Introduction
Pertussis is a worldwide, cyclic acute respiratory infection caused by Bordetella pertussis. Prior to widespread coverage of the pertussis vaccine (PV), pertussis was the primary cause of death among infants and young children [1]. The PV resulted in a marked decrease in the morbidity and mortality rates since the 1950s [1]. However, despite widespread implementation of these different immunisation programmes and associated levels of uptake, pertussis continues to persist. Since the 1980s, there has been increased incidence and shifts in age-specific incidence towards a higher representation of adolescents and adults have been reported in many developed countries with high vaccine coverage, which has been labelled so-called ‘re-emergence of pertussis’. Available evidence indicates that there is a more rapid waning of immunity, and possibly a reduced impact on transmission, with acellular pertussis vaccine (APV) relative to whole-cell pertussis vaccine (WPV) [1–Reference Tartof3].
To further control pertussis, the International Consensus Group on Pertussis Immunization [Reference Campinsmartí4] and the Global Pertussis Initiative [Reference Forsyth5] have both recommended a universal adolescent booster vaccination combined with targeted immunisation of those adults most likely to have contact with babies, including parents, close family members, healthcare workers and day-care workers; universal immunisation of young adults and routine use of tetanus–diphtheria–pertussis vaccine (Tdap) rather than tetanus toxoid and diphtheria vaccine (Td) for all adult booster immunisations. In Canada, Germany, Belgium and the United States, two booster shots of APV products are recommended for 4–7-year-old children and 11–17-year-old adolescents after four doses of routine vaccination [Reference Plotkin6]. In Australia, a cocoon programme offering parents of new babies a government-funded dose of a pertussis-containing vaccine was implemented [Reference Rowe7]. In the United States, the United Kingdom, Switzerland and Australia, a maternal vaccination programme was offered to prevent cases in young infants [Reference Areta8].
In China, pertussis vaccination prepared from WPV was available to children as part of the publicly funded National Immunization Programme from 1978 through 2007, protecting infants who were 2 months of age or older. Due to safety and effectiveness, APV replaced the whole-cell product in 2008. Vaccination against pertussis was recommended at 3, 4 and 5 months, and between 18 and 24 months of age. The vaccination coverage survey conducted in 2008 showed that in children aged 1–2 in China, 99.10% had an immunisation certificate, and 99.38% had an immunisation card, with an overall vaccination coverage of 99.42% [Reference Cao9].
The incidence of pertussis in China decreased from between 100 and 200 cases per 100 000 population during the pre-vaccination era of the 1960s and 1970s [Reference Li10] to a historic low of less than one case per 100 000 population from 2004 to 2016 [Reference Ning11–Reference Yin13]. In China, pertussis cases were usually identified via passive hospital reports. Although two types of diagnostic criteria [14, 15] were used in 1995–2006 and 2007–2016, respectively, there were only minor changes to surveillance methods in China during these time periods. Most cases were clinically diagnosed and laboratory methods were not routinely used in hospitals [Reference Huang16].
As the capital city of Shandong province in the east of China, Jinan achieved the goal of immunisation coverage among 85% of targeted children at county and township levels in 1991 and 1996, respectively. From then on, PV coverage in Jinan has consistently been greater than 95% (CDC, unpublished data). According to the national immunisation schedule, WPV was introduced into the Expanded Programme on Immunisation (EPI) as a government-purchased vaccine (category 1 vaccine) from 1978 to 2009 in Jinan. Category 1 vaccines refer to free vaccines that are mandatory for children in China to be vaccinated in accordance with the government's EPI. Between 2002 and 2007, APV was introduced as a category 2 vaccine that refers to vaccines that are not for routine use, are optional for residents and must be privately bought in China. In 2008, APV became a category 1 vaccine, making it free for the fourth dose, and WPV was still in use for prior doses one to three. In 2009, WPV was free for the first dose and APV for the following two to four doses. From 2010 to the present time, only APV is available. Before the PVs were introduced from 1956 through to 1977, the mean reported incidence of pertussis in Jinan was 67.61 cases per 100 000 population, with a peak value of 303.93 in 1963. During the vaccination era from 1978 to 2016, the incidence decreased from 14.84 cases per 100 000 population to a historic low of 0.02 per 100 000 in 2003, increased to 3.08 in 2014, then continued to increase to 4.74 in 2015 and 6.85 in 2016.
