Vitamin A and its metabolites are essential for the functioning of the immune system( Reference Raverdeau and Mills 1 ). Vitamin A supplementation (VAS) is recommended for children from 6 months to 5 years of age in populations with high risk of vitamin A deficiency (VAD) to reduce VAD-related morbidity and mortality( 2 ). High-dose VAS in the neonatal period (NVAS) is not WHO policy but has been tested in several randomised controlled trials (RCT) and is currently being considered as a policy, at least for sub-groups( Reference Haider and Bhutta 3 , 4 ). Our research group in Guinea-Bissau has conducted several of the NVAS trials, and found that NVAS had sex-differential effects on mortality, being associated with slightly lower mortality in males, but higher mortality in females( Reference Benn, Diness and Roth 5 – Reference Benn, Fisker and Napirna 7 ). The negative effect in females increased with increasing length of follow-up, and other studies have indeed corroborated this pattern; in all existing trials with follow-up to 12 months of age, females who had received NVAS had higher mortality than females who had received placebo at birth from 6 to 12 months of follow-up( Reference Benn, Aaby and Fisker 8 ). We have hypothesised that this could be due to a negative interaction between NVAS and subsequent diphtheria–tetanus–pertussis (DTP) vaccination (recommended in three doses at 6, 10 and 14 weeks of age) in females( Reference Benn, Aaby and Fisker 8 , Reference Benn, Rodrigues and Yazdanbakhsh 9 ). This observation is epidemiologically founded. Explorative immunological studies may generate hypotheses, which could guide the design of further mechanistic studies. In the present explorative study, we took advantage of two existing trial cohorts to assess sex differences in the immunological response to NVAS, which could help explain the sex differences in the mortality effects.
The early measles vaccine (MV) trial was a RCT testing the effect of providing an additional dose of early (i.e. 4–6 months of age) dose of MV in Guinea-Bissau, West Africa. Children were enrolled at the age of 4–6 months after the third dose of DTP and randomised to early MV or no early MV in addition to the usual MV at the age of 9 months (Fig. 1)( Reference Aaby, Martins and Garly 10 ). Before randomisation, a blood sample was collected from a subgroup of the children for an immunological study( Reference Jensen, Sondergaard and Andersen 11 ). Independent