Introduction
The development of the textile industry has many benefits. However, along with that development, people risks the environment becoming polluted by wastewater from industry, chemical factories, textile dyeing, etc. Azo dyes are utilized widely, primarily because of their low cost and significant color fastness (Hassan Alzain et al., Reference Hassan, Victor, Karim and Mona2023). Aromatic amines, the primary by-products of untreated azo dyes released into the environment, have been classified as compounds with significant potential to induce cancer, genetic mutations, and ecological imbalance (Rania Al-Tohamy et al., Reference Rania, Sameh, Fanghua, Kamal, Yehia, Tamer, Haixin, Yinyi and Jianzhong2022). Traditional methods of solving problems related to water treatment can be costly and cause secondary pollution (Chengyue et al., Reference Chengyue, Chuanzhi, Yong, Feng and Jianshe2022; Muhammad et al., Reference Muhammad, Norah, Lamia, Afifa, Walid and Munawar2022; Yanmin et al., Reference Yanmin, Yongsheng, Zhaoli, Tiantian, Qiangshan and Peng2022). Therefore, it is urgent to research and manufacture advanced materials that can treat environmental pollutants effectively. ‘Bleaching’ refers to the process of using chemicals or other agents to lighten or remove color from a material. Meanwhile, ‘mineralization’ refers to the process by which organic matter is converted into minerals. Mineralization of dyes typically refers to the degradation or breakdown of dye molecules into simpler, inorganic compounds. This process can metabolize or break down complex organic molecules such as dyes. The result of mineralization is the conversion of these organic dye molecules into inorganic end products. In the context of environmental science and waste-water treatment, the mineralization of dyes is important for reducing the environmental impact of dye-containing effluents. When dyes are released into water bodies or waste water, their persistence can be harmful. Mineralization processes play a role in breaking down these dyes into less harmful or inert substances, reducing the color and potential toxicity of the effluent. Recently, photocatalysis has been considered an effective solution to the problems above (Henrique et al., Reference Henrique, Andréa and Caue2010; Tammanoon et al., Reference Tammanoon, Varanya, Tanyaporn, Khuanjit and Suwat2021). Natural clays such as montmorillonite (Mnt), zeolite, kaolinite, and vermiculite with a porous structure, large surface area, and significant cation exchange capacity (CEC) have attracted much attention in recent years due to their potential applications in many different fields (Miao et al., Reference Miao, Zhimin, Buxing, Jianling, Xin, Jimin and Zhenyu2006; Ke et al., Reference Ke, Jingyi, Jie, Yumin and Wenxi2010; Zhaohui et al., Reference Zhaohui, Nicholas, Joseph, Jianle and Guocheng2018). Mnt is an aluminosilicate clay with a 2:1 layer structure, which can adsorb organic substances on the surface or in the space between its sheets by interacting or replacing exchangeable cations (Neelaveni et al., Reference Neelaveni, Santhana, Ramya, Sonia and Shanthi2019; Bang Tam et al., Reference Bang Tam, Thu Loan, Hon Nhien, Chi-Nhan, Mai Loan, Patrick and Dang Mao2021; Xinyu et al., Reference Xinyu Zhang, Yuhang, Yingying, Guoying and Zhenbo2021; Bang Tam et al., Reference Bang Tam, Loan Thu, Do Trung, Nhien Hon, Truong Huu and Chi-Nhan2022; Bang Tam et al., Reference Bang Tam, Thu Loan, Do Trung, Nhien Hon, Quoc Kien, Huu Truong and Chi-Nhan2023). However, Mnt has a disadvantage: it will reverse to desorption to the medium when the adsorption equilibrium is reached. Therefore, the ability to modify Mnt to improve and enhance the adsorption capacity is needed urgently at present (Alireza et al., Reference Alireza, Murat, Semra and Samira2016; Alireza et al., Reference Alireza, Kıransanc, Semra and Mohsen2017). Titanium dioxide (TiO2) pillared montmorillonite (PILM) was synthesized successfully by impregnating a TiO2 sol into the interlayers of Mnt using TiCl4 as the precursor of TiO2 (Gao et al., 2018). However, the effect of Mnt content on photocatalytic efficiency has not been studied. Due to their many exciting properties, porous heterostructures based on TiO2 and clay minerals have attracted more attention. These nanocomposites can be used as photocatalysts to treat waste water contaminated by toxic organic compounds (Ewa et al., Reference Ewa2021; Kelechi et al., Reference Kelechi, Kennedy, Kalu and Nnamdi2021; Joanna et al., Reference Joanna, Dariusz and Beata2022).