The reasons for the re-emergence of pertussis in Jinan from 2014 through 2016 are not well understood. In previous studies, protection effectiveness of WPV and APV was mostly calculated using case–control studies and field trials of mass vaccination [Reference Tartof3, Reference Cherry17–Reference Stehr22]. In China, there were only case–control studies of immunogenicity and immune persistence of APV with small sample sizes. Therefore, further research on the pertussis vaccination programme in Jinan, China is necessary. In this study, we used a long-term macroscopic evaluation to examine the impact of PV schedule changes. Interrupted time series (ITS) design and segmented regression analysis were utilised to analyse PV immunisation strategies based on the trends of pertussis incidence rates from 1956 through to 2016 in Jinan.
Methods
Study design
As a large-scale intervention, it is difficult to evaluate the effect of a mass vaccination intervention by a randomised controlled trial on the population level without randomisation or any control. We conducted a longitudinal study of the reported pertussis incident between 1956 and 2016 with a single group of ITS design for evaluating the impacts before and after mass vaccination interventions.
Data source
This study was conducted using the data of annually reported pertussis incidence in Jinan. The data of reported cases and permanent population in 1956–2003 was collected from the ‘Annual Report on the Incidence of Infectious Diseases in Jinan City'. Data from 2004–2016 came from the ‘Infectious Disease Reporting Information Management System’, one subsystem of China's ‘Information System for Disease Control and Prevention’.
Case definition
The cases were all hospital reported patients living in Jinan City for more than 3 months. In China, two types of diagnostic criteria were used in 1995–2006 and 2007–2016, respectively. In 1995 [14], the clinical case definition required one of the symptoms to be either a cough for at least 14 days, a paroxysmal spasmodic cough, a cough with vomiting, unknown paroxysmal asphyxia in infants or on the basis of an epidemiologic link. This confirmation could be based on laboratory results, for example, a positive culture for B. pertussis or a significant fourfold increase in a specific serum antibody. In 2007 [15], a suspected case was confirmed with an acute cough lasting more than 14 days, paroxysms of coughing, inspiratory ‘whoop’, posttussive vomiting or direct contact (epidemiology linkage). A clinical case was defined by a suspected case with a significantly increasing calculation of leucocyte and lymphocyte in peripheral blood. The laboratory-confirmed case was confirmed as a clinical case with isolation of B. pertussis from phlegm or nasopharyngeal secretion, or the serum specific antibody of the convalescence period was four times higher than the acute phase.
Outcome variable
Y t was the main outcome variable that corresponds to the annual incidence rates of hospital reported pertussis, including suspected, clinical and laboratory-confirmed cases. Annual pertussis incidences were calculated at each time point by the number of cases per year obtained from the resident population, divided by the resident population in the year, then multiplied by 100 000 inhabitants.
Intervention definitions
According to the immunisation schedules in Jinan, the history of intervention can be subdivided into seven stages from 1956 to 2016. Stage A was the pre-vaccination period from 1956 to 1977. Stage B was the timeframe of which the WPV vaccination programme was introduced. Annual average coverage during this period was 67.61%. Stage C was a WPV vaccination period between 1991 and 2001, with an average coverage of more than 90%. Stage D, between 2002 and 2007, was the WPV vaccination period, with the introduction of APV as a category 2 vaccine. In stage E, APV was introduced as a category 1 vaccine for the fourth dose in 2008. In stage F, APV replaced WPV for doses 2 through to 4 as the category 1 vaccine in 2009. In stage G, APV replaced all four doses of WPV from 2010 to 2016 (Table 1).
