The metabolic syndrome is a combination of medical disorders that increase the risk of developing CVD and other chronic ailments. Recently, the number of affected individuals has been rapidly increasing worldwide, because of a shift towards dietary excess, lack of exercise and increasing stress, causing social problems. Visceral fat-type obesity is one underlying reason for the metabolic syndrome. Excessive visceral fat accumulation disrupts the production of adiponectin, plasminogen activator inhibitor type 1, TNF and NEFA, which induces insulin resistance linked with high blood glucose, high blood pressure and dyslipidaemia. To prevent the metabolic syndrome, it is important to improve lifestyle habits and maintain the balance of energy intake and consumption. One idea attracting increasing attention is the possibility of using specific food factors as supplements.
Lactoferrin (LF) is an Fe-binding glycoprotein which is found at highest concentrations in mammary breast milk. It is multi-functional, with anti-bacterial, antiviral, immunostimulatory, antioxidant and cancer-preventive potential(Reference Tomita, Bellamy and Takase1–Reference Sekine, Ushida and Kuhara5) and because LF is a natural component of breast milk which is ingested by infants, it is considered to be highly safe. Thus it has been approved as a food additive in Japan, and is included in the ‘generally recognized as safe’ (GRAS) category in the USA. Firstly, we focused on the anti-bacterial activity of LF, and found potent anti-pathogenesis activities against periodontal disease(Reference Suzuki, Kigawa, Ono, Thuda, Shimazaki and Tanaka6). In the course of conducting oral care research on LF, we also noted another highly interesting effect. Our results pointed to a new function – reducing the visceral fat that is the key cause of the metabolic syndrome – discovered through animal studies. There have been reports on the influence of LF on lipid metabolism. In the study of Takeuchi et al. (Reference Takeuchi, Shimizu and Ando7), bovine LF reduced plasma TAG and NEFA accompanied by decreases in hepatic cholesterol and TAG contents in rodents. Tamano et al. reported a significant decrease of serum TAG to 72 % of the control level(Reference Tamano, Sekine and Takase8). However, these reports were the results of animal experiments, and, to our knowledge there, has up till now been no human clinical trial aimed at determining the influence of LF on lipid metabolism. Furthermore, there has been no examination of the effects of LF on visceral fat accumulation. Therefore, we conducted the present investigation, focusing on lipid metabolism and visceral fat in a human clinical study. Since orally administered proteins are generally degraded by pepsin in the stomach, we used enteric-coated LF (eLF) tablets as the test food in the present evaluation of LF activity in a randomised double-blind placebo-controlled trial.
Experimental methods
Design and subjects
This trial was performed during the period of January 2008 to May 2008 with volunteers at the Moriyama Hospital in the Kanto District in Japan. The protocol was approved by the institutional board and the trial was conducted in accordance with the Helsinki Declaration under the supervision of clinical investigators. The subjects provided informed consent, including their permission for the findings to be published. Inclusion criteria were healthy Japanese men and women more than 20 years of age, with a BMI>25 kg/m2, and a visceral fat area>100 cm2, who were considered to be visceral fat-type obese, but had not been treated at an out-patient department and had no serious disease.
This was a randomised double-blind placebo-controlled trial, consisting of a 2-week run-in period and an 8-week treatment period. After the run-in period, the subjects were allocated to two groups designated as the eLF group (daily ingestion of three eLF tablets; 300 mg/d as bovine LF) and the control group (ingestion of three enteric-coated placebo tablets). Randomisation was stratified by age, sex, and visceral fat area measured at the time of the run-in period at hospital (control group, six men and seven women; eLF group, five men and eight women).