Several recent studies have investigated the synthesis of TiO2–clay composites using various methods, e.g. Aydin et al. (Reference Aydin, Alireza, Mehrangiz and Semra2018) synthesizing TiO2/Mnt nanocomposites by a simple hydrothermal method with tetraethyl orthotitanate as a precursor of Ti. The TiO2/Mnt was applied as a catalyst for the sonocatalytic degradation of ciprofloxacin. Under the following conditions, pH 6, catalyst dosage of 0.2 g L–1, initial CIP concentration of 10 mg L–1, and ultrasonic power of 650 W L–1, a degradation efficiency of 65.01% was obtained (Aydin et al., Reference Aydin, Alireza, Mehrangiz and Semra2018). Jiao et al. (Reference Jiao, Ting, Yanqing, Jianlong, Ruohua, Guoping and Junhui2018) prepared TiO2-pillared Mnt nanocomposites (TM) from Mnt with tetrabutyl titanate by a sol-gel method. The samples were placed in a chamber for 336 h under a 500 W UV lamp. Although the exposure time is long and the radiation power is high, the degradation efficiency is only 55% (Aydin et al., Reference Aydin, Alireza, Mehrangiz and Semra2018). Amit et al. (Reference Amit, Manisha, Akansha and Soumen2017) synthesized bentonite clay-based TiO2 with titanium (IV) butoxide under microwave conditions to degrade methylene blue under a 64 W UV lamp. The greatest degree of photocatalytic activity was achieved using bentonite containing 50% TiO2 by weight because of its relatively large specific surface area and pore volume (Amit et al., Reference Amit, Manisha, Akansha and Soumen2017). hydrothermally with tetrabutyl titanate for use as photocatalysts for methylene blue degradation at various pH values, reaction temperatures, and dwelling times; 30% TM achieved a methylene-blue removal efficiency of 95.6% (Yonghui et al., Reference Yonghui, Baoji, Qiuling, Zhiming, Yange and Basandorj2022). Many previous studies have prepared TiO2–clay nanocomposites by impregnating TiO2 either on the clay surface or between its layers with various precursors, such as titanium isopropoxide, titanium (IV) butoxides, titanyl sulfate (TiOSO4) or titanium tetrachloride (TiCl4) (Aydin et al., Reference Aydin, Alireza, Semra, Canan and Peyman2017; Kelechi et al., Reference Kelechi, Kennedy, Kalu and Nnamdi2021).
The difference in this study compared with previous studies is that the Mnt was denatured with TiO2 anatase (Merck) by a simple wet stirring method without annealing temperature. Mnt is purified from bentonite (Vietnam) by a chemical method (patented by the Intellectual Property Office of Vietnam with code B071-2013-02346 SC in 2017). Previous studies have not adequately explored how the Mnt content in nanocomposites affects photocatalytic degradation efficiency. Moreover, research on the photocatalytic degradation of azo-based organic dyes, such as rhodamine B (RhB), remains limited. This present study addressed these gaps by demonstrating a method that achieves high degradation efficiency of azo-organic dyes while minimizing the intermediate chemicals required in the synthesis process. Additionally, the Mnt/TiO2 nanocomposite developed in this study is anticipated to achieve high photodegradation efficiency with reduced contact time and lower excitation source power compared with existing methods.
Materials and methods
Materials
The present study used TiO2 (Merk KgaA, Germany; anatase phase), rhodamine B (Sigma-Aldrich, Singapore), bentonite (Lam Dong Province, Vietnam), ethanol (Merck KgaA, Germany), and NaCl (Merck KgaA, Germany).
Preparation of Mnt
The Mnt-purification steps in this study were similar to those used in previous studies (Ha Thuc et al., Reference Ha Thuc, Grillet, Reinert, Ohashi, Ha Thuc and Duclaux2010; Nguyen Van et al., Reference Nguyen Van, Chu Van, Luu Hoang, Vo Nguyen and Ha Thuc2020; Bang Tam et al., Reference Bang Tam, Thu Loan, Hon Nhien, Chi-Nhan, Mai Loan, Patrick and Dang Mao2021, Bang Tam et al., Reference Bang Tam, Loan Thu, Do Trung, Nhien Hon, Truong Huu and Chi-Nhan2022; Bang Tam et al., Reference Bang Tam, Thu Loan, Do Trung, Nhien Hon, Quoc Kien, Huu Truong and Chi-Nhan2023;). Bentonite was dispersed in water and stirred mechanically for 24 h. NaCl was then added and stirred mechanically for 3 h. The suspension was centrifuged and washed several times with deionized water and ethanol – the suspension was centrifuged and dried at 60°C for 72 h to obtain pure Mnt.
Preparation of Mnt–TiO2 nanocomposite
In this study, the amount of TiO2 used was calculated based on the CEC of Mnt by Eqn (1) (Johannes et al., Reference Johannes, Emil and Bambang2019):
where CEC is the cationic exchange capacity of Mnt (CEC = 0.00097 mol g–1; Ha Thuc et al., Reference Ha Thuc, Grillet, Reinert, Ohashi, Ha Thuc and Duclaux2010); A is the weight of Mnt (g); B is the molecular weight of TiO2 (g mol–1); and n is the ratio to be used (Tushar and Bergaya, Reference Tushar and Bergaya2006).
The steps for synthesizing Mnt–TiO2 nanocomposites were as in previous publications (Bang Tam et al., Reference Bang Tam, Thu Loan, Hon Nhien, Chi-Nhan, Mai Loan, Patrick and Dang Mao2021; Bang Tam et al., Reference Bang Tam, Loan Thu, Do Trung, Nhien Hon, Truong Huu and Chi-Nhan2022). Mnt was added slowly to 100 mL of deionized water and stirred until the Mnt was wholly dispersed in the solution. Nanocomposites of TiO2 and Mnt were formed by stirring TiO2 in Mnt suspension for 4 h with various mass ratios, m Mnt:m TiO2 = 10:1, 5:1, and 1:1; corresponding to the samples denoted MT10-1, MT5-1, and MT1-1. These mixtures were sonicated for 1 h. The products obtained were centrifuged and dried at 80°C for 24 h to form the Mnt–TiO2 nanocomposites.
Characterization
The elemental composition of the nanocomposites was determined by energy-dispersive X-ray spectroscopy (EDX; HORIBA H-7593, Hitachi, Japan). Scanning electron microscopy (SEM: S4800, Hitachi, Japan) was used to examine the samples’ surface morphology. Crystal characteristics and bonding vibrations were analyzed by the following methods, respectively: X-ray diffraction analysis (XRD; D2 PHASER diffractometer, Bruker, Germany) with Ni-filtered CuK radiation (λ=1.540 Å) at 40 kV, 40 mA, scan rate 0.030° s–1 and scan angle 5–80°2θ) and Fourier transform infrared spectroscopy (FTIR; Nicolet iS 50 (ThermoFisher, USA) in the range from 4000 to 400 cm−1). Surface areas of Mnt and MT10-1 were analyzed using the Brunauer–Emmett–Teller (BET) method with N2 adsorption-desorption isotherms at 77.3 K on the Nova 1000e (Quanta chrome) instrument (FL, USA). Their pore volume and diameter were analyzed using the Barrett–Joyner–Halenda (BJH) method. The absorption and photocatalytic properties were measured with UV-Vis absorption spectroscopy (Jasco V-670, Tokyo, Japan). The by-products of RhB degradation were analyzed via the LCMS method (XEVO-TQD, Milford, MA, USA).
Adsorption/photocatalysis experimental set-up
The photocatalytic decomposition of Rhodamine B (RhB) was carried out in a closed system consisting of an excitation source (a UVC lamp; 254 nm, 15 W) placed 7 cm above the surface of the solution (Fig. 1). Initially, 10 mg of the photocatalyst was dispersed in 100 mL of 10 ppm RhB. Before turning on the UVC lamp, the mixture was stirred magnetically in the dark for 60 min to reach adsorption–desorption equilibrium. The solution was then irradiated by a UVC lamp for 210 min. The photocatalytic reaction occurred during this time. Every 30 min, 10 mL of solution was extracted and stored in centrifuge tubes.

Figure 1. Experimental set-up of RhB photodegradation under the UVC light source.