Table 1. The incidences, coverages and immunisation strategies of pertussis in Jinan, China, 1956–2016

* P value of χ2 test: the average of incidence compared with the next period.
Vaccination interventions were identified into five statuses according to the shifts of immunisation strategy including (1) Intervention 1: stage A (1956–1977) was the pre-intervention area and stages B and C (1978–2001) were considered as the WPV intervention stage. We regarded the year 1978 as the PV intervention time point. (2) Intervention 2: stage B (1991–2001) was considered as the WPV-intervention period with coverage of more than 90%. Stage C (2002–2007) was set as the APV (self-funded vaccine and low coverage) vaccination intervention stage. Year 2002 was regarded as the beginning of APV vaccination intervention. (3) Intervention 3: stages C and D were considered as the pre-intervention period with WPV vaccination. Stages E–G were reckoned as the APV vaccination intervention era. Year 2008 was regarded as the beginning of APV vaccination intervention for the replacement of APV for the fourth dose of WPV. (4) Intervention 4: stages C–E (1991–2008) were considered the pre-intervention period of the WPV vaccination and stages F and G (2009–2016) as the APV vaccination intervention stage. Year 2009 was regarded as the beginning of APV vaccination intervention for the replacement of APV for the second−fourth dose of WPV. (5) Intervention 5: stages C–F (1991–2009) were considered as the pre-intervention period of the WPV vaccination and stage G as the APV vaccination intervention stage. Year 2010 was regarded as the beginning of all four doses of APV vaccination intervention (Table 1).
Statistical analysis
The segmented regression model is most commonly used to assess ITS data. It is fitted through to a least squares regression line to each segment of the independent variable and time, thus assuming a linear relationship between time and the outcome within each segment [Reference van Seben23, Reference Taljaard24]. We used segmented regression analysis to assess the significance of changes in levels and trends of reported pertussis incidence before and after the vaccination interventions, including implementation of PV and alternative strategies. The level is the value of the series at the beginning of a given time interval (i.e. the y-intercept for the first segment and the value immediately following each change point at which successive segments join). A change in level constitutes an abrupt intervention effect. The trend is the rate of change of a measure (in other words, the slope) during a segment. A change in trend is defined by an increase or decrease in the slope of the segment after the intervention as compared with the segment preceding the intervention. The regression model was set as follows:

where Y t is the reported pertussis rate per year. Year was a continuous variable indicating time in years from the beginning of the observation period at time (t). We used log 10-transformation first, then used a moving average to pre-process the raw incident data, which can be seen in Figure 1. This was completed to remove the cycles and better capture the trends. Then we analysed using interrupted time-series analysis [Reference Rohani25]. Intervention was an indicator variable for time (t) occurring either before (intervention = 0) or after (intervention = 1) the mass vaccination. Postslope was a continuous variable counting the number of years at time (t) after the intervention, which was equal to Year × Intervention. β0 estimates the baseline level of the outcome at time zero. β1 estimates the overall linear trend of yearly change in the pertussis rate. β2 estimates the change in level in the rate immediately after the intervention from the end of the preceding stage. β3 estimates the long-term trend change in the outcome after the intervention, compared with the trend before the intervention. ɛ t is the error term at time (t), representing the random variability not explained by the model. The Durbin–Watson (DW) method was applied to test the stability of time series data.
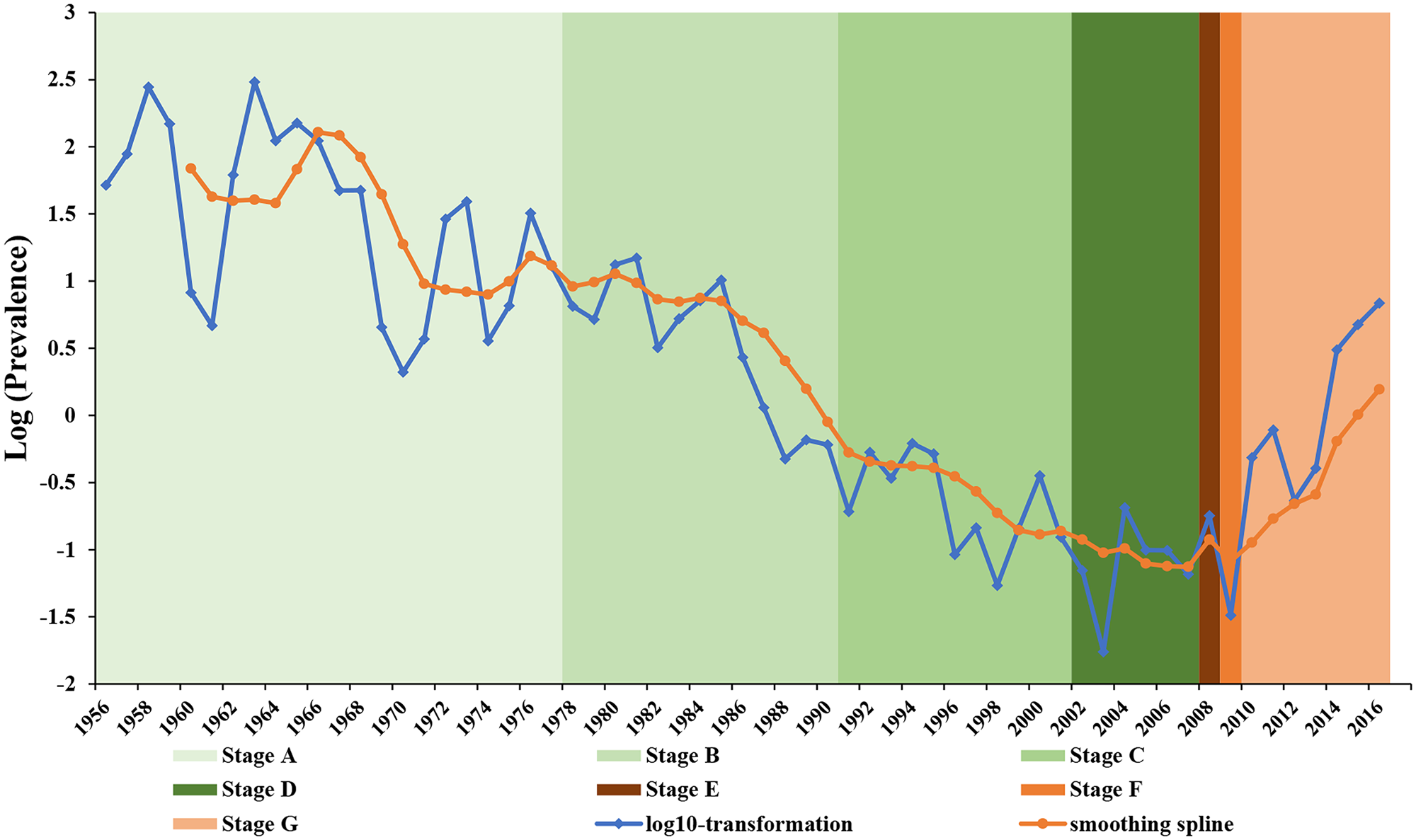
Fig. 1. Pertussis reported incidence rates in Jinan, 1956–2016. Blue line depicts the trend in log-transformed per capita pertussis incidences in Jinan, China from 1956 to 2016. Orange line depicts smoothing spline in backward moving average with five periods. Seven colour shaded regions in the figure present stages A–G with different immunisation schedules consistent with Table 1.
To describe how the immunisation strategies affect the all-age pertussis incidence, we analysed the incidences of birth cohorts from 2005 to 2016 (Table 2). All statistical analyses were conducted using SAS statistical software (ver 9.3). The statistical significance level was set at 0.05.