The test tablets that we used in the present study were eLF tablets, containing LF 100 mg/tablet, and control enteric-coated tablets, containing lactose instead of LF. Other constituents of each tablet were crystalline cellulose, carboxymethylcellulose-Ca, sucrose ester, silicon dioxide, shellac, sorbitol, arginine, dextrin and long pepper powder. In this formulation, LF molecules are protected from proteolytic digestion in the stomach, since the tablets are coated with an acid-resistant material, shellac, which dissolves easily under the neutral pH conditions in the intestine. The enteric-coated properties of this formulation were checked by the standard disintegration test to satisfy the criterion for the Japanese Pharmacopoeia.
The subjects consumed three tablets of the test material per d for 8 weeks. The time for ingestion of the test tablets was not limited, but it was recommended that the subjects take three test tablets after their evening meal/before sleep, to maintain compliance.
Energy and fat intake was not limited throughout the trial period, but supplemental food products or medications known to influence lipid or carbohydrate metabolism were prohibited. The subjects were instructed to maintain their usual dietary intake and physical activity.
The subjects visited the medical institution at 4-week intervals after the run-in period. Eating and drinking, except for water, were prohibited from 21.00 hours on the day before the visit until various measurements were completed.
Anthropometry, measurements of circulatory parameters, fasting blood sampling for biochemical and haematological parameters, and interviews were performed at − 2 (before treatment), 0, 4 and 8 weeks. Computed tomography (CT) was performed at 0 and 8 weeks to measure the abdominal fat area.
Anthropometric and vital sign measurements
Height (only at − 2 weeks), body weight, waist circumference and hip circumference were measured at each visit. BMI was calculated from the height and body weight. Waist and hip circumference, at the umbilical level and at the level of the greatest posterior protuberance (maximal gluteal circumference), respectively, were measured using a non-elastic anthropometric tape measure. Systolic blood pressure, diastolic blood pressure and pulse rate were assessed using an Hg manometer with subjects in a seated position after resting quietly for 10 min.
Evaluation of the abdominal fat level
The abdominal fat level, including total fat area, visceral fat area and subcutaneous fat area, were measured from CT images by Pronto-Xi/Si (Hitachi, Tokyo, Japan), using Fat Pointer software (version 2; Hitachi Medico Co., Tokyo, Japan), under X-ray conditions of a tube voltage of 120 kVp (peak voltage) and 100 mA(Reference Tokunaga, Matsuzawa and Ishikawa9). Several CT images around the umbilicus were obtained and single scan images at the precise point of the umbilicus were used for analysis.
Blood biochemical examination
The serum total cholesterol (cholesterol oxidase method(Reference Richmond10)), HDL-cholesterol (selective inhibition method(Reference Sugiuchi, Uji and Okabe11)), LDL-cholesterol (enzymic method(Reference Kanno, Sakurabayashi and Saito12)), TAG (enzymic method after eliminating endogenous free glycerol(Reference Eggstein and Kreutz13)), total lipid (sulfo-phospho-vanillin method(Reference Frings and Dunn14)), NEFA (enzymic method(Reference Okabe, Uji and Nagashima15)), total protein (biuret method(Reference Gornall, Bardawill and David16)), albumin (bromcresol green (BCG) method(Reference Doumas, Watson and Biggs17)), glutamic oxaloacetic transaminase (standard methods established by the Japan Society of Clinical Chemistry (JSCC)(18)), glutamic pyruvate transaminase (standard methods established by the JSCC(18)), lactate dehydrogenase (standard methods established by the JSCC(18)), alkaline phosphatase (standard methods established by the JSCC(18)), γ-glutamyl transferase (standard methods established by the JSCC(18)), total bilirubin (enzymic method(Reference Otsuji, Mizuno and Ito19)), direct bilirubin (enzymic method(Reference Otsuji, Mizuno and Ito19)), creatinine (enzymic method(Reference Tanganelli, Prencipe and Bassi20)), blood urea N (enzymic method(Reference Tabacco, Meiattini and Moda21)), uric acid (uricase–peroxidase method(Reference Gochman and Schmitz22)), creatine kinase (standard methods established by the JSCC(18)), C-reactive protein (immunonephelometry(Reference Férard, Goester and Klumpp23)), blood glucose (glucose oxidase–peroxidase method(Reference Sugiura and Hirano24)), glycosylated HbA1c (latex particle agglutination method(Reference John25)) and insulin (enzyme immunoassay (EIA) method(Reference Yoshioka, Taniguchi and Kawaguchi26)) were measured in fasting blood samples. Non-HDL-cholesterol was calculated from the value of total cholesterol and HDL-cholesterol. The albumin:globulin ratio was calculated from the value of total protein and albumin.