The RhB adsorption experiment for 210 min was performed the same way as above but without UVC irradiation. The solution in the centrifuge tubes was centrifuged and measured with a UV-Vis spectrometer (Jasco V-670) in the 400–700 nm region, a step of 0.2 nm, and a scan rate of 400 nm min–1. Solution concentrations before and after adsorption/photocatalysis were used to calculate the decolorization efficiency using Eqn (2):
where H is the decolorization efficiency (%); C 0 is the concentration of the RhB initially (mg L–1); and C is the concentration of the RhB (mg L–1) at time t (min).
Results and Discussion
Structure confirmation of nanocomposites
The nanocomposites with different Mnt contents were denoted MT10-1, MT5-1, and MT1-1, corresponding to mass ratios of m Mnt:m TiO2 = 10:1, 5:1, and 1:1, respectively. The EDX spectra revealed that Mnt consisted of the following elements: Al, C, Fe, K, Mg, Na, O, Si, K, and Fe (Bang Tam et al., Reference Bang Tam, Thu Loan, Hon Nhien, Chi-Nhan, Mai Loan, Patrick and Dang Mao2021; Xinyu et al., Reference Xinyu Zhang, Yuhang, Yingying, Guoying and Zhenbo2021; Bang Tam et al., Reference Bang Tam, Thu Loan, Do Trung, Nhien Hon, Quoc Kien, Huu Truong and Chi-Nhan2023) (Fig. 2a). MT10-1, MT5-1, and MT1-1 also had the same elements as Mnt and Ti element peaks with different intensities depending on the mass ratio (Fig. 2b–d). As the ratio of m Mnt:m TiO2 decreases, the wt.% of Si/Ti also decreases because the amount of Ti in the nanocomposite increases (Amit et al., Reference Amit, Akansha and Soumen2018) (Fig. 2e). This showed that the ratio mMnt: mTiO2 affected the Ti content in the Mnt /TiO2 nanocomposite.

Figure 2. EDX spectra of Mnt (a), MT10-1 (b), MT5-1 (c), MT1-1 (d), and Si/Ti (wt.%) comparison (e) for Mnt, MT10-1, MT5-1, and MT1-1.
Variation in surface morphology of Mnt and nanocomposites observed by SEM
The surface of the pure Mnt had flake particles with an average size of 400–500 nm (Jiao et al., Reference Jiao, Ting, Yanqing, Jianlong, Ruohua, Guoping and Junhui2018; Phetladda et al., Reference Phetladda, Apisit, Weerapat, Pinit and Weekit2018; Bang Tam et al., Reference Bang Tam, Thu Loan, Hon Nhien, Chi-Nhan, Mai Loan, Patrick and Dang Mao2021) (Fig. 3a). After modification with TiO2 at the ratio m Mnt:m TiO2 = 10:1 (Fig. 3b; MT10-1), the Mnt surface was covered with tiny particles with a 30–45 nm diameter. These TiO2 particles were randomly dispersed on the surface and between the Mnt sheets (Aydin et al., Reference Aydin, Alireza, Mehrangiz and Semra2018; Bang Tam et al., Reference Bang Tam, Thu Loan, Hon Nhien, Chi-Nhan, Mai Loan, Patrick and Dang Mao2021; Menelisi et al., Reference Menelisi, Manoko and John2021). As the mass ratio of Mnt:TiO2 decreases, the density of TiO2 nanoparticles on the surface and the space between the Mnt sheets increases (Fig. 3c; MT5-1). When the ratio m Mnt:m TiO2 was 1:1, it was almost impossible to see the Mnt sheets because the amount of TiO2 nanoparticles in the nanocomposite was too great (Fig. 3d; MT1-1).

Figure 3. SEM images of raw Mnt (a), MT10-1 (b), MT5-1 (c), and MT1-1 (d).
The XRD patterns of raw Mnt, TiO2, MT10-1, MT5-1, and MT1-1 (Fig. 4) revealed that TiO2 nanoparticles had reflection planes belonging to the anatase phase with diffraction peaks at 25.3°, 37.9°, 47.8°, 54.3°, 55°, and 62.7°2θ, respectively, for the planes (101), (004), (200), (105), (211), and (204). The (101) reflection plane at 25.3°2θ was the characteristic peak of the anatase phase and has the greatest intensity (Bang Tam et al., Reference Bang Tam, Thu Loan, Hon Nhien, Chi-Nhan, Mai Loan, Patrick and Dang Mao2021; Mohamed et al., Reference Mohamed, Wojciech, Sylwester and Wojