Although dietary intervention has been recognised as useful in the treatment of type 2 diabetes mellitus (T2DM), current guidelines emphasise the role of macronutrients in glycaemic control in T2DM patients, giving little attention to micronutrients(1). The benefits of dietary Ca and dairy products to control T2DM have been suggested(Reference Pittas, Lau and Hu2–Reference Pasin and Comerford4).
Adequate Ca consumption seems to improve glucose homeostasis in adults with T2DM, particularly among low habitual consumers (<600 mg/d)(Reference Pittas, Lau and Hu2). That effect seems to be mainly due to the consumption of fat-free dairy products(Reference Candido, Ton and Alfenas5) associated with energy-restricted diets(Reference Abargouei, Janghorbani and Salehi-Marzijarani6). A meta-analysis of randomised clinical trials indicated that increasing dairy product intake resulted in greater body weight (BW) and body fat losses, while lean mass loss was attenuated in subjects on weight loss diets(Reference Stonehouse, Wycherley and Luscombe-Marsh7). However, the effect of different dairy sources could not be distinguished (e.g. milk v. cheese v. yoghurt)(Reference Stonehouse, Wycherley and Luscombe-Marsh7).
The effects of dairy components on BW and body composition in athletes and healthy overweight and obese adults have been extensively investigated(Reference Zemel, Thompson and Milstead8–Reference Devries and Phillips19). However, few studies have examined the effect of dairy foods on individuals with T2DM(Reference Pittas, Lau and Hu2, Reference Pasin and Comerford4). Clinical evidences described in a systematic review indicated that dairy foods and dairy proteins (mainly whey protein) consumption might improve insulin secretion in T2DM adults(Reference Pasin and Comerford4). However, due to the different doses consumed, different dairy sources, and the short-term nature of the clinical trials it is difficult for us to make effective dietary recommendations based on the results obtained in the studies included in that review(Reference Pasin and Comerford4).
Considering the relevance of glycaemic control to prevent T2DM complications and the scarcity of well-controlled studies concerning the role of dairy products and Ca consumption in T2DM, we compared the effects of low-Ca and high-Ca fat-free milk consumption on obesity and glycaemic control in subjects with T2DM.
Subjects and methods
Subjects
Eligible subjects were adults of both sexes with T2DM, treated with only diet or with diet plus oral hypoglycaemic agent (Metformin), that had the metabolic syndrome(Reference Alberti, Eckel and Grundy20), had low habitual Ca intake (<600 mg/d), were between 20 and 59 years of age, had a dietary restraint<14(Reference Stunkard and Messick21), had a light to moderate physical activity level (PAL)(Reference Pardini, Matsudo and Araujo22) and had T2DM for at least 1 year. Exclusion criteria were (1) smokers; (2) use of Ca, vitamin D, Zn or Mg supplements or medication that affects the metabolism of these micronutrients; (3) use of drugs (except hypoglycaemic drugs), herbs or diets for weight loss; (4) hormone replacement therapy; (5) menopause or in post-menopause; (6) recent weight gain or loss (±5 kg) over the previous 3 months; (7) recent change in PAL over the previous 3 months; (8) aversion or intolerance to the shakes provided during the study; (9) consumption of more than 12 g of alcohol/d for women and 24 g/d for men; (10) eating disorders; (11) endocrine (except T2DM and obesity), kidney or liver pathology; (12) Ca malabsorption; (13) history of recurrent nephrolithiasis; (14) history of gastric surgery or current gastric disease including gastroparesis; (15) consumption of more than 350 mg of caffeine/d; (16) pregnancy or lactation; (17) anaemia; and (18) changes in the medication type or dosage during the study. Sample size was calculated according to Mera et al. (Reference Mera, Thompson and Prasad23), considering the glycated Hb (HbA1c) as the main variable. Our eligible subjects’ baseline mean and standard deviation HbA1c values were applied, considering a desirable reduction of 1 % to decrease microangiopathy and neuropathy occurrence(1). The statistical power was set up at 90 %. Therefore, and by applying a P value <0·05, the sample size required in our study was equivalent to fourteen volunteers per session. Four men and ten women completed the study (49·5 (sd 8·6) years old, and BMI of 29·4 (sd 4·5) kg/m2).
The study protocol was approved by the ethics committees of the Federal University of Viçosa, Brazil. All subjects provided their fully informed and written consent before participation.
Study design
Details of the present study design were described previously(Reference Gomes, Costa and Alfenas24). In short, subjects completed a randomised double-blinded, crossover clinical trial of two 12-week sessions separated by a washout period of 8 weeks. Subjects were initially randomly assigned to high-Ca fat-free milk session (HC) (equivalent to about three fat-free milk portions/d) or low-Ca control session (LC) session in 1:1 ratio. Participants and data analysts were blinded. An energy-restricted diet (restriction of 2092 kJ/d) containing 800 mg of dietary Ca/d was prescribed. Diets were prescribed according to the American Diabetes Association nutrition recommendations(1) and considering the nutritional composition of the breakfast shakes provided during the study. Subjects commuted daily to the laboratory, where they consumed a breakfast shake containing 700 mg (HC) (equivalent to approximately three servings of fat-free milk) or 6·4 mg (LC) of Ca. A complete description of the nutritional composition of these shakes can be seen elsewhere(Reference Gomes, Costa and Alfenas24). All other meals were consumed in free-living condition in both sessions. Subjects were instructed to maintain constant PAL and medication use during the study. HC- and LC-prescribed diets presented similar contents of macronutrients, vitamin D, P, Mg, Zn and dietary fibre. HC-prescribed diet contained 1500 mg and LC had 800 mg of Ca/d.
Our breakfast shakes contained 17·1±0·2 g of protein. HC shakes contained 20 % casein and 80 % whey protein, while LC shakes contained 100 % whey protein. We included only whey protein in the LC because micellar casein (as present in milk) could not be used since it contains Ca and hydrolysed casein is less bioavailable. In milk, about 30 % of Ca exists as free ionic Ca and the remaining approximately 70 % is complexed with casein in micellar calcium phosphate. Besides, hydrolysed casein coagulates in the stomach, being less available for enzymatic hydrolysis and less absorbed in the intestine.
PAL, food intake, body composition (fat mass (FM) and fat-free mass), anthropometric (BW, waist circumference (WC), waist:hip ratio) and biochemical variables (serum Ca, P, Mg, glucose, uric acid, HbA1c, vitamin D, insulin, fructosamine and parathormone (PTH) concentrations) were evaluated at baseline and after 12 weeks of each dietary experimental session.
Physical activity assessment
PAL was assessed at baseline and after 12 weeks of each experimental session using the long format International Physical Activity Questionnaire, version 6, validated for the Brazilian adult population(Reference Pardini, Matsudo and Araujo22).
Food intake assessment
Food intake was assessed at baseline and after 12 weeks of each experimental session by 3 d dietary records. The amounts of foods were converted into grams for energy intake, macronutrients, Ca, P, Mg, Zn and dietary fibre intake analyses using DietPro, version 5.1i (July 2015).
Breakfast shakes
HC and LC shakes presented similar macronutrients, vitamin D, Na and dietary fibre contents, differing mainly in Ca content. Ingredients and nutrient composition of the two shakes have been published elsewhere(Reference Gomes, Costa and Alfenas24). HC shakes contained fat-free milk powder (Itambé® enriched with Fe; vitamins A, C and D; and Ca) reconstituted in water (250 ml). To ensure similarity to HC shakes, LC shakes contained whey protein (BemVital®, Diacom), sucrose, sodium chloride (Cisne®) and a powder supplement containing Fe (iron chelate) and vitamins A (retinol acetate), C (ascorbic acid) and D3 (cholecalciferol).
Anthropometric and body composition measurements
BW was assessed using an electronic platform scale (Model 2096 PP) with a capacity for 150 kg and precision of 50 g. Height was measured using a stadiometer with a scale of 0–220 cm, precision 0·1 cm (SECA 206, Seca). WC and hip circumferences were measured using a flexible inelastic tape. WC was measured at the midpoint between the lowest rib and the iliac crest with a precision of 0·1 cm, and hip circumference was measured at the greatest circumference between the anterior iliac crest and the greatest trochanter. Waist:hip ratio was calculated by dividing WC by hip circumference.
Body composition was assessed using a Prodigy densitometer (GE Lunar Medical Systems). Scans were analysed using EnCoreTM, version 13.5.
Biochemical assays
Venous blood samples were obtained after 12 h of overnight fasting. Serum total Ca concentrations were assessed by the Caarsenazo III method (intra-assay and inter-assay CV of 0·9 and 1·5 %, respectively) (Mira Plus, Roche Diagnostic Systems). A colorimetric assay (Bioclin kit, Quibasa Basic Chemical Ltda) was used to measure P and Mg concentrations, with intra-assay and inter-assay CV of 2·7 and 2·5 % for P, and 1·2 and 1·3 % for Mg, respectively. PTH concentrations were measured using an electrochemiluminescence immunoassay (intra-assay and inter-assay CV of 2·8 and 3·3 %, respectively) (Elecsys Modular-E-170, Roche Diagnostics Systems). 25-Hydroxivitamin D concentrations were determined using a chemiluminescent microparticle immunoassay (Architect i2000, Abbott Diagnostics), with intra-assay and interassay CV of 5·1 and 7·2 %, respectively. IR was calculated using the updated homeostatic model assessment of insulin resistance (HOMA2-IR) index, which considers a more accurate physiological basis to predict homeostatic response than the HOMA-IR index. The HOMA2 index estimates β-cell function (% B) and insulin sensitivity (% S), and it was obtained using HOMA Calculator, version 2.2.2, available at https://www.dtu.ox.ac.uk/homacalculator/.
Serum total Ca concentrations were assessed by the Caarsenazo III method (Mira Plus, Roche Diagnostic Systems). Serum P and Mg concentrations were measured using a colorimetric assay (Bioclin kit, Quibasa Basic Chemical Ltda). Serum PTH concentrations were measured using an electrochemiluminescence immunoassay (Elecsys Modular-E-170, Roche Diagnostics Systems). Serum 25-hydroxivitamin D concentrations were determined using a chemiluminescentmicroparticle immunoassay (Architect i2000, Abbott Diagnostics).
Statistical analysis
Statistical analyses were conducted using the Statistical Package for Social Sciences for Windows, version 20.0 (IBM). All variables’ distribution normality was assessed according to the Shapiro–Wilk test at 5 % significance. Variance homogeneity was tested using the Levene test. An independent-samples t test was conducted to identify possible differences between the subjects that started with either HC (n 7) or LC (n 7) at baseline. To verify the efficacy of the washout period, a paired t test was conducted between the initial baseline data and the post-washout baseline data. Baseline data were calculated before each intervention period. Once the efficacy of the washout period was verified, the initial baseline and post-washout baseline data were combined, and the final data from each study arm (n 14 each for the two sessions) were combined. Then, data within sessions were analysed using the paired t test or Wilcoxon rank sum test, pairing results from the same individual before (baseline) and after (12 weeks) each dietary intervention (LC or HC session), considering P values less than 0·05 as significant. Data on changes from the baseline over the 12 weeks of the intervention (deltas, i.e. the final value minus the baseline value) were compared between the sessions using the paired t test or Wilcoxon rank sum test, with Bonferroni correction for multiple comparisons. The criterion of significance adopted was P<0·025, two tailed.
Results
Food intake and Ca homeostasis markers result have been published elsewhere(Reference Mera, Thompson and Prasad23). In short, Ca (P = 0·000), P (P = 0·000), Mg (P = 0·002) and fibre intake (P = 0·013) increased after HC and remained unchanged after LC. The Ca:P ratio was 0·49 and 0·66 at baseline, and 0·74 and 0·62 post-treatment for the HC and LC, respectively. Dietary fibre (P = 0·001), Ca (P = 0·000) and P (P = 0·000) intake increased after HC compared with LC. HC final serum 25-hydroxyvitamin D concentrations were higher (P = 0·001), and PTH concentrations were lower (P = 0·003) compared with baseline values. PTH (P = 0·002) and Mg (P = 0·015) decreased, whereas 25-hydroxyvitamin D increased (P = 0·000) in HC compared with LC.
Anthropometry and body composition
All the anthropometric variables and FM reduced after the HC session but did not change after the LC session compared with baseline (Table 1). HC promoted a greater reduction in BW, FM, WC and waist:hip ratio (Fig. 1). Percentage FM and WC decreased in HC, while it remained unchanged in LC (Table 1). Conversely, fat-free mass percentage increased in HC and remained unchanged in LC (Table 1).
Table 1. Anthropometry, body composition and biochemical data at baseline and after 12 weeks in the high-calcium fat-free milk (HC) and low-calcium control (LC) experimental sessions (n 14)
(Mean values and standard deviations)
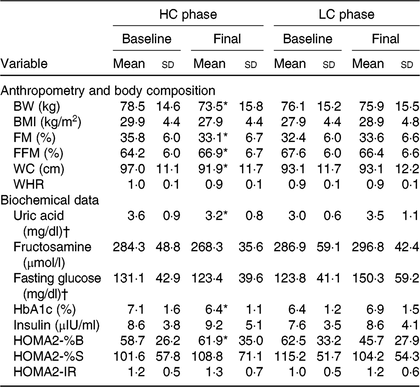
BW, body weight; FM, fat mass; FFM, free-fat mass; WC, waist circumference; WHR, waist:hip ratio; HbA1c, glycated Hb; HOMA2-%B, homeostatic model assessment-2 β-cell function; HOMA2-%S, HOMA-2 insulin sensitivity; HOMA2-IR, HOMA-2 of insulin resistance.
* Mean value was significantly different from baseline (P<0·05). BW (P = 0·034), FM (P = 0·008), FFM (P = 0·036), WC (P = 0·000), uric acid (P = 0·001), HbA1c (P = 0·000), HOMA2-%B (P = 0·014). Calculated from paired t test (or Wilcoxon rank sum test) for comparing baseline with the 12-week value in each experimental session.
† To convert uric acid in mg/dl to to μmol/l, multiply by 59·48. To convert glucose in mg/dl to mmol/l, multiply by 0·0555.
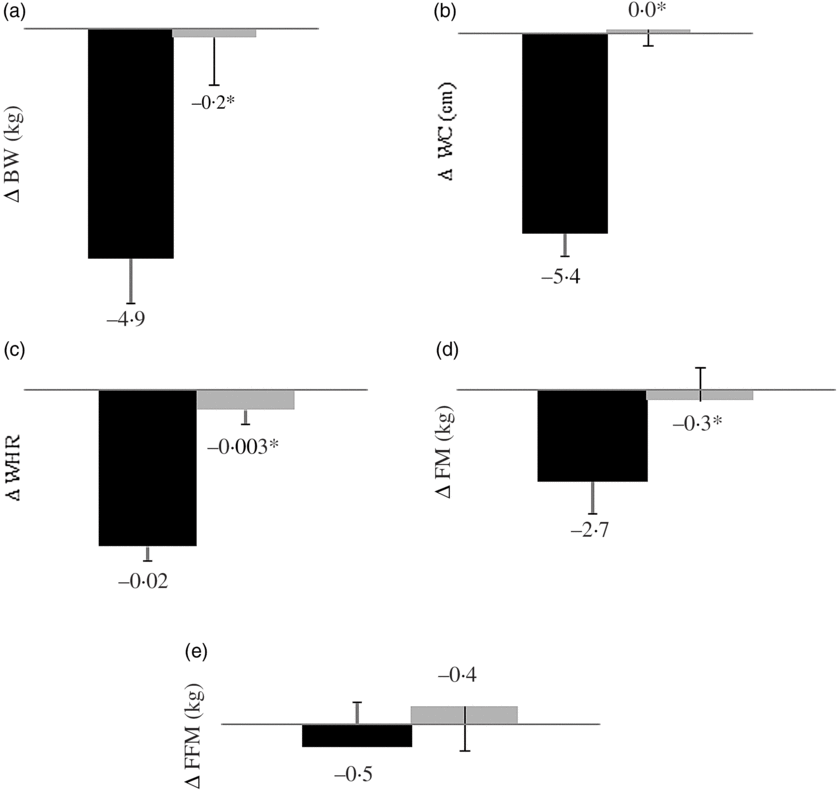
Fig. 1. Body weight (BW) (a), waist circumference (WC) (b), waist:hip ratio (WHR) (c), fat mass (FM) (d) and fat-free mass (FFM) (e) changes from baseline according to study session (n 14). During 12 consecutive weeks subjects consumed a high-calcium fat-free milk (700 mg of calcium/d; ▪) or a low-calcium control (6·4 mg of calcium/d; ![]() ) shake for breakfast. Energy-restricted diets (restriction of 2092 kJ/d, 800 mg of calcium/d) were prescribed. Values are means, with their standard errors represented by vertical bars. Except for FFM, all variables differed between sessions (*P<0·025, paired t test with Bonferroni correction for multiple comparisons). BW (P = 0·019), FM (P = 0·000), WC (P = 0·000) and WHR (P = 0·004). Delta (Δ) was calculated subtracting the final value (after a 12-week intervention) from the baseline value.
) shake for breakfast. Energy-restricted diets (restriction of 2092 kJ/d, 800 mg of calcium/d) were prescribed. Values are means, with their standard errors represented by vertical bars. Except for FFM, all variables differed between sessions (*P<0·025, paired t test with Bonferroni correction for multiple comparisons). BW (P = 0·019), FM (P = 0·000), WC (P = 0·000) and WHR (P = 0·004). Delta (Δ) was calculated subtracting the final value (after a 12-week intervention) from the baseline value.
Glycaemic profile and calcium homeostasis
HC final HOMA2-%B was higher, and final serum uric acid and HbA1c concentrations were lower when compared with baseline values (Table 1). A comparison of changes over 12 weeks to the baseline (deltas) revealed that serum uric acid, fasting glucose and HbA1c decreased, whereas HOMA2-%B increased in HC when compared with LC (Table 2).
Table 2. Biochemical data changes from baseline† in response to high-calcium fat-free milk (HC) and low-calcium control experimental session (LC) (n 14)
(Mean values and standard deviations)
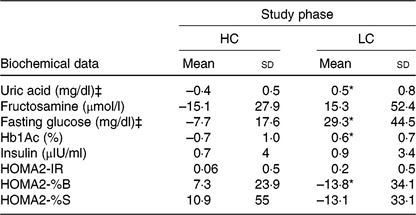
HbA1c, glycated Hb; HOMA2-IR, homeostasis model assessment-2 of insulin resistance; HOMA2-%B, HOMA-2 β-cell function; HOMA2-%S, HOMA-2 insulin sensitivity.
* P<0·025.
† Changes from baseline were calculated by subtracting the final value from the baseline value. Uric acid (P = 0·008); fasting glucose (P = 0·020); Hb1Ac (P = 0·001); HOMA2-%B (P = 0·016). P value was estimated by paired t test or Wilcoxon rank sum (both with Bonferroni correction for multiple comparisons).
‡ To convert uric acid in mg/dl to to μmol/l, multiply by 59·48. To convert glucose in mg/dl to mmol/l, multiply by 0·0555.
Discussion
In the present study, the consumption of about three servings of fat-free milk and 1200 mg of dietary Ca/d (the amount consumed in the HC session) was more effective to control obesity (BW, FM and WC reduction), and the variables related to the glycaemic control (decrease in serum uric acid, HbA1c and fasting glucose and increase in HOMA2-%B) than the control diet (low Ca consumption, approximately 525 mg/d) in individuals with T2DM and low habitual Ca intake (less than 600 mg/d). Possible mechanisms that explain our results are summarised in Fig. 2.
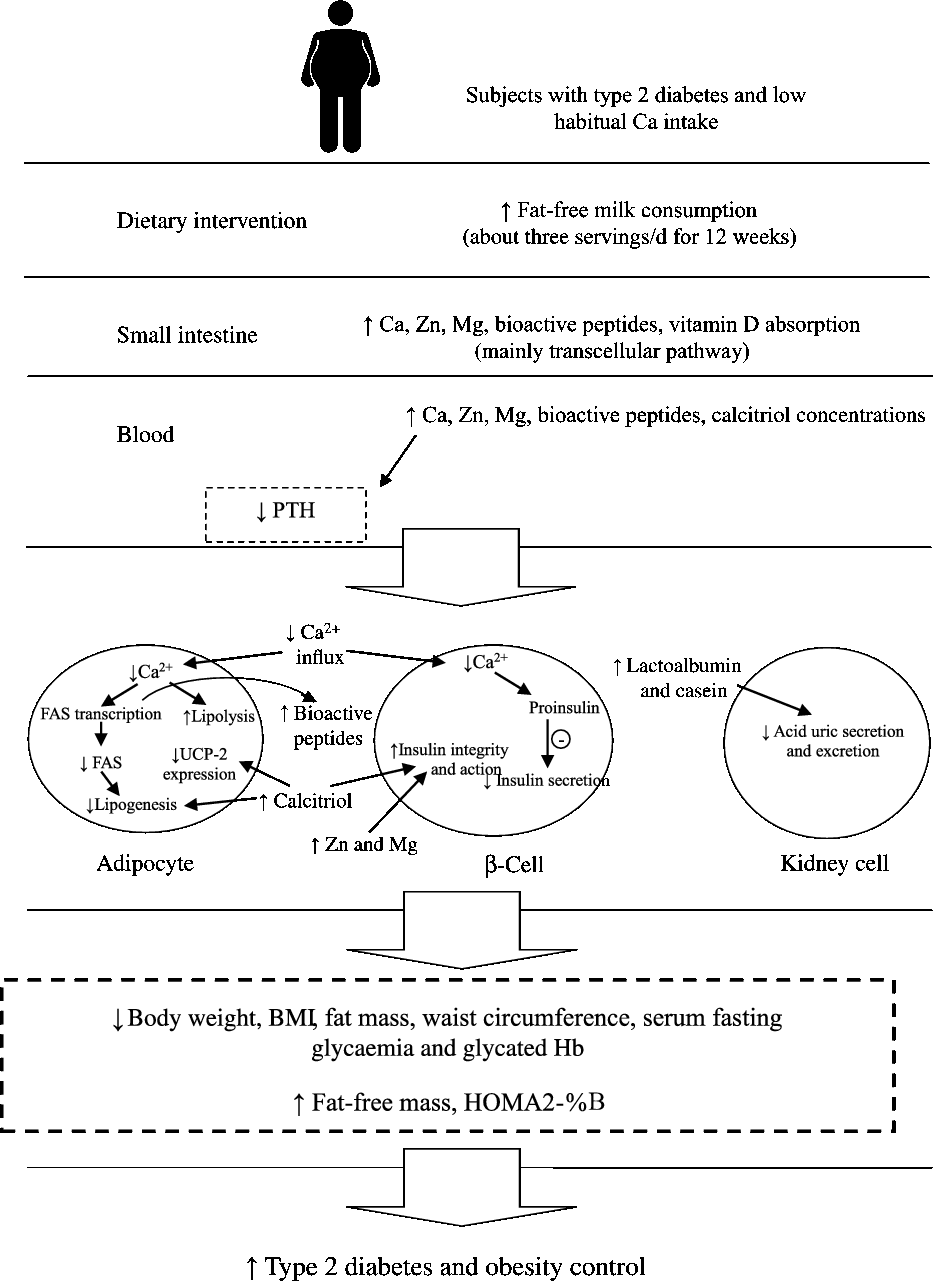
Fig. 2. Possible mechanisms that explain the effects of increased fat-free milk consumption on diabetes and obesity control, based on our results. FAS, fatty acid synthase; HOMA2-%B, homeostasis model assessment-2– β-cell function; PTH, parathormone; UCP-2, uncoupling protein 2.
Some authors reported that diets higher in dairy foods lead to beneficial effects on weight loss(Reference Zemel, Thompson and Milstead8), body fat(Reference Zemel, Richards and Mathis13, Reference Zemel, Teegarden and Loan14, Reference Torres and Sanjuliani18) and WC reduction(Reference Zemel, Richards and Mathis13–Reference Stancliffe, Thorpe and Zemel15, Reference Torres and Sanjuliani18), besides insulin sensitivity increase(Reference Rideout, Marinangeli and Martin25, Reference Maki, Nieman and Schild26), while others have observed no effects in these variables(Reference Bowen, Noakes and Clifton9–Reference Thompson, Holdman and Janzow12, Reference Jones, Eller and Parnell17, Reference Shalileh, Shidfar and Haghani27). Methodological differences in these clinical trials can explain these controversial results, as described later.
First, the Ca dose (1000(Reference Rideout, Marinangeli and Martin25) to 2400 mg of Ca/d(Reference Bowen, Noakes and Clifton9)) and the supplementation period (6 weeks(Reference Maki, Nieman and Schild26) to 1 year(Reference Gunther, Legowski and Lyle10, Reference Rideout, Marinangeli and Martin25)) differed greatly between studies. However, the habitual Ca intake of the subjects seems to have more decisive effect in the results than the doses or the supplementation periods. Some authors did not report the subjects’ habitual Ca intake(Reference Gunther, Legowski and Lyle10, Reference Thompson, Holdman and Janzow12). In other cases, daily habitual Ca consumption was greater than or between 700 and 800 mg/d(Reference Bowen, Noakes and Clifton9, Reference Harvey-Berino, Gold and Lauber11, Reference Jones, Eller and Parnell17). In these studies, no effect on adiposity or glycaemic profile was verified(Reference Bowen, Noakes and Clifton9–Reference Thompson, Holdman and Janzow12, Reference Jones, Eller and Parnell17). On the other hand, when habitual Ca intake at baseline was less than or between 600 and 700 mg/d, beneficial effects on body composition and on insulin sensitivity were observed(Reference Zemel, Thompson and Milstead8, Reference Zemel, Richards and Mathis13–Reference Stancliffe, Thorpe and Zemel15, Reference Torres and Sanjuliani18, Reference Rideout, Marinangeli and Martin25, Reference Maki, Nieman and Schild26). Zemel et al. (Reference Zemel, Teegarden and Loan14) emphasised the importance of selecting subjects with low habitual intake of Ca (less than 600 mg/d) for the benefits of increased Ca consumption to occur. Our subjects consumed 488·9 (sd 233·4) mg of Ca/d at baseline (Table 1).
Furthermore, studies comparing the effects of a high intake of dairy products v. a control diet (low in dairy products) have showed significant BW and central fat reductions when subjects were concomitantly submitted to energy restriction(Reference Zemel, Thompson and Milstead8, Reference Zemel, Richards and Mathis13, Reference Zemel, Teegarden and Loan14, Reference Torres and Sanjuliani18). The beneficial effects of increased Ca intake seem to be more significant when it is associated with moderate energy intake reduction(Reference Abargouei, Janghorbani and Salehi-Marzijarani6, Reference Stonehouse, Wycherley and Luscombe-Marsh7). However, in our study we observed that although the energy restriction prescribed was not followed by the subjects, the increased consumption of fat-free milk enhanced weight loss, improved body composition and promoted glycaemic control. Aside from that, consumption of low-fat dairy products seems to have more effect on T2DM control than the consumption of whole dairy products(Reference Candido, Ton and Alfenas5). We and other authors have observed positive effects of Ca supplementation through low-fat dairy products on body composition and on glycaemic profile(Reference Zemel, Richards and Mathis13, Reference Zemel, Teegarden and Loan14, Reference Torres and Sanjuliani18, Reference Rideout, Marinangeli and Martin25, Reference Maki, Nieman and Schild26).
In most of the studies in which dairy Ca did not affect BW, adiposity and IR, habitual Ca intake was equal to or higher than 700 mg/d(Reference Bowen, Noakes and Clifton9–Reference Thompson, Holdman and Janzow12, Reference Jones, Eller and Parnell17). Although some authors tested the effect of energy restriction(Reference Bowen, Noakes and Clifton9, Reference Harvey-Berino, Gold and Lauber11, Reference Thompson, Holdman and Janzow12, Reference Jones, Eller and Parnell17) and/or of low-fat dairy consumption(Reference Bowen, Noakes and Clifton9, Reference Harvey-Berino, Gold and Lauber11, Reference Thompson, Holdman and Janzow12), the habitual Ca consumption seems to be the main determinant of the outcomes. Other features, such as the use of low vitamin D content dairy products(Reference Bowen, Noakes and Clifton9), lack of statistical power(Reference Harvey-Berino, Gold and Lauber11) and intervention based only on nutritional counselling and using food records to assess food intake (since dairy product was not provided in the laboratory, there is no guarantee that the treatment was actually applied)(Reference Gunther, Legowski and Lyle10), may also partially explain the lack of positive effects in some studies.
About 25–35 % of the ingested Ca is absorbed in the intestine via paracellular (passive transport) and transcellular (active transport) pathways(Reference Kopic and Geibel28). Transcellular Ca absorption is mediated by calcitriol and occurs mainly in duodenum and jejunum(Reference Kopic and Geibel28). The rate of paracellular pathway is almost constant, while the transcellular pathway is more efficient under conditions of dietary Ca restriction(Reference Kopic and Geibel28). Our participants were low habitual Ca consumers (<600 mg/d), so probably the transcellular Ca uptake increased when they consumed more Ca (HC session). So, adding three servings of fat-free milk per d (HC session) could be of interest in clinical practice, since Western diet is typically rich in Na and P (minerals that reduce Ca absorption), and poor in Ca and vitamin D(Reference Calvo and Tucker29). However, the long-term effect of that is unknown, since the paracellular Ca absorption tends to increase under normal Ca consumption(Reference Kopic and Geibel28).
Possible mechanisms involving Ca on weight loss and glycaemic control are not clear. It has been suggested that a low-Ca intake increases serum PTH and calcitriol (1,25-dihydroxyvitamin D), resulting in a Ca2+ influx into adipocytes via either receptor- or voltage-mediated Ca2+ channel activation(Reference Zemel, Shi and Greer30). High intracellular Ca2+ concentration stimulates fatty acid synthase gene expression, and consequently results in the stimulation of fatty acid synthase activity, increasing de novo lipogenesis(Reference Zemel, Shi and Greer30). In addition, elevated Ca2+ concentration inactivates Ca-dependent kinases and Ca/calmodulin-dependent protein kinase, which phosphorylate phosphodiesterase, resulting in reduced cAMP concentrations and, consequently, in inhibition of hormone-sensitive lipase(Reference Larsson, Jones and Göransson31). Consequently, a low-Ca diet induces lipogenesis and inhibits lipolysis, leading to body fat accumulation and IR(Reference Zemel, Shi and Greer30). On the other hand, a high-Ca intake seems to reduce lipogenesis and promote lipolysis and increased thermogenesis, increasing fat oxidation and energy expenditure(Reference Zemel, Shi and Greer30). In our study, the breakfast shakes offered in both sessions contained 3·5 μg of vitamin D, which is about 20 % of the recommendation for adults(3). However, it seems that vitamin D from dairy shake (HC) was more bioavailable than the vitamin D3 supplemented (LC), since serum vitamin D only increased in HC. The role of calcitriol in lipogenesis has been discussed. Concomitant supplementation of Ca and vitamin D increases the intestinal absorption of Ca(Reference Candido and Bressan32) and BW loss(Reference Pittas, Lau and Hu2). In adipocytes, vitamin D suppresses the differentiation of preadipocytes, reducing lipogenesis(Reference Candido and Bressan32). We observed a decrease in serum PTH and an increase in serum 25-hydroyvitamin D after HC, suggesting the occurrence of these mechanisms.
Our breakfast shakes contained 17·1±0·2 g of protein. However, HC shakes contained 20 % casein and 80 % whey protein, while LC shakes contained 100 % whey protein. We included only whey protein in the LC because micellar casein (as present in milk) could not be used since it contains Ca(Reference Gaucheron33) and hydrolysed casein is less bioavailable(Reference Gaucheron33). In milk, about 30 % of Ca exists as free ionic Ca, and the remaining approximately 70 % is complexed with casein in micellar calcium phosphate(Reference Gaucheron33). Besides, hydrolysed casein coagulates in the stomach, being less available for enzymatic hydrolysis and less absorbed in the intestine(Reference Dugan and Fernandez34). Milk proteins and bioactive peptides have been associated with increased satiety, increased thermogenesis, muscle protein loss sparing and enhanced glycaemic control(Reference Acheson, Blondel-Lubrano and Oguey-Araymon35). Branched-chain amino acids, especially leucine, seem to increase body fat loss by increasing fat oxidation, stimulating muscle protein synthesis and thus reducing lean tissue loss(Reference Sun and Zemel36). Although casein and whey protein have similar amounts of leucine, when whey protein is ingested alone, its rapid intestinal transit reduces the absorption of branched-chain amino acid absorption(Reference Sun and Zemel36). When whey protein is ingested with casein, which occurs naturally in milk protein, it increases intestinal transit time favouring the absorption of branched-chain amino acids(Reference Sun and Zemel36). Therefore, in our study, the casein present in milk may have been essential to increase the uptake of leucine, which may have contributed to the lean tissue preservation and body fat reduction in HC.
The effects of Ca on glycaemic control seem to be associated with insulin secretion, since Ca stimulates the conversion of proinsulin into insulin and promotes insulin release by the pancreatic β-cells(Reference Pittas, Lau and Hu2). Thus, an inadequate Ca intake may alter the balance between extracellular and intracellular β-cell Ca pools, which may interfere with the secretory function of pancreatic β-cells(Reference Pittas, Lau and Hu2). In the present study, the increase in HOMA2-%B suggests an increase in insulin secretion after HC session. HOMA2-%B is considered the major contributor to the variability of HbA1c in people with T2DM(Reference Monnier, Colette and Thuan37). In addition, HC session decreased HbA1c concentrations compared with baseline and to LC session, improving our subjects’ glycaemic control. This reduction can be due to the direct (i.e. stimulation of insulin secretion) and indirect (i.e. BW loss and reduction of FM) effects of Ca on insulin sensitivity. Other milk components, such as Zn, Mg and vitamin D, play a key role on insulin action. Zn is involved in insulin synthesis, storage and secretion as well as in insulin hexametric form conformational integrity(Reference Yahya, Yahya and Saqib38). Mg acts in insulin secretion and is a cofactor for multiple enzymes involved in carbohydrate metabolism(Reference Yahya, Yahya and Saqib38). In pancreatic β-cells, vitamin D activates the transcription of the insulin gene and the insulin receptor gene(Reference Candido and Bressan32). In addition, the presence of a vitamin D response element in the human insulin gene promoter suggests a potential influence of vitamin D on glucose homeostasis(Reference Candido and Bressan32). Therefore, our results cannot be attributed to dairy Ca only, as the synergistic effects of different components of fat-free dairy products may have influenced our results. The amount of Ca added to HC shakes (700 mg) was established bearing in mind the need to offer a relatively high load of Ca without affecting its applicability in clinical practice. These shakes were very well tolerated by the subjects. However, we cannot assure that the results obtained in the present study will be observed if instead of being consumed once that same amount of Ca was consumed in small amounts throughout the day.
We demonstrated that HC session reduced serum acid uric concentrations compared with baseline and with LC session. Milk proteins (lactalbumin and casein) have uricosuric effect(Reference Ghadirian, Shatenstein and Verdy39). In diabetic patients, serum acid uric has been considered an early marker of impaired glucose metabolism and a good predictor of cardiovascular risk(Reference Verdoia, Barbieri and Schaffer40). Hyperglycaemia and hyperinsulinaemia resulting from IR decline renal function and increase uric acid production(Reference Neupane, Dubey and Gautam41). Concomitantly, high serum uric acid concentrations inhibit insulin signalling and induce IR(Reference Zhu, Hu and Huang42). Uric acid also inhibits endothelial nitric oxide bioavailability and activates rennin-angiotensin system, resulting in renal dysfunction, hypertension and dyslipidaemia(Reference Neupane, Dubey and Gautam41). In our study, the HC session decreased HbA1c and increased HOMA2-%B, suggesting that the attenuation of IR contributed to lower serum acid uric concentrations. Dietary fibre intake increased after the HC session. However, the recommendation for dietary fibre (14 g fibre/4184 kJ)(43) was achieved only in the HC session. The difference in dietary fibre between the experimental diets was approximately 9·4 g/d after the interventions (24·6 (sd 4·8) g/d in HC and 15·2 (sd 6·7) g/d in LC). An increase in fibre intake equivalent to 10 g/d contributes to a weight loss of only 39 g/year(Reference Du, Van der and Boshuizen44) and the consumption of 42·5 g of fibre/d can affect glycaemic control(Reference Silva, Kramer and de Almeida45), which refutes the possibility that fibre interfered in our results.
The low compliance to the dietary prescription is a limitation of the present study. This was a free-living study in which unfortunately it is not possible to strictly control the subjects’ food consumption, although they have been carefully instructed. The small sample size of our study limited the statistical power to conduct a multivariate statistical analysis. However, the randomisation process was carefully conducted by us. Because of that, the intervention groups (HC and LC) presented similar baseline body composition, besides clinical, biochemical and anthropometric data.
We conclude that the consumption of about three servings of fat-free milk (700 mg of dietary additional Ca/d) and 1200 mg of dietary Ca/d for 12 weeks promoted greater weight loss and glycaemic control in individuals with T2DM and low-habitual Ca consumption (<600 mg/d) than did the low-Ca diet (approximately 525 mg/d). These findings may be useful in the dietary treatment of T2DM.
Acknowledgements
We thank Instituto Federal do Sudeste de Minas (IF Sudeste MG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Bioclin, Quibasa Basic Chemical Ltda for the financial support.
J. M. G. G., J. A. C. and R. C. G. A. formulated the research question, designed the study, carried it out, analysed the data and wrote the article. P. V. M. R. assisted in the article writing.
The authors declare no conflicts of interest.







