, p. 11, fig. 1.6.
- Reference Rolfe and Moore1969
Dioxycaris argenta; Rolfe, p. 325, fig. 150.4.
- Reference Briggs1976
Dioxycaris argenta; Briggs, p. 13, pl. 6, figs. 3, 4.
- Reference Robison and Richards1981
Dioxycaris argenta; Robison and Richards, p. 7, pl. 3, figs. 4–6.
- Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b
Dioxycaris argenta; Kimmig et al., p. 614, fig. 4j.
- Reference Whitaker, Schiffbauer, Briggs, Leibach and Kimmig2022
Dioxycaris argenta; Whitaker et al., p. 6, fig. 2C.
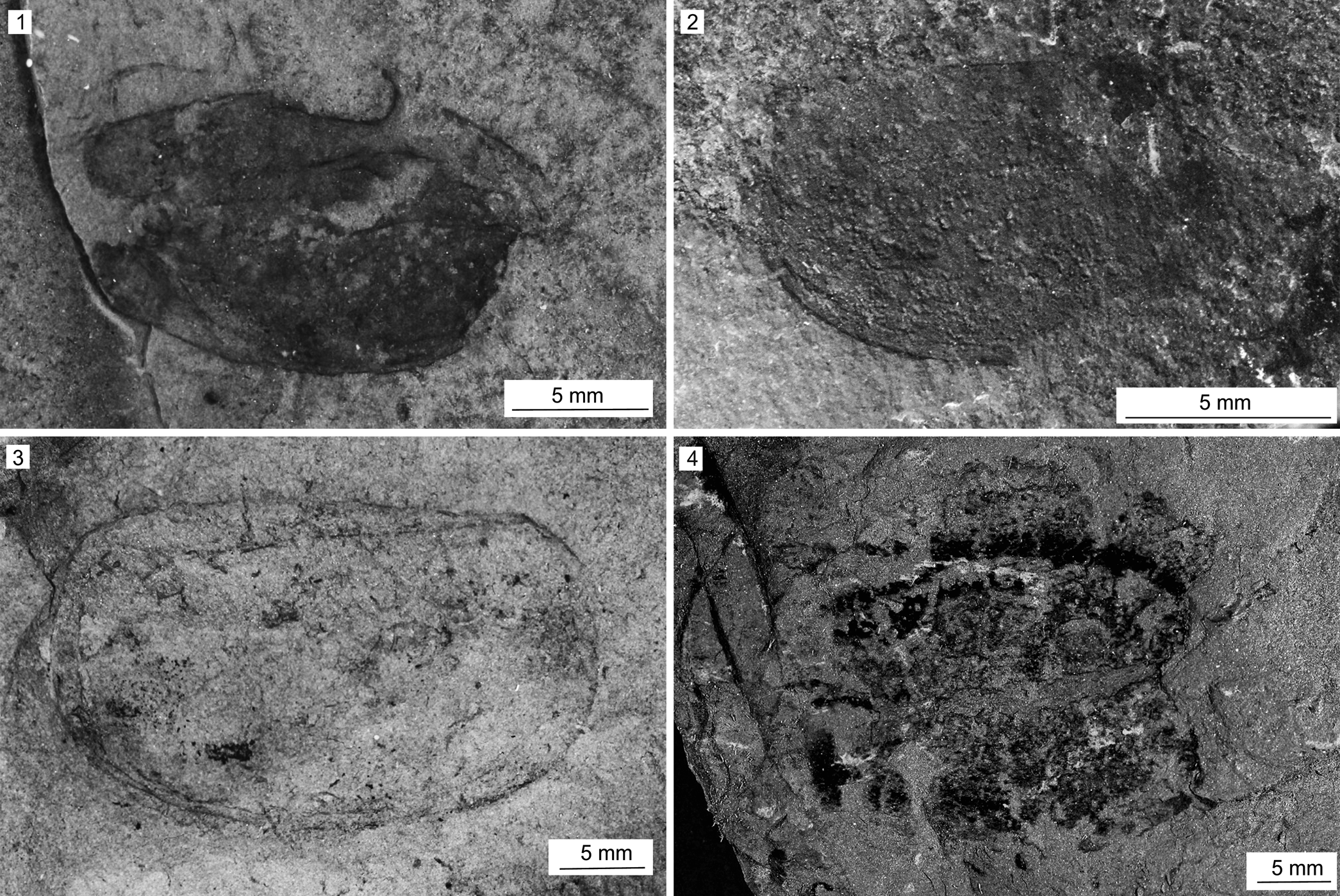
Figure 10. Dioxycaris argenta Walcott, Reference Walcott1886 from the Spence Shale Member, Langston Formation (Cambrian: Wuliuan), Idaho and Utah, USA: (1) KUMIP 314048, Antimony Canyon, collected by the Gunther family; (2) MCZ IP 164073 (part), Antimony Canyon, collected by Lloyd Gunther; (3) KUMIP 491904, Miners Hollow, collected by the Gunther family; (4) KUMIP 558518 (part), Spence Gulch, collected by Julien Kimmig.