of the early MV trial, some participants had previously participated in an RCT of NVAS( Reference Benn, Diness and Balde 6 ). Using the blood samples collected as a baseline in the early MV trial (before allocation to early MV or no early MV)( Reference Jensen, Sondergaard and Andersen 11 ), we analysed the association between NVAS and in vitro cytokine responses in the 4- to 6-month-old infants who had participated also in the NVAS trial.
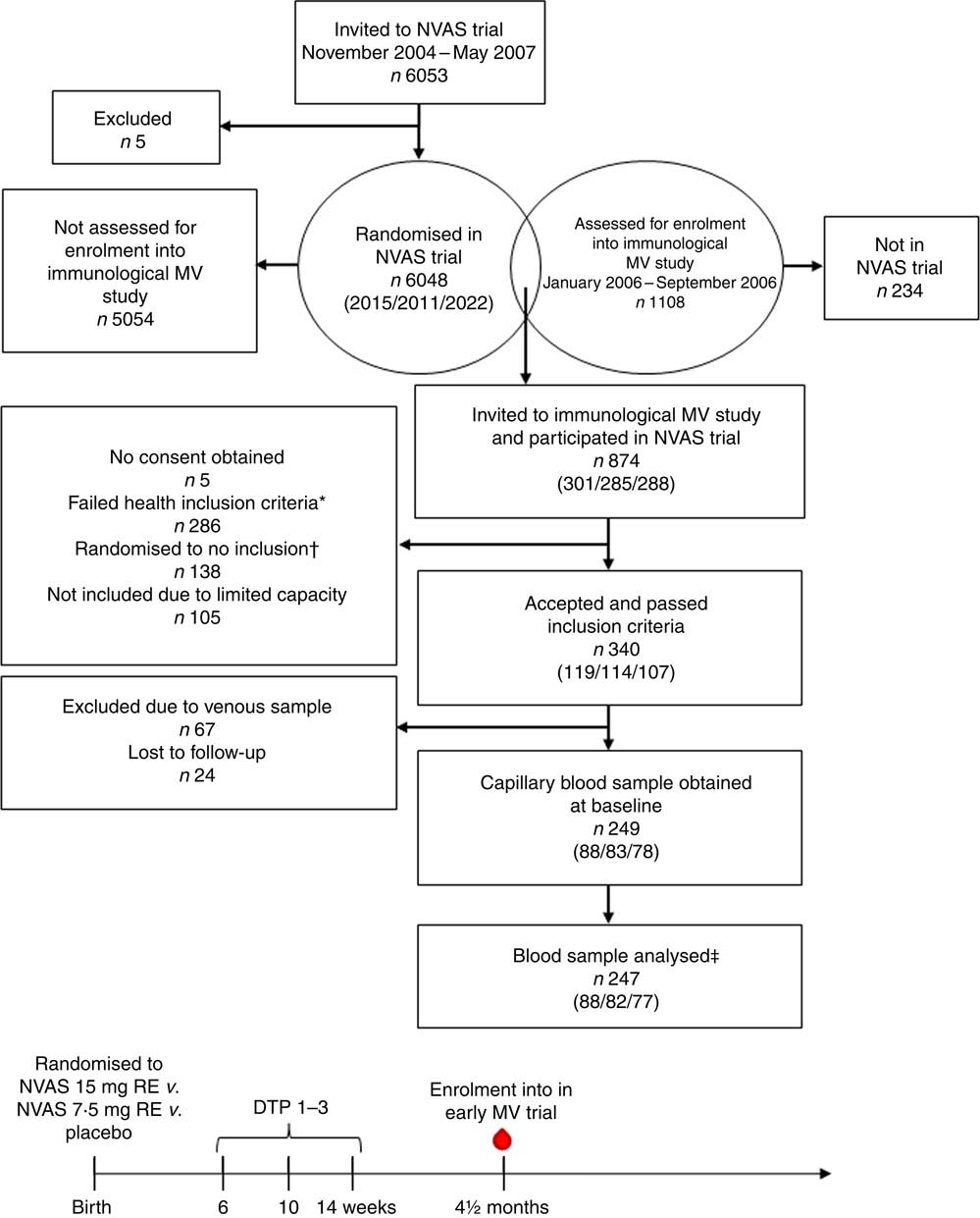
Fig. 1 Flow chart of participants and timeline of studies. Numbers in parentheses indicate the number of infants randomised to either of the three treatment arms: 30mg retinol equivalents (RE) (50000 IU vitamin A)/7·5mg RE (25000 IU vitamin A)/placebo. NVAS, neonatal vitamin A supplementation; MV, measles vaccine; DTP, diphtheria–tetanus–pertussis vaccine; ![]() , blood sample used in the present study. * Health exclusion criteria were as follows: current fever or diarrhoea reported by the mother; an axillary temperature above 37·5°C; a respiratory rate at 60/min or above; or current infection diagnosed by the examining physician. † As the Early MV trial randomised infants 1:2 to early MV v. no early MV at 4·5 months of age, half of the children in the control arm of the early MV trial were randomised off inclusion to the immunological MV sub-group study, in order to achieve an equal number of infants in the immunological study in the early MV and no early MV groups, respectively. ‡ Of the total analysed blood samples, 240 had a plasma sample for ex vivo inflammatory marker analysis, and 208 had an in vitro-stimulated blood sample for cytokine response analysis.
, blood sample used in the present study. * Health exclusion criteria were as follows: current fever or diarrhoea reported by the mother; an axillary temperature above 37·5°C; a respiratory rate at 60/min or above; or current infection diagnosed by the examining physician. † As the Early MV trial randomised infants 1:2 to early MV v. no early MV at 4·5 months of age, half of the children in the control arm of the early MV trial were randomised off inclusion to the immunological MV sub-group study, in order to achieve an equal number of infants in the immunological study in the early MV and no early MV groups, respectively. ‡ Of the total analysed blood samples, 240 had a plasma sample for ex vivo inflammatory marker analysis, and 208 had an in vitro-stimulated blood sample for cytokine response analysis.