Dietary diary and daily living records
The subjects recorded the content of their meals in a dietary diary for 3 d before the visits at 0, 4 and 8 weeks. Based on the information in the diaries, dietitians analysed the daily energy intake, fat intake and fat:energy ratio, using Standard Tables of Food Composition in Japan, the 5th revised and enlarged edition(27), and mean values for the 3 d were calculated. In addition, the subjects recorded their compliance of the test tablet intake, and daily activities, including eating habits and exercise, measured by a passometer (Spalding cumulative passometer PS453; Tokyo Compass Mfg. Co. Ltd, Tokyo, Japan) every day from 0 to 8 weeks, in a daily living record using a simple checklist. The clinical investigators provided feedback of the daily living record to the subjects to encourage a constant level of daily activity. Physical conditions and adverse effects were examined by a physician in the interview at each visit.
Statistical analysis
Data presented for all test parameters are mean values and standard deviations. Results are expressed either in actual values or changes from 0 to 4 weeks (Δvalue at week 4) or 0 to 8 weeks (Δvalue at week 8). To compare the week 0 values for the two groups, an unpaired t test (two-sided) was employed. An intergroup comparison by repeated-measures ANOVA was performed using actual values from week 0 to week 8. Post hoc analysis was conducted by ANCOVA, the Dunnett test, or the paired t test. The statistically significant level was set at P < 0·05. The data were analysed using JMP (version 5.0.1a; SAS Institute Inc., Cary, NC, USA).
Results
A total of thirty subjects volunteered to participate in the study. Of the subjects, two withdrew agreement and were excluded from the original thirty subjects enrolled before the release of the double-blinding. In addition, two subjects (one in the control group and one in the eLF group) were discontinued because of job relocation or pressure of work. Data were analysed using the per-protocol samples of twenty-six subjects (control group, six men and seven women; eLF group, five men and eight women). The flow of participants in the trial is shown in Fig. 1. The baseline characteristics of the study subjects did not differ significantly between the groups (Table 1). Compliance of test tablet intake in the eLF group and the control group was 98·0 and 96·7 %, respectively.

Fig. 1 Flow of participants in a study investigating the effect of enteric-coated lactoferrin (eLF) on body weight and abdominal fat area level in Japanese men and women.
Table 1 The baseline (week 0) characteristics of the study subjects
(Mean values and standard deviations)

eLF, enteric-coated lactoferrin.
* Except for sex, t test between groups.
† Square test between groups.
Table 2 shows the daily energy, protein, carbohydrate and fat intakes. No significant differences were found between the two groups. Daily living records indicated that exercise levels were maintained at a constant level during the study.
Table 2 Daily energy, protein, carbohydrate and fat intakes
(Mean values and standard deviations)

eLF, enteric-coated lactoferrin.
* Repeated-measures ANOVA.
Table 3 shows changes in anthropometric parameters and circulatory parameters. Body weight (P < 0·05 at week 4, P < 0·01 at week 8), BMI (P < 0·05 at week 4, P < 0·01 at week 8), waist circumference (P < 0·01 at week 8) and hip circumference (P < 0·05 at week 8) decreased significantly in the eLF group by the Dunnett test as compared with week 0. Body weight (P = 0·037 at week 4, P = 0·013 at week 8), BMI (P = 0·041 at week 8) and hip circumference (P = 0·032 at week 8) were statistically different between the eLF and control groups as analysed by ANCOVA. There was also a tendency for a greater reduction in waist circumference (P = 0·073 at week 8) in the eLF group than with the placebo. No significant differences in circulatory parameters were found between the groups.