Holotype
Probably right valve (USNM 15401) from the lower member of the Ophir Shale, Cottonwood Canyon, Utah, USA (Walcott, Reference Walcott1886, pl. 8, fig. 5).
Diagnosis
Carapace bivalved with hinge line. Valves subelliptical with slight posteroventral expansion; anterodorsal process of moderate size, posterodorsal process large; lateral surfaces and free margins lacking spines. (Robison and Richards, Reference Robison and Richards1981)
Occurrence
KUMIP 314048 and MCZ IP 164072, 164073 originate from the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Antimony Canyon locality (GPS: 41.561, -112.006), Wellsville Mountains, Box Elder County, Utah. KUMIP 491904 originates from the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Miners Hollow locality (GPS: 41.602, -112.033), Wellsville Mountains, Box Elder County, Utah. KUMIP 558518 originates from + 6 m from the base of the exposed Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Spence Gulch locality (GPS: 42.306, -111.515), Bear River Range, Bear Lake County, Idaho.
Description of new material
Valve subelliptical in outline; posterior portion moderately wider than anterior, with maximum width at approximately two-thirds along length of hinge line from anterior margin. Maximum length 25 mm, maximum width 11 mm; smallest 15 mm length, 10 mm width. Posterior process slightly larger than anterior process, exhibiting angle of ~110°. Anterior angle hinge line straight. Circular muscle scar inconspicuous in larger valves; located on one smaller specimen (Fig. 10.3) close to anterior margin and three times its diameter from hinge line.
New material
Two complete carapaces in dorsal view and two isolated valves in lateral view (KUMIP 314048, 491904, 558518, MCZ IP 164072, 164073).
Other material
Three isolated valves in lateral view (KUMIP 135145–135147).
Remarks
Dioxycaris argenta is the most common bivalved panarthropod in the Spence Shale, however the valves and carapaces seemingly have a tendency to decompose more rapidly than those of other bivalved panarthropods. Although this might have to do with varying taphonomic conditions in different Spence Shale outcrops (Whitaker et al., Reference Whitaker, Schiffbauer, Briggs, Leibach and Kimmig2022), it also suggests that the valves might have been thinner than those of other bivalved panarthropods. With the specimens described herein, there are now at least nine specimens known from the Spence Shale, five of them from Antimony Canyon. This value likely underestimates the relative abundance of this taxon, because many fragmentary specimens from the Spence Shale doubtless represent fragments of D. argenta.
Order Bradoriida Raymond, Reference Raymond1935
Family uncertain
Genus Walcottella Ulrich and Bassler, Reference Ulrich and Bassler1931
Type species
Walcottella apicalis Ulrich and Bassler, Reference Ulrich and Bassler1931 from the middle Cambrian of the Grand Canyon, Arizona, USA.
Walcottella? sp. indet.
Figure 11.1–11.4
Occurrence
KUMIP 579390 originates from + 5 m from the base of the exposed Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Spence Gulch locality (GPS: 42.306, -111.515), Bear River Range, Bear Lake County, Idaho. KUMIP 579391 originates from + 1 m from the base of the exposed Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Spence Gulch locality (GPS: 42.306, -111.515), Bear River Range, Bear Lake County, Idaho. YPM 530096 originates from the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Antimony Canyon locality (GPS: 41.563, -112.018), Wellsville Mountains, Box Elder County, Utah.