Methods
Setting
The study took place at the Bandim Health Project in Guinea-Bissau (www.bandim.org), which follows a population of about 100 000 people in a health and demographic surveillance system. The study was defined by two existing trial cohorts, involving a group of children who had participated in first an NVAS trial, and then, independent of the NVAS trial, participated in the early MV trial, including an immunological MV sub-group study( Reference Jensen, Sondergaard and Andersen 11 ).
Neonatal vitamin A supplementation trial
From 2004 to 2008, an RCT of NVAS was conducted in the study area randomising normal-birth weight (>2500 g) infants 1:1:1 to receiving 15mg retinol equivalents (RE) (50000 IU vitamin A), 7·5mg RE (25000 IU vitamin A) or placebo at birth; all received concurrently bacille Calmette–Guérin (BCG) vaccine( Reference Benn, Diness and Balde 6 ).
Early measles vaccine trial
From 2003 to 2007, an RCT of early MV was conducted, testing the effect on mortality of giving an additional MV earlier than the generally recommended MV at 9 months of age( Reference Aaby, Martins and Garly 10 ). In brief, infants who had received three scheduled DTP vaccinations were eligible for enrolment and randomisation to early MV (Edmonston-Zagreb) at 4–6 months of age or no early MV (all infants were to receive the recommended MV at 9 months). Thus, at enrolment, infants had DTP3+oral polio vaccine 3 as their last vaccines.
Immunological measles vaccine sub-group study
The immunological MV sub-group study to investigate the non-specific immunological effects of MV (clinicaltrials.gov: no. NCT00168545) took place in 2006. From January through September 2006, immediately before randomisation to early MV or no early MV at 4·5 months of age, a physician examined children for eligibility. To avoid that acute illness influenced plasma biomarkers and stimulated cytokine production, children with the following characteristics were excluded from the immunological study: current fever or diarrhoea reported by the mother; an axillary temperature above 37·5°C; a respiratory rate at 60/min or above; or current infection diagnosed by the physician. BCG scarification was assessed and a blood sample was taken by finger prick. The present study is based on this baseline (pre-MV) sample from the early MV trial. Some children had a venous instead of capillary blood sample obtained, but we excluded venous samples because we found differences in the cytokine responses in venous and capillary blood( Reference Eriksson, Sartono and Martins 12 ). Analytical details can be found in( Reference Jensen, Sondergaard and Andersen 11 ). In brief, whole blood was diluted 1:9 with RPMI-1640 containing streptomycin (100 mg/ml), penicillin A (100 IU/ml), glutamate (2 mm) and pyruvate (1 mm) (all Gibco). The diluted blood was stimulated with lipopolysaccharide (LPS, 1 ng/ml; Sigma-Aldrich) (a Toll-like receptor (TLR)4 ligand), (S)-(2,3-bis-(palmitoyloxy)-(2-RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH, trihydrochloride (Pam3CSK4, 100 ng/ml; Cayla-InvivoGen Europe, a TLR2 ligand), phytohaemagglutinin (PHA, 2 μg/ml; Wellcome Diagnostics), tetanus toxoid (TT, 1·5 Lf/ml), purified protein derivative (PPD) from Mycobacterium tuberculosis (10 μg/ml; Statens Serum Institut) or medium alone. Concentrations of cytokines were measured from supernatants using the Luminex platform (Luminex 100; Luminex Corp.) (lower limit of detection (LLD) given in parenthesis): TNF-α (10 pg/ml), IFN-γ (5 pg/ml), IL-5 (3 pg/ml), IL-10 (5 pg/ml), IL-13 (10 pg/ml) and IL-17 (10 pg/ml) (BioSource). In unstimulated plasma, concentrations of the following compounds were measured using the Luminex assay kit (Fluorokine MAP Multiplex Human Cytokine Panel A; R&D Systems): TNF-α (LLD: 5 pg/ml), IL-10 (5 pg/ml), IL-6 (7 pg/ml), monocyte chemoattractant chemokine protein (MCP)-1 (10 pg/ml), IL-8 (2·8 pg/ml) and IL-1Ra (30 pg/ml); concentrations of soluble urokinase plasminogen activator receptor (suPAR) was measured by ELISA (suPARnostic standard ELISA, ViroGates)( Reference Jensen, Sondergaard and Andersen 11 ).