Table 2. The pertussis incidences of birth cohorts from 2005 to 2016 (per 100 000)

Results
In the pre-vaccination era, the reported incidence of pertussis ranged between 2.10 and 303.93 cases per 100 000 population during 1956–1977, with an average incidence of 67.61 cases per 100 000, which reduced to an average value of 4.80 per 100 000 population with a range between 0.47 and 14.84 during 1978–1990, reduced to 0.27 (0.05–0.62) in 1991–2001 and 0.11 (0.02–0.21) in 2002–2007. In the period of the shift from WPV to APV, the incidence was 0.18 in 2008 and 0.03 in 2009, respectively. In the APV vaccination intervention stage (2010–2016), the mean value of incidence raised to 2.58 (0.23–6.85). There were statistically significant differences between the average incidence of one policy period and the subsequent period, except for the difference between 2002–2007 and 2008. Table 1 shows the average coverage rose from 75.71% of three doses of PV during 1978–1990, to 90.52% during 1991–2001 and reached 99.53% in 2010–2016.
Figure 2 shows the distribution of annual pertussis cases by five different age groups (under 1 year, 1–2, 3–6, 7–14 and 15–59 years). From 2005 to 2016, the age distribution of cases remained relatively stable with between 83.33% and 100% of all cases attributed to infants younger than 6-year-old, especially the cases under 1 year. In the APV vaccination intervention stage (2010–2016), the percentage of cases under 1 year dropped from 86.67% in 2010 to 28.83% in 2016, with increasing proportions of cases in those aged 1–6 years from 13.33% in 2010 to 56.24% in 2016. Cases among those aged 7–14 and 15–59 years had been reported since 2014, with the cumulative percentage of cases in both age groups ranging from 8.74% to 14.93% (Fig. 2).

Fig. 2. Age distribution of annual pertussis cases in Jinan, 2005–2016.
Intervention 1: WPV vaccination as the intervention in 1978–2001
As presented in Table 3, the segmented regression analysis results indicate that before the vaccination intervention era, the trend of reported incidence of pertussis declined by 1.10 cases per 100 000 population every year (P = 0.049). The significant declining trends existed in every model by approximately one case per 100 000 population. When WPV was introduced in 1978, there was a slight decline in the incidence of 1.11 cases per 100 000 population (P = 0.0743) immediately. This statistically significant trend continued to 2001 when incidence decreased by 1.21 cases per 100 000 population per year (P < 0.0001) (Table 3 and Fig. 3a).
Table 3. Estimated level and trend changes of reported pertussis incidence before and after PV vaccination inventions

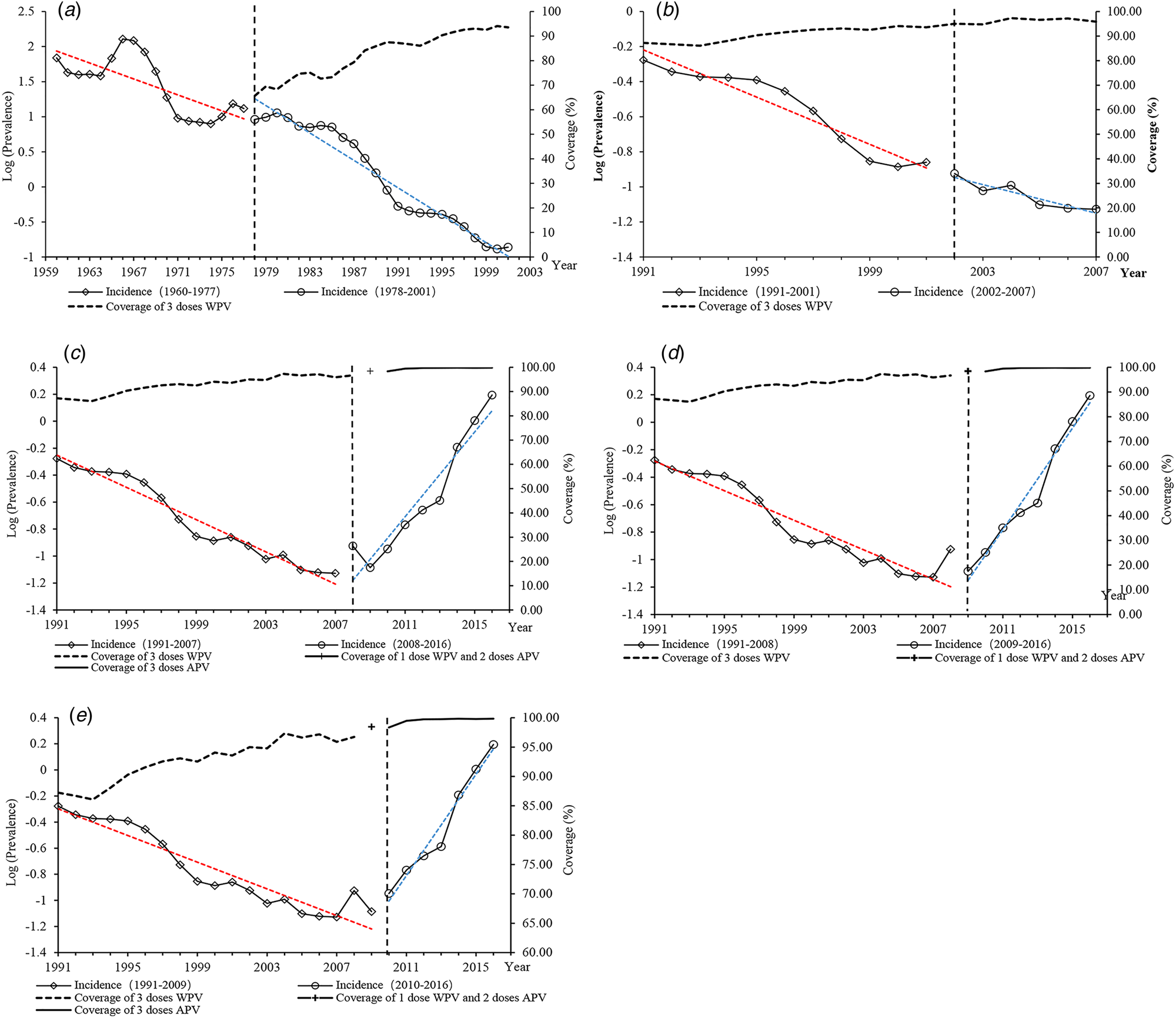
Fig. 3. ITS scatter plot of the annual incidence rate of pertussis. (a) Left: A pre-vaccination period in 1960–1977; right: vaccination period with WPV for 1–4 doses in 1978–2001. (b) Left: WPV vaccination period in 1991–2001; right: WPV vaccination period and APV as the second category vaccine in 2002–2007. (c) Left: WPV vaccination period in 1991–2007; right: APV vaccination period in 2008–2016 and APV replaced the fourth dose of WPV in 2008. (d) Left: WPV vaccination period in 1991–2008; right: APV vaccination period in 2009–2016 and APV replaced the 2–4 dose of WPV in 2009. (e) Left: WPV vaccination period in 1991–2009; right: all-APV vaccination period in 2010–2016.
Intervention 2: the introduction of APV (as the category 2 vaccine) as the intervention in 2002–2007
In 2002–2007, APV was introduced as the category 2 vaccine. In 2002, there was a slight decrease in the level of incidence of 1.06 cases per 100 000 population, and an increase in trend in the incidence of 1.06 cases per 100 000 population from 2002 through 2007. The result indicated that there was no significant change in level (P = 0.703) and trend (P = 0.267) after the intervention (Table. 3 and Fig. 3b).
Intervention 3: APV vaccination (WPV for 1–3 doses and APV for fourth dose in 2008) as the intervention in 2008–2016
In 2008, APV was introduced as the category 1 vaccine for the fourth dose. There was an immediate decline in the incidence of 1.98 cases per 100 000 population and there was no significance (P = 0.774). However, there was a significant increasing trend from 2008 through 2016 of 1.63 cases per 100 000 population per year (P < 0.0001) (Table 3 and Fig. 3c).
Intervention 4: APV vaccination (WPV for 1 dose and APV for dose 2–4 in 2009) as the intervention in 2009–2016
In 2009, when APV replaced WPV for the doses 2–4, the incidence declined immediately by 1.98 cases per 100 000 population (P = 0.773). However, the trend increased by 1.77 cases per 100 000 population per year and was significant (P < 0.0001) from 2009 to 2016 (Table 3 and Fig. 3d).
Intervention 5: APV vaccination as the intervention in 2010–2016
When APV replaced WPV for doses 1–4 in 2010, there was an immediate decrease in the incidence of 1.08 cases per 100 000 population (P = 0.733). There was a significant increasing trend in the incidence by 1.78 cases per year from 2010 through 2016 (P < 0.0001) (Table 3 and Fig. 3e).
The incidence in the birth year of each cohort increased from 2.80 per 100 000 in the 2005 cohort, and to 182.31 per 100 000 in the 2016 cohort. After the last receipt of all-WPV schedules, the incidence was lower than 3 cases per 100 000 population. This lasted for 8 years in the 2005 cohort, 7 years in the 2006 cohort and 6 years in the 2007 cohort. However, in the 2008 cohort the incidence began to increase in 2013, 4 years after the last receipt of APV. In the 2009–2011 cohort, the lasting years after the fourth dose and before the increase of incidence was only 1–2 years. Two years after the all-APV schedule was recommended in 2012, the incidences further increased for all cohorts in 2014 and the periods of protection effect were inapparent. In 2016, yearly incidences for every cohort reached their peak (Table 2).
Discussion
It is chosen as the strongest quasi-experimental approach for evaluating the longitudinal effects of interventions. Therefore, outcomes before and after implementation of the intervention were collected and compared, while accounting for potential confounders and potential secular data trends [Reference Wagner26, Reference Bernal27].
In the model for the intervention of WPV mass vaccination, there were level and trend changes that decreased at the rate of 1.11 and 1.21 per 100 000 population with the intervention from 1978 to 2001, respectively. It indicated that WPV mass vaccination could effectively reduce the incidence of pertussis. Numerous scientific pieces of evidence demonstrate that a primary series of WPV or APV, regardless of vaccine type or manufacturer, can provide a high level of protection against pertussis [1].
Our study found that a combination of 1–3 doses WPV and a fourth dose APV in 2008, and a combination of 1 dose WPV and 2–4 doses APV in 2009 had the highest impacts, with an immediate level decrease of 1.98 cases per 100 000 population in 2008 and 2009, respectively. The results indicate that the impact of a mixed schedule including both WPV and APV was better than four doses of only WPV. This can be interpreted as a result of increased compliance to the vaccination schedule, promoted by the perceived safety of APV, as this would guarantee vaccine efficacy [Reference Plotkin6]. Related studies found that the incidence of side effect was significantly lower in children with basic immunisation and immune-strengthening using APV than in WPV [1].
Despite decreasing level changes in the annual rate immediately after the APV intervention, compared to the WPV vaccination era, changes in trend were increasing at the rate of 1.63 cases per 100 000 population in 2008–2016, 1.77 cases per 100 000 population in 2009–2016 and 1.78 cases per 100 000 population in 2010–2016, respectively. The changes in trend may be associated with the WPV-induced immunity persistence that is longer than APV's [Reference Smits28]. Evidence from a systemic meta-analysis suggests that the average duration of protective immunity to pertussis after the fifth dose of APV is 3–4 years [Reference McGirr29]. However, a mathematical model analysis has shown a longer duration of DTaP immunity in the United States than the meta-analysis. The result demonstrates that APV confers imperfect, but has long-lived protection [Reference Domenech de Celles30]. There is also ample evidence that APV confers long-term protection [Reference Domenech de Celles31–Reference Domenech de Celles34].