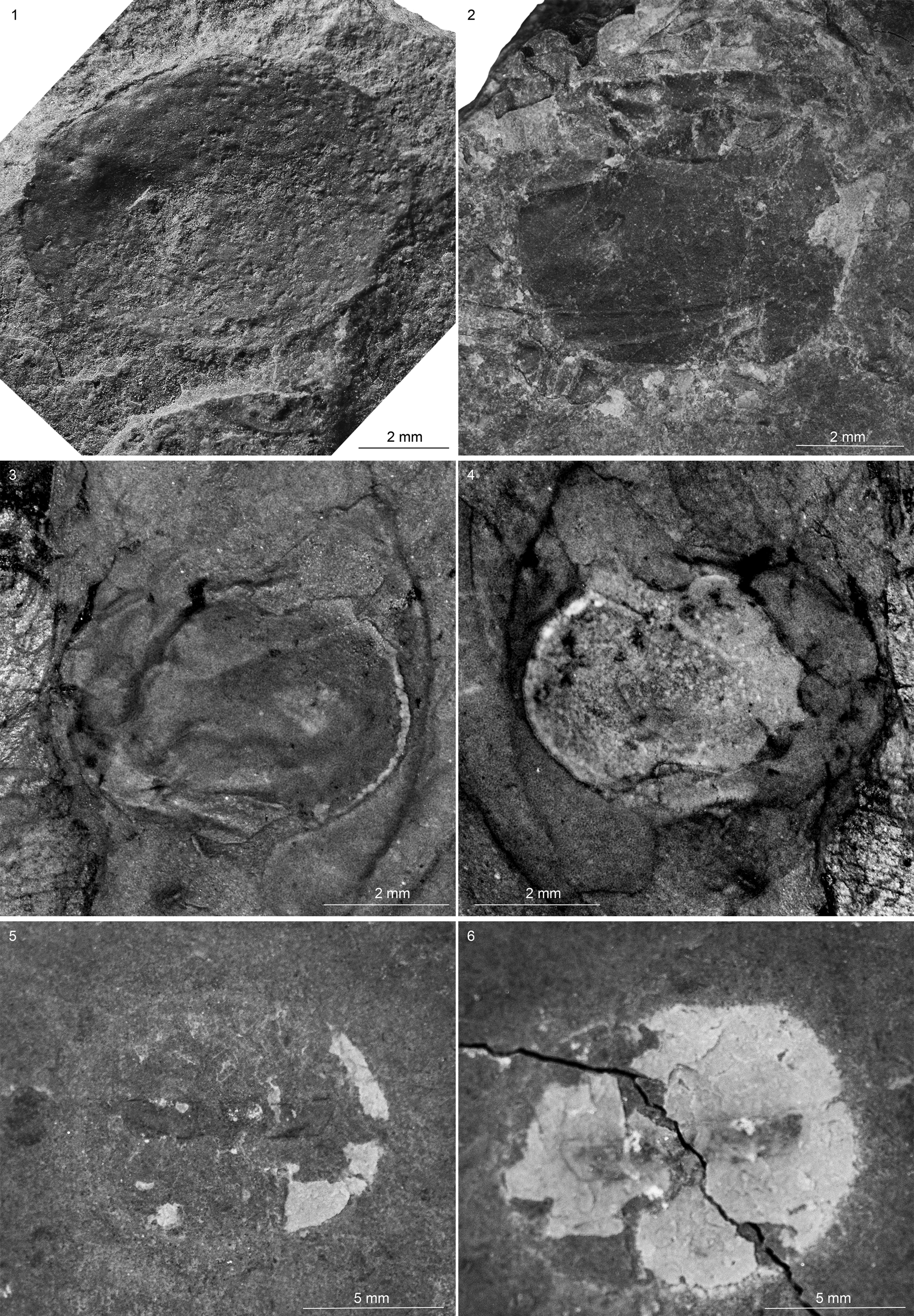
Figure 11. Bradoriids from the Spence Shale Member, Langston Formation (Cambrian: Wuliuan), Idaho and Utah, USA: (1) Walcottella? sp. indet. (YPM 530096), Antimony Canyon, collected by Lloyd Gunther; (2) Walcottella? sp. indet. (KUMIP 579390), Spence Gulch, collected by Rhiannon LaVine; (3, 4) Walcottella? sp. indet. (KUMIP 579391, part and counterpart), Spence Gulch, collected by Rhiannon LaVine; (5, 6) Bradoriida gen. indet. sp. indet. (KUMIP 585568, part and counterpart), High Creek, valve in lateral view, likely part of a coprolite, collected by Julien Kimmig.