Statistical methods have been described previously( Reference Jensen, Sondergaard and Andersen 11 ). Cytokine data were log-transformed and analysed with Tobit regression using multiple imputations to account for measurements below the LLD of the assay, and estimates were back-transformed providing geometric mean ratios (GMR). Where >50 % of measurements were below the LLD of the respective analytes, Poisson regression was used, providing proportion ratios (PR) of measurements above LLD as done previously( Reference Jensen, Fisker and Andersen 13 , Reference Jensen, Larsen and Biering-Sorensen 14 ). A summary of the proportion of measurements >50 % and the geometric means can be found in the online Supplementary Table S1.
For distributions with <50 % observations below LLD, the ratios of IFN-γ to IL-5, IFN-γ to IL-10 and TNF-α to IL-10 were analysed and reported as GMR-ratios (GMRR).
Exploratory data analysis indicated that there was no difference in the effect of 7·5mg RE v. 15mg RE overall or stratified by sex; hence, the two doses were analysed collectively as NVAS v. placebo.
To test the effect of NVAS on overall cytokine responsiveness irrespective of stimulation, a collective test for each of the in vitro cytokines was performed for distributions with <50 % measurements below LLD, except for cytokines where the effect estimates across the different stimulations in the particular analysis were too heterogeneous (P<0·05) to be combined.
The effect of NVAS was analysed overall and stratified by sex.
Ethics
In both the NVAS trial and the immunological MV sub-group study, the mothers were informed by a trained field worker about the studies in their own language, Portuguese Creole, and, provided consent was given, they were asked to sign or fingerprint the consent form. The studies adhered to the Helsinki Declaration. The protocols of the respective studies were approved by the Ministry of Health in Guinea-Bissau, and the Danish Central Ethical Committee gave its consultative approval.
Results
In total, the study included 247 infants, who enrolled in both the NVAS RCT and in the immunological MV sub-group study to the early MV trial (Fig. 1). The infants included in the present analysis only represent a fraction of the participants in the NVAS trial. To assess whether a particular selection of the cohort had taken place, we therefore compared background characteristics of the herein analysed infants with the participants enrolled in the NVAS trial during the same period, but not included in the present study. There were only very subtle differences between the analysed infants and the non-analysed infants, the former being slightly smaller (online Supplementary Table S2), and, importantly, of the analysed infants, the NVAS and placebo groups were similar with respect to characteristics presented in Table 1, which could influence the immune system at the age of 4–6 months (Table 1).
Table 1 Population characteristics (Percentages and numbers; median values and 10th–90th percentiles; mean values and standard deviations)
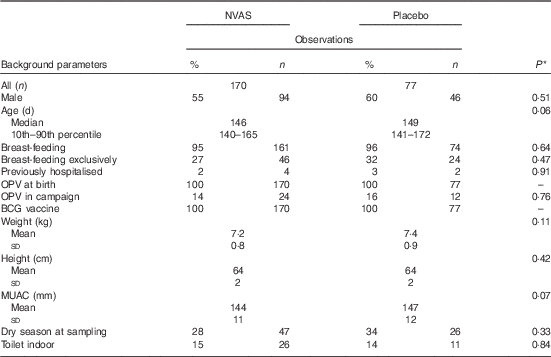
NVAS, neonatal vitamin A supplementation; OPV, oral polio vaccine; BCG, bacille Calmette–Guérin; MUAC, mid-upper arm circumference.
* Statistical test comparing NVAS with placebo using Kruskal–Wallis test for non-normal numerical data, Student’s t test for normal numerical data and χ 2 test for categorical data.
Sex differences in the placebo group
In the placebo group, there were strong differences between males and females. Females were less likely than males to have a BCG scar (71 v. 93 %, proportion ratio of no scar: 1·21; 95 % CI 1·05, 1·40). Females produced lower IFN-γ responses and higher IL-10 responses than males did, resulting in lower pro- to anti-inflammatory TNF-α:IL-10 ratios and IFN-γ:IL-10 ratios, reaching significance for the collective cytokine test (online Supplementary Fig. S1). There were no significant sex differences in plasma cytokine levels in the placebo group (data not shown), except for higher IL-1Ra levels in females (GMR: 1·19; 95 % CI 1·00–1·40).