Table 3 Changes in anthropometric and circulatory parameters after taking enteric-coated lactoferrin (eLF) or control tablets for 8 weeks
(Mean values and standard deviations for thirteen subjects per group)

Mean value was significantly different from that at baseline (week 0): * P < 0·05, ** P < 0·01 (Dunett's test).
† The value is the change from week 0 to week 4.
‡ The value is the change from week 0 to week 8.
§ Repeated-measures ANOVA.
∥ ANCOVA.
Table 4 shows changes in abdominal fat areas. Visceral fat area and total fat area decreased significantly over time (P < 0·01 at week 8; paired t test) in the eLF group. The decreases in visceral fat area, subcutaneous fat area and total fat area at week 8 from baseline were − 14·6, − 13·4 and − 28·0 cm in the eLF group, and − 1·8, − 9·9 and − 11·7 cm in the control group, respectively. A significant difference between the eLF and the control visceral fat area was found at week 8 (P = 0·0089), as analysed by ANCOVA.
Table 4 Abdominal fat area after taking enteric-coated lactoferrin (eLF) or control tablets for 8 weeks
(Mean values and standard deviations for thirteen subjects per group)

** Mean value was significantly different from that at baseline (week 0) (P < 0·01; paired t test).
† The value is the change from week 0 to week 8.
‡ Repeated-measures ANOVA.
§ ANCOVA.
Tables 5 and 6 show changes in blood lipid parameters and biochemical parameters. No significant differences were found between the two groups. No adverse effects of eLF were apparent.
Table 5 Changes in serum lipid parameters after taking enteric-coated lactoferrin (eLF) or control tablets for 8 weeks
(Mean values and standard deviations for thirteen subjects per group)

* Repeated-measures ANOVA.
Table 6 Changes in biochemical parameters after taking enteric-coated lactoferrin (eLF) or control tablets for 8 weeks
(Mean values and standard deviations for thirteen subjects per group)
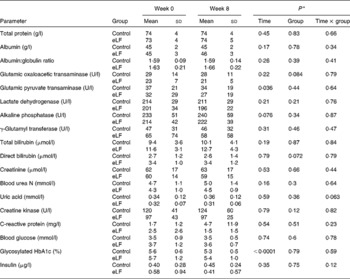
* Repeated-measures ANOVA.
Discussion
The present investigation of effects of eLF tablets (as 300 mg LF/d for 8 weeks) on visceral fat accumulation in Japanese men and women with abdominal obesity demonstrated a significant benefit as compared with the placebo regarding the visceral fat area and a tendency for greater improvement in anthropometric data.
Major sources of exogenous LF in our daily diet are dairy products from bovine milk. LF contents in bovine colostrum and normal milk are 1000 and 20–350 μg/ml, respectively. During the milk pasteurisation process, LF is inactivated by heating, but unpasteurised dairy products, such as natural cheese, contain approximately 300 mg LF per 100 g. Therefore, during the treatment period, the daily intake of LF for the eLF group was almost the same as approximately 100 g of natural cheese per d(Reference Shimazaki and Yoshimoto28), although LF in natural cheese naturally will be degraded in the stomach. Under our conditions, 8 weeks of oral administration of eLF tablets significantly reduced the accumulation of visceral fat, compared with control, and total fat area, hip circumference, body weight and BMI were also significantly decreased by eLF. No adverse events were observed with regard to safety parameters. In addition to its known anti-bacterial, anti-virus, immunostimulatory, antioxidant and cancer-preventive properties(Reference Tomita, Bellamy and Takase1–Reference Sekine, Ushida and Kuhara5), the present results thus point to a novel functional significance of eLF in reducing visceral fat.
Recently, Moreno-Navarrete et al. (Reference Moreno-Navarrete, Ortega and Bassols29, Reference Moreno-Navarrete, Ortega and Bassols30) reported the circulating LF concentration to be inversely associated with BMI, the waist:hip ratio and the fasting TAG and glucose concentrations, and positively with insulin sensitivity. They speculated that the preservation of LF production leads to decreased free lipopolysaccharide concentration and maintenance of an adequate lipid profile(Reference Moreno-Navarrete, Ortega and Bassols29, Reference Moreno-Navarrete, Ortega and Bassols30). Metabolic endotoxaemia initiates obesity and insulin resistance(Reference Cani, Amar and Iglesias31), and LF is known to bind to and inactivate lipopolysaccharide(Reference Wakabayashi, Takase and Tomita32). Although, in the present study, we administered bovine LF extrinsically, there is a possibility that circulating LF could rise with oral administration of eLF, and neutralise the action of lipopolysaccharide. Takeuchi et al. described LF to be transported into the blood circulation from the intestine via the lymphatic pathway in adult rats(Reference Takeuchi, Kitagawa and Harada33) and Fischer et al. detected immunoreactive LF in the serum, liver, kidneys, gall bladder, spleen and brain of mice after administration by gastric intubation(Reference Fischer, Debbabi and Blais34). These reports support our hypothesis described above.
With regard to another possible mechanism underlying the effects of eLF on visceral fat, the anti-adipogenic action of LF should be considered. Two studies have demonstrated such activity by LF on pre-adipocytes. Yagi et al. (Reference Yagi, Suzuki and Takayama35) and Moreno-Navarrete et al. (Reference Moreno-Navarrete, Ortega and Ricart36) reported that LF inhibits adipogenic differentiation of the MC3T3-G2/PA6 cell lines and the 3T3-L1 cell line(Reference Yagi, Suzuki and Takayama35, Reference Moreno-Navarrete, Ortega and Ricart36). We also confirmed those actions of LF against pre-adipocytes isolated from rat mesenteric fat using a primary culture system. Moreover, we proved that trypsin-degraded LF retained this activity, whereas pepsin-degraded LF did not, suggesting an important role for enteric coating in enabling the LF to bypass the stomach's digestive action in order to exert its anti-adipogenic action (T Ono, S Morishita, C Fujisaki, M Ohdera, M Murakoshi, N Iida, H Kato, K Miyashita, M Iigo, T Yoshida, K Sugiyama and H Nishino, unpublished results).
LRP1, a known LF receptor, may be a key factor for the anti-adipogenic action of LF. Dietary lipids are carried in chylomicron remnants which are taken up into the liver mainly via LRP1. Crawford & Borensztajn reported that LF inhibits the plasma clearance of chylomicrons in the mouse(Reference Crawford and Borensztajn37). Moreover, Hofmann et al. reported that LRP1 is expressed in visceral fat and modulates postprandial lipid transport and glucose homeostasis in mice(Reference Hofmann, Zhou and Perez-Tilve38). These findings suggest that LF may bind LRP1 to block incorporation of lipid in the visceral fat. Further experimentation should be conducted to validate these hypotheses, as well as to determine the LF distribution after administration of eLF.
In summary, this trial clarified that the ingestion of eLF for an 8-week period can reduce visceral fat in men and women without the need for any lifestyle change. Additional analysis, with larger sample sizes, of this potential to prevent obesity and decrease risk of the metabolic syndrome is clearly warranted.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors' contributions were as follows: M. M. and N. S. designed the present study and performed the laboratory analysis; N. I., M. O., M. I., T. Y., K. S. and H. N. provided advice for the design; T. O. and N. S. contributed to interpretation of the data and statistical analysis; M. M. helped T. O. to write the manuscript. All authors read and approved the final version of the manuscript.
The authors have no conflict of interest associated with the present study.