Description
The valve is elongate-ovate in outline and a possible anterocentral node is present in KUMIP 579391 and YPM 530096 (Fig. 11). Faint ornamentation appears to be present on KUMIP 579390, a valve ~7 mm in length and 5 mm in width. Possible anterodorsal node or nodes are present. A narrow marginal ridge extends continuously from the posterior end of hinge to at least the intersection of the possible node(s). KUMIP 579391 is 8 mm in length and 5 mm in width.
YPM IP 530096: Carapace postplete; valve ~11 mm length, 8 mm width, ovate in outline. Hinge line well-developed, midanterior surface showing marked indentation, possibly indicative of single anterior lobe. Faint furrow demarcating a moderately wide margin.
Material
Three isolated valves (YPM 530096, and part and counterpart of KUMIP 579390, 579391).
Remarks
All three specimens are flattened and show signs of weathering. KUMIP 579390 and 579391 are partially obscured by matrix and overlapping nondescript skeletal elements. The valve outline is more symmetrical than that of Anabarochilina cf. A. australis Hinz-Schallreuter, Reference Hinz-Schallreuter1993, described from the Cambrian Marjum and Weeks formations of Utah, and Liangshanella burgessensis Sieveter and Williams, Reference Sieveter and Williams1997 from the Burgess Shale of Canada (Sieveter and Williams, Reference Sieveter and Williams1997). However, the outline and the position of the possible anterocentral node is similar to that of Walcottella apicalis Ulrich and Bassler, Reference Ulrich and Bassler1931 from the Cambrian (Wuliuan) Bright Angel Shale of Arizona. For these reasons, we tentatively assign these specimens to Walcottella. It is worth noting that the Bright Angel Shale specimens are preserved in three dimension and these specimens are flattened.
Bradoriida gen. indet. sp. indet.
Figure 11.5, 11.6
Occurrence
KUMIP 585568 originates 1 m from the top of the Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, High Creek locality (GPS: 41.896, -111.711), north of Logan, Wasatch Range, Cache County, Utah.
Description
Valve almost semicircular in outline, 10 mm long, 8 mm wide. Hinge probably short. Anterior process short; posterior process unknown. Narrow marginal ridge.
Material
Isolated valve with part and counterpart (KUMIP 585568).
Remarks
The valve and the trilobite were likely digested and are part of a coprolite or regurgitite that appears to have lost its soft parts. Coprolites are common in the Spence Shale (Kimmig and Strotz, Reference Kimmig and Strotz2017) and bradoriid shells have been identified in coprolites from other deposits (Kimmig and Pratt, Reference Kimmig and Pratt2018).
Subphylum Artiopoda Hou and Bergström, Reference Hou and Bergström1997
Superclass Trilobitomorpha Størmer, Reference Størmer1944
Order Nektaspida Raymond, Reference Raymond1920
Family Naraoiidae Walcott, Reference Walcott1912
Genus Naraoia? Walcott, Reference Walcott1912
Type species
Naraoia compacta Walcott, Reference Walcott1912, by original designation.
Other species
Naraoia spinifer Walcott, Reference Walcott1931; N. spinosa Zhang and Hou, Reference Zhang and Hou1985; N. bertensis Caron et al., Reference Caron, Rudkin and Milliken2004; N. taijiangensis Peng, Zhao, and Sun, Reference Peng, Zhao and Sun2012; N. magna Mayers et al., Reference Mayers, Aria and Caron2019; N. arcana Mayers et al., Reference Mayers, Aria and Caron2019.
Diagnosis
Naraoiid nektaspids with trunk length/total length ratio < 0.65; cephalic and trunk margins smooth or spinose. (Mayers et al., Reference Mayers, Aria and Caron2019)
Occurrence
Laurentia, China, and South Australia, lower and middle Cambrian, Series 2 and Miaolingian, Stage 3 to Drumian.
Naraoia? sp. indet.
Figure 12
Occurrence
Wuliuan Spence Shale Member (Glossopleura walcotti Biozone) of the Langston Formation, Two Mile Canyon locality (GPS: 42.166, -112.209), near Malad, Oneida County, Idaho.
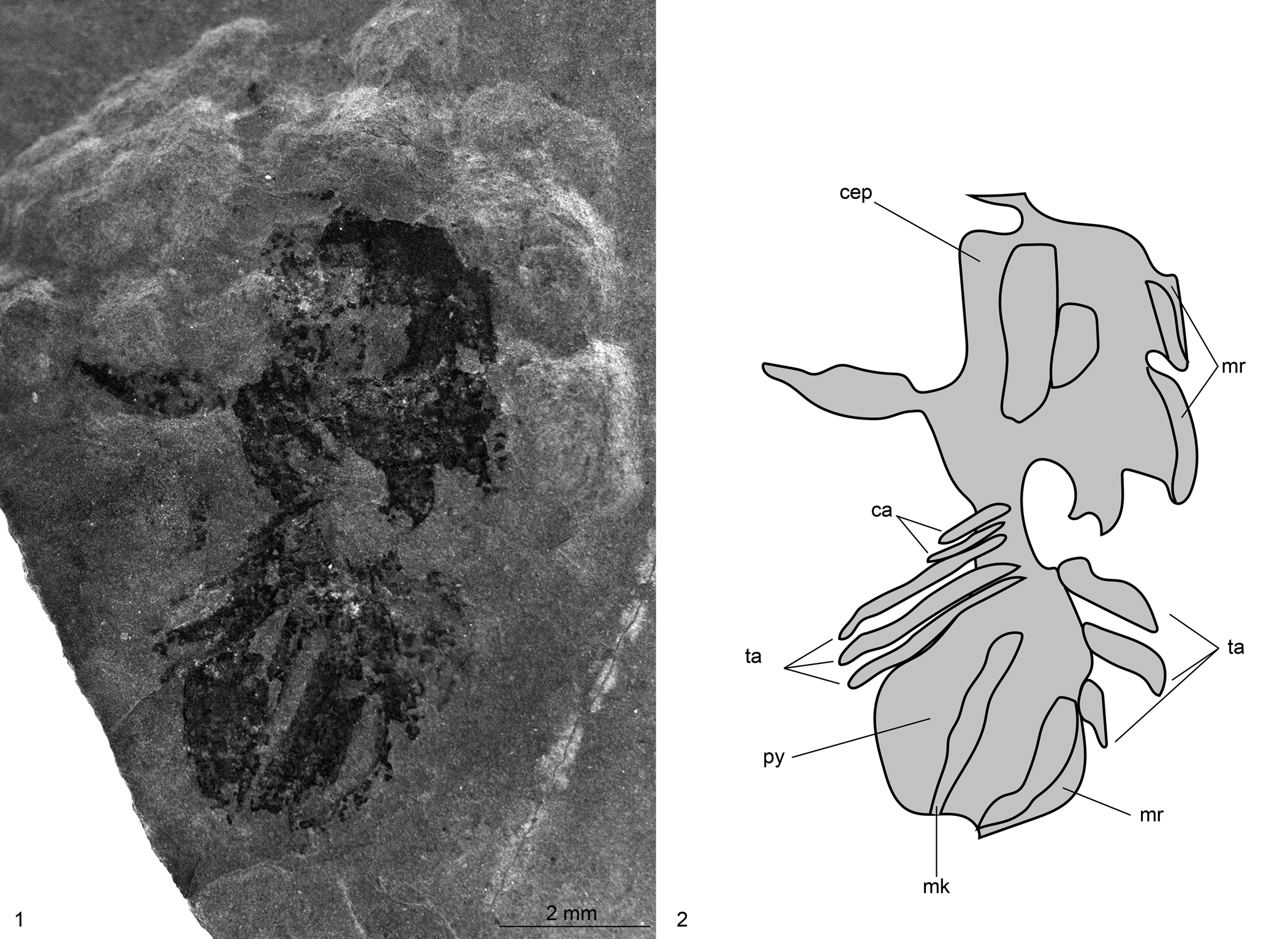
Figure 12. Naraoia? sp. indet. (IMNH 555/1725), collected by L.J. Krumenacker from the Two-Mile Canyon locality, Spence Shale Member, Langston Formation (Cambrian: Wuliuan), Idaho, USA: (1, 2) specimen in dorsal view and explanatory drawing. ca, cephalic appendages; cep, cephalon; mk, median keel; mr, marginal rim; py, pygidium; ta, thoracic appendages.