Effects of neonatal vitamin A supplementation
Females receiving NVAS were significantly more likely to have BCG scars than the placebo group (96 v. 71 %, PR: 1·24; 95 % CI 1·09–1·42). There was no such difference among males (PR: 1·02; 95 % CI 0·95–1·10, P=0·01 for interaction between NVAS and sex).
In males, NVAS was associated with a significantly increased in vitro IL-10 to PPD (GMR: 1·99; 95 % CI 1·10, 3·58), to LPS (GMR: 1·45; 95 % CI 1·02, 2·06), and for all stimulations analysed collectively, but not in females (Fig. 2). The interaction between NVAS and sex was significant for IL-10 responses analysed collectively (P=0·002). Moreover, the association of NVAS with the TNF-α:IL-10 ratios was sex-differential with NVAS tending to increase the rations in females, but decreasing them in males, resulting in significant interactions between NVAS and sex for PPD (P=0·05), TT (P=0·04), medium alone (P=0·03) and for the ratios analysed collectively irrespective of the stimulation (P=0·02). A similar pattern was observed for the IFN-γ:IL-10 and IFN-γ:IL-5 ratios, where NVAS was associated with reduced ratios in males exclusively, significantly so for IFN-γ:IL-10 to PHA (GMRR NVAS-to-placebo: 0·40; 95 % CI 0·16, 0·96) and PPD (GMRR: 0·33; 95 % CI 0·13, 0·88), albeit the interaction with sex was not significant. In males but not in females, IL-17 responses to PHA (GMR: 1·69; 95 % CI 1·10, 2·59) and for all stimulations analysed collectively were higher in NVAS than placebo recipients (Fig. 2).
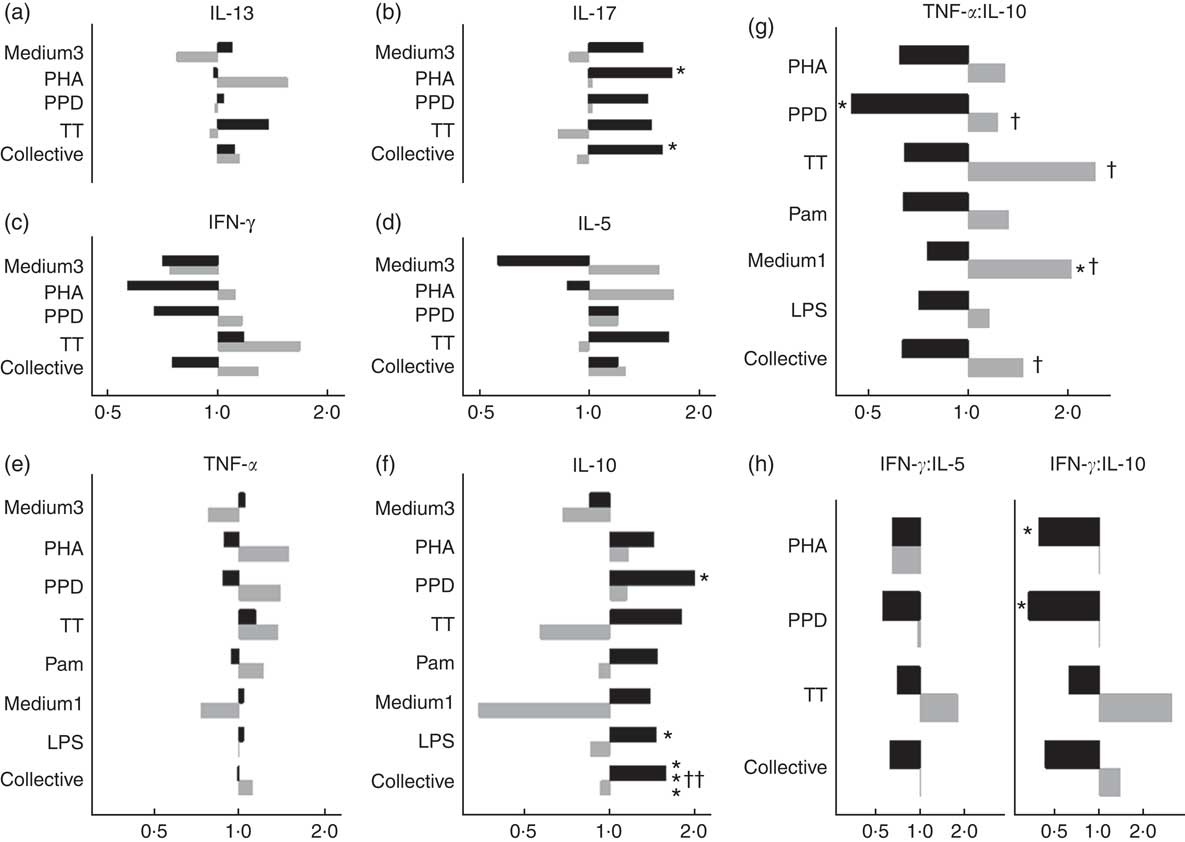
Fig. 2 Effects of neonatal vitamin A supplementation on in vitro cytokine responses at the age of 4–6 months, stratified by sex. (a–f) Geometric mean ratios (GMR) of cytokine responses, comparing NVAS with placebo, stratified by sex. For responses of IL-13, IL-17, IL-10, IFN-γ and IL-5 in the medium 3 condition and IL-17 to purified protein derivative from Mycobacterium tuberculosis (PPD), >50 % of measurements were below the lower detection limit; hence, these outcomes were analysed as the frequency of measureable values by Poisson regression, and reported as proportion ratios of measurements being above the lower limit. (g and h) GMR-ratios (GMRR) of cytokine responses TNF-α:IL-10 (g) or IFN-γ:IL-5 and IFN-γ:IL-10 (h), comparing NVAS with placebo; only cytokine distributions with >50 % detectable observations were included in the analysis. A GMR or GMRR >1 can be interpreted as an increasing effect of NVAS on cytokine concentrations or ratios, respectively. Note that