Although globally there are a wide variety of APV vaccination schedules that are recommended, for every additional year after the last dose of APV, the odds of pertussis increase by 1.33 times [Reference McGirr29]. A multi-centre clinical trial showed that the efficacies of most APV products in America were significantly different, which suggested that the source, method of manufacture and included antigens of the vaccine should be considered in the comparative study [Reference Edwards35]. In China, the now available APV consists predominantly of FHA and inactivated PT and are known as Takeda-type vaccines [36]. A study of the serological effects of two types of PV developed by Chinese producers showed that higher levels of anti-PT antibody and anti-FHA antibody were detected in sera from children boosted with three doses APV than that boosted with three doses WPV in 1 month and 6 months after vaccination [Reference Sun37].
In the all-WPV era, the imperfect, but nevertheless effective vaccines conferred a slow waning protection and strong herd immunity. In the 2005–2007 cohort, a shorter phase of low incidence after the last dose of WPV can be observed. This could be due to the epidemic cycle. As the cohort population is growing older, they may act as a reservoir of transmission to young children. Substantial pertussis circulation among adults and adolescents would inevitably lead to the increased incidence among infants, especially those too young to get immunised [Reference Domenech de Celles34, Reference Gay38]. When APV sequentially replaced WPV since 2008, the lasting low incidence after APV vaccination began growing shorter in the 2008–2016 cohort. Since 2014, incidences in all cohort had further increased, which may lead to the speculation that APV usage in China provided short-lived immunity. The high transmissibility of pertussis [Reference Wood2], the ‘end-of-honeymoon’ effect of WPV vaccination [Reference Plotkin6], persisted circulation [Reference Winter39] and the relatively high risk of disease in groups with high contact rates, such as schoolchildren [Reference Domenech de Celles31] may also be cause for the increasing incidences of pertussis in Jinan city in recent years. The association between pertussis vaccination policy and incidences is intricate, making their interpretation difficult. However, the individual variability of primary vaccine failure and vaccine duration failure of APV should be considered as well [Reference Domenech de Celles30].
Although surveillance data may underestimate disease incidence, biases would be expected to apply equally to local reporting and vaccination patterns over time. At the same time, this study did not control potential variables including social and economic factors, migration [Reference Sun40], vaccine production and only considered factors such as the implementation of different vaccines and vaccination programmes. Multi-factor analysis should further be researched.
The results of this study show that the impact of WPV may be less than that of a combination of WPV and APV, and the short-term influence of APV is better than that of WPV; however, the duration of APV-induced immunity is not ideal. Analogous to the developed countries with high vaccine coverage, the incidence of pertussis has increased in Jinan. As long as currently available APVs are in use, it is likely that the ‘new normal’ will be higher disease incidence throughout pertussis cycles [Reference Winter39]. Furthermore, some inventions in other countries that had a similar rise in pertussis incidence had a reference value. In the UK, a maternal vaccination programme was offered to prevent cases in young infants who were not yet eligible for the vaccine [Reference Areta8]. The International Consensus Group on Pertussis Immunization [Reference Campinsmartí4] and the Global Pertussis Initiative [Reference Forsyth5] both have recommended universal adolescent booster vaccination combined with targeted immunisation of those adults most likely to have contact with babies, including parents close family members, healthcare workers and day-care workers; universal immunisation of young adults and routine use of Tdap rather than Td for all adult booster immunisations. However, despite the limitations of current PV, timely and complete immunisation with available PV is still the best practice against pertussis. Additional evaluation of vaccines and development of improved PVs and vaccination schedules in Jinan, China is necessary.
Acknowledgements
We thank Shicheng Yu for his excellent statistical support and Professor Zundong Yin, Lijie Zhang, Yixing Li, Guijun Ning and Maddison Naulty for critically reviewing and language polishing.
Conflict of interest
There is no conflict of interest among the authors.
Ethical standards
The ethics approval was obtained from the ethics committee in Jinan Centre of Disease Control and Prevention.