Description
Dorsoventrally compressed, 9.8 mm long (reconstructed pre-disarticulation), preserving cephalon and trunk (partly separated, with greatest degree of separation on right side of specimen). Cephalic shield ovoid in outline, 4.9 mm long, 3.9 mm at widest point 0.5 mm from posterior end. Left portion of cephalon broken off, separated from main body of cephalon (Fig. 12). Genal angles rounded, spineless. Narrow marginal rim preserved on sides of cephalon. One cephalic appendage pair preserved at posterior end of cephalon, partly overlapped by cephalic shield and oriented posteriorly. Partial left antennule appears to be preserved.
Trunk elongated, suboval in outline, 4.9 mm length, 3.8 mm at widest point 1.6 mm from anterior end. Median keel apparent and appears to run entire length of pygidium, 0.4 mm at widest point. Faint marginal rim, 0.2 mm wide, borderng pygidium.
Three appendage pairs apparent on each side of specimen with same morphology as preserved cephalic appendages; right side appears to preserve exopods in addition to endopods. These angled posteriorly, with those on left side more completely preserved. Longest limb, occurring most posteriorly on left side, 1.7 mm length, 0.2 mm at its widest point.
Material
One specimen in dorsal view (IMNH 555/1725).
Remarks
Preservation of this specimen is poor, and it appears to be a molt because the left part of the cephalon is partially broken off. The specimen preserves sufficient anatomical detail—including the subequally divided dorsal section, the single transverse articulation between the cephalon and the trunk, and the narrow marginal rim of the cephalon—that it can be attributed to Naraoia? sp. indet. The specimen is considered to likely belong to Naraoia also based on the dorsal carapace being divided into two subequal parts that are separated by a single transverse articulation.
Discussion
Panarthropods are by far the most diverse group of animals in the Spence Shale and the new material adds 10 species, bringing the total diversity to 67 species. This makes the Spence Shale the second most diverse panarthropod fauna of the Cambrian of Laurentia, only surpassed by the Burgess Shale of Canada. Unsurprisingly, the most abundant and diverse panarthropods in the Spence Shale are radiodonts and bivalved panarthropods, but the distribution of these two groups differs. So far, radiodonts are restricted to the shales of the Wellsville Mountains, whereas bivalved panarthropods are also found at the High Creek and Spence Gulch localities, in shallower water carbonates (Maxey, Reference Maxey1958; Liddell et al., Reference Liddell, Wright and Brett1997; Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b; unpublished data, Kimmig, Reference Kimmig, Elias and Alderton2021). This difference in distribution could have several explanations: (1) it might be that hurdiid radiodonts preferred deeper-water environments, an explanation indeed offered for their rarity in early Cambrian deposits (Wu et al., Reference Wu, Pates, Ma, Lin, Wu, Zahng and Fu2022); (2) there could be taphonomic factors that allowed radiodonts to be preserved in the Wellsville Mountains strata that were not present at other localities (Whitaker et al., Reference Whitaker, Schiffbauer, Briggs, Leibach and Kimmig2022); or (3) this could be due to collection biases, because the Wellsville Mountains localities have been extensively excavated, whereas the other localities have not been sampled to the same extent (Whitaker and Kimmig, Reference Whitaker and Kimmig2020). This difference in distribution also applies in general to the abundance of panarthropod and other species in the Spence Shale, because the Wellsville Mountain localities, especially Antimony Canyon and Miners Hollow, are the only known localities for many of the species described (Conway Morris et al., Reference Conway Morris, Selden, Gunther, Jamison and Robison2015; Legg and Pates, Reference Legg and Pates2017; Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b; Whitaker and Kimmig, Reference Whitaker and Kimmig2020).
The soft-bodied panarthropod fauna was previously thought to be limited to the Spence Shale in the Wellsville Mountains and the material presented herein demonstrates that this is definitely not the case. It is now apparent that soft-bodied panarthropods can be preserved in all of the environments present in the Spence Shale, from the shallower-water carbonates of High Creek and Spence Gulch, to the more distal deposits of Two Mile Canyon.
Conclusions
The Spence Shale preserves a unique panarthropod fauna in the Miaolingian of Laurentia, because radiodonts are the most abundant soft-bodied panarthropods, comprising approximately one-third of the identified specimens (Fig. 13). Most of these specimens are hurdiids. Additionally, the Spence Shale not only preserves arthropod species that are found in the Wuliuan-age Burgess Shale of Canada, i.e., Branchiocaris pretiosa, Dioxycaris argenta, Thelxiope cf. T. palaeothalassia, and Tuzoia retifera, but also species that are otherwise confined to the Drumian deposits of Laurentia, i.e., Buccaspinea cooperi Pates et al., Reference Pates, Lerosey-Aubril, Daley, Kier, Bonino and Ortega-Hernández2021a and Tuzoia guntheri.

Figure 13. Identified soft-bodied arthropods in the IMNH, KUMIP, MCZ, ROM, USNM, and YPM Spence Shale collections by specimen counts.
The new panarthropod specimens described herein also provide additional information on the distribution of soft-bodied taxa in the Spence Shale and suggest that other exposures beyond those currently sampled might also preserve soft-bodied fossils. For instance, most of the collecting in the Spence Shale has been done in the Wellsville Mountains (Whitaker and Kimmig, Reference Whitaker and Kimmig2020), and thus, the majority of soft-bodied fossils are known from this region (Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019b; Whitaker and Kimmig, Reference Whitaker and Kimmig2020). It might be that much of the diversity of the Spence Shale remains to be discovered, and species currently known from one or a few specimens—e.g., Armilimax Kimmig and Selden, Reference Kimmig and Selden2021, Shaihuludia Kimmig et al., Reference Kimmig, LaVine, Schiffbauer, Egenhoff, Shelton and Leibach2023, Siphusauctum O'Brien and Caron, Reference O'Brien and Caron2012 (see Kimmig et al., Reference Kimmig, Strotz and Lieberman2017), Utahscolex Whitaker et al., Reference Whitaker, Jamison, Schiffbauer and Kimmig2020, and Totiglobus Bell and Sprinkle, Reference Bell and Sprinkle1978 (see Wen et al., Reference Wen, Babcock, Peng and Robison2019b)—might be more abundant than previously thought.
Acknowledgments
We would like to thank J. Cundiff (MCZ), C. Green (MCZ), S. Losso (Harvard University), and S. Butts (YPM) for taking and providing pictures of Spence panarthropods in their collections. N. López Carranza (KUMIP) is thanked for accessioning material. We thank J. Ortega-Hernández (MCZ) for use of photography equipment. J. Cundiff (MCZ) provided curatorial support for the loan of radiodont specimens. We also thank the U.S. Department of Agriculture Forest Service for permits, and N. López Carranza and L. Schlenker (KUMIP) for assistance obtaining these, J. Young for access to his land, and J. Skabelund for access to his lease. L. Gunther and P. Reese are thanked for collecting and donating specimens. JK was supported by a Paleontological Society Arthur James Boucot Research Grant, a Western Interior Paleontological Society Karl Hirsch Memorial Grant, and an Association of Earth Science Clubs of Greater Kansas City Research Grant. AW was supported by an Association of Earth Science Clubs of Greater Kansas City Research Grant, a University of Kansas Biodiversity Institute Panorama Grant, a Geological Society of America Graduate Student Research Grant, and a Paleontological Society Kenneth E. and Annie Caster Student Research Award. SP was supported by a Herchel Smith Postdoctoral Fellowship. We thank D. Fu and an anonymous reviewer, associate editor T. Hegna, editor O. Vinn, and managing editor J. Kastigar for their comments and help that significantly improved the manuscript.
Declaration of competing interests
The authors declare that no competing interests exist.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.fn2z34v0h.


