the x-axes for IL-13, IL-17 and IFN-γ and IL-5 are identical. Medium3, medium1: culture with medium alone for 3 or 1 d, respectively; PHA, phytohaemagglutinin; TT, tetanus toxoid; collective: cytokine responses were analysed collectively, grouped across the different stimulations, excluding outcomes with <50 % measurements within detection ranges of the assay; Pam, palmitoyl(3)-cysteine-serine-lysine(4); LPS, lipopolysaccharide; ![]() , Males;
, Males; ![]() , females. Statistical test for effect of NVAS: * P<0·05; *** P<0·001; statistical test for interaction between NVAS and sex: † P<0·05; †† P<0·01.
, females. Statistical test for effect of NVAS: * P<0·05; *** P<0·001; statistical test for interaction between NVAS and sex: † P<0·05; †† P<0·01.
For plasma biomarkers, NVAS was associated with increased IL-1Ra in males (GMR: 1·16; 95 % CI 1·01, 1·34), but not in females (GMR: 0·96; 95 % CI 0·81, 1·14, P for interaction=0·10). NVAS was not associated with other plasma cytokine levels in either sex (data not shown).
Discussion
The present study indicates long-lasting effects of NVAS on responses to BCG vaccination and immunological responsiveness of peripheral blood cells persisting at 4–6 months of age in a sex-dependent manner. NVAS v. placebo was associated with increased frequency of BCG scars in females, making scar frequencies in NVAS-receiving females comparable to those of boys in general. NVAS was also associated with more pro-inflammatory cytokine responses in females, whereas the opposite tendency was seen in males. Sex differences in NVAS effects were found for responses to several recall antigens and for baseline secretion in non-stimulated cells, suggesting generalised sex-dependent immune-modulatory properties of NVAS. Except for IL-1Ra, plasma inflammatory markers were not associated with NVAS, indicating that the effects observed for in vitro responses were not due to differences in systemic inflammation at the time of bleeding.
Strengths and weaknesses
The investigation of immunological effects of NVAS was conducted in a relevant cohort of infants in a low-income high disease burden setting, with a documented high proportion of individuals with low vitamin A levels( Reference Danneskiold-Samsoe, Fisker and Jorgensen 15 ), and hence a potential target for VAS distribution. The data were generated in a cohort of infants previously randomised to VAS or placebo, and an overall balanced distribution of background factors remained in the present group of participants at blood sampling.
The immunological study, however, was not planned before conducting the NVAS trial; the samples were collected in connection to a subsequent immunological MV sub-group study of shorter duration and smaller size, inevitably implying that only a minor sub-group of the NVAS trial participants were followed up. Therefore, a biased selection of individuals cannot be ruled out, although the available background characteristics from the NVAS trial do not indicate any particular selection. The immunological assay was designed to explore potential non-specific immunological effects of early MV on cytokine recall responses to heterologous vaccines in addition to typical innate stimulation, investigating Th1-, Th2- and Th17-related responses. Although the assessment of the thirty different stimulation-cytokine combinations entailed a considerable risk of chance findings, we did not use adjustment for multiple testing owing to the explorative nature of the study. The findings should be interpreted with this potential caveat in mind. However, the overall rather consistent sex-differential pattern of NVAS dependent cytokine responses is unlikely to be a spurious association owing to multiple testing. By inherent limitations of the assay, cellular sources of the measured cytokines could not be elucidated, hence hampering a mechanistic interpretation.
Comparison with other studies
Sex differences in basic immunological parameters in infancy have been reported previously, including higher NK cell counts and increased inflammatory in vitro responses to LPS and mitogen stimulation in males( Reference Klein and Flanagan 16 ). A recent Gambian study in a cohort where VAS is routinely given at 6 months (KL Flanagan, personal communication) found reduced type-1 T-cell reactivity in females compared with males at 10 months of age (1 month after DTP vaccination), as evidenced by lower IL-12p70 responses and IFN-γ:IL-4 ratios in unspecific T-cell stimulation( Reference Noho-Konteh, Adetifa and Cox 17 ). This seemingly contrasts the elevated IFN-γ:IL-5 ratios to TT and PPD in females compared with males among the NVAS recipients in the present fully DTP vaccinated cohort. However, in likening with the DTP arm of the Gambian study, the present study found no sex differences among NVAS recipients in the TNF-α:IL-10 ratios to LPS, TT or PPD.
In addition to sex differences per se, sex differences in immunological effects of VAS in children have also been described, although the evidence of the effects is not unambiguous in the literature. A previous study from Guinea-Bissau reported no effect of NVAS on BCG scar frequencies at 2 and 6 months overall or by sex, as both males and females had high scar frequencies also in the placebo group. NVAS was associated with increased in vitro IFN-γ responses to PPD in males at 6 weeks, but had no effect on IL-10 responses to PPD; NVAS was also associated with a transiently lower proportion of tuberculin skin test responders at 2 months among males, but not females( Reference Diness, Fisker and Roth 18 ). Another study from Guinea-Bissau evaluating the immunological effect of NVAS 6 weeks after administration found the strongest effects of NVAS in DTP-vaccinated males. NVAS reduced TNF-α and IL-10 secretion in medium alone in males with prior DTP, but not in females( Reference Jorgensen, Fisker and Sartono 19 ). A third immunological study from Guinea-Bissau investigating the effect of VAS v. placebo administered together with MV in >6-month-old children found that VAS tended to decrease all measured cytokine responses to PHA (TNF-α, IL-10, IL-2, IL-5 and IFN-γ) in females, but not in males, six weeks after administration; this, however, was exclusively found in infants, who had not previously received VAS( Reference Jensen, Fisker and Andersen 13 ). Overall, these previous findings are not unequivocally corroborated by the present study at 4·5 months of age, in which all infants had received three doses of DTP; herein, NVAS was associated with anti-inflammatory responses in males, but with pro-inflammatory responses in females.
Part of the discrepancy may be attributed to differences in age of VAS receipt, as VAS at birth v. VAS at 6 months may have different sex-dependent health effects, or differences in age of sampling and interaction with vaccination( Reference Benn, Aaby and Arts 20 ). In the immunological MV sub-group study from which the present study data used the baseline samples, infants were randomised to receiving early MV or no early MV (equal distribution of NVAS and placebo infants). We have previously published that six weeks after randomisation, MV was associated with increased TNF-α:IL-10 ratios in infants who had previously received NVAS, whereas MV tended to decrease the ratios in children without previous NVAS. This pattern, however, was not significantly different between males and females( Reference Jensen, Sondergaard and Andersen 11 ). Hence, MV may enhance the pro-inflammatory effect of NVAS in females, whereas MV may ameliorate the anti-inflammatory effect of NVAS in males. The latter would be in keeping with the Gambian infant study, in a setting where VAS is routinely distributed at 6 months, and where MV at 9 months of age was associated with enhanced pro-inflammatory marker levels in plasma and responses to TLR4 agonist and PPD stimulation in males but not in females 1 month after MV compared with the pre-MV baseline( Reference Noho-Konteh, Adetifa and Cox 17 ).
In the trial of early MV using all-cause mortality as an outcome, NVAS-receiving females tended to benefit from early MV (as compared with no early MV), whereas early MV was detrimental for NVAS-receiving males( Reference Aaby, Martins and Garly 10 ). A combined analysis of three NVAS RCT in Guinea-Bissau, including the present, found that the overall mortality effect of NVAS was significantly sex-differential, with increased female mortality( Reference Benn, Diness and Balde 6 ), which may be caused by a negative interaction with DTP( Reference Benn, Rodrigues and Yazdanbakhsh 9 ). Hence, in females, NVAS v. placebo may be associated with increased pro-inflammatory responsiveness and an increased mortality risk after DTP, whereas the increased pro-inflammatory responsiveness after MV in NVAS recipients seems not to be associated with increased mortality risk in females. Further studies are needed to potentially reconcile these seemingly contrasting immunological and survival effects of NVAS and vaccines.
Interestingly, in the same setting, NVAS has also been associated with an increased risk of atopy in older children. In two follow-up studies in Bissau in children aged 3–9 years and 8–10 years who previously enrolled in trials of VAS at birth (in low-birth weight and normal-birth weight infants, respectively), NVAS v. placebo was associated with increased risk of atopy (skin prick test) and wheezing, although in the latter study this association was exclusively seen in females( Reference Kiraly, Benn and Biering-Sorensen 21 , Reference Aage, Kiraly and Da 22 ). Studies on the potential associations between cytokine responses and subsequent risk for development of atopic diseases are not unambiguous; however, a large body of evidence indicates that Th2-biased and IFN-γ-reduced cytokine response profiles in cord blood and infancy are be associated with increased risk of development of atopy in childhood (e.g. ( Reference Allam, Zivanovic and Berg 23 , Reference Stern, Guerra and Halonen 24 )); for example, in a prospective cohort study, decreased IFN-γ:IL-4 and IFN-γ:IL-10 response ratios of PMA/ionomycin-stimulated PBMC in very young infants with eczema were associated with increased risk of asthma at 4 years of age( Reference Sarria, Mattiello and Yao 25 ). In the present cytokine study, NVAS tended to drive cytokine responses towards a Th2-bias, but in males only, potentially suggesting sex-differential associations between NVAS, cytokine patterns and atopic disease.
In conclusion, the present study corroborates sex-differential effects of VAS on the immune system, finding that NVAS was associated with an increased scar formation after BCG vaccination and increased pro- to- anti-inflammatory cytokine response ratios in females, but not in males, emphasising the importance of analysing VAS effects by sex.
Acknowledgements
The authors thank Jesper Eugen-Olsen for suPAR analysis (suPARnostic standard ELISA, ViroGates, Denmark).
The present study received support from Novo Nordisk Foundation, Fonden til Lægevidenskabens Fremme, Danish Medical Research Council, Augustinus Fonden, Beckett fonden, Dagmar Marshalls fond and the Aase og Ejnar Danielsens fond. P. A. holds a research professorship grant from the Novo Nordisk Foundation. C. S. B. is funded by an European Research Council (ERC) Starting Grant (ERC-2009-StG-243149). Research Center for Vitamins and Vaccines (CVIVA) is funded by the Danish National Research Foundation (DNRF108). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
K. J. J. analysed the data and wrote the manuscript; M. J. S. and C. M. supervised the field work and collection of blood samples; M. J. S. handled blood samples in the laboratory and performed in vitro stimulations; A. A. supervised data analysis; P. A. and C. S. B. conceived the research idea and designed the randomised trials; C. E., C. S. B. and M. J. S. designed the immunological study. All authors read and approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517002938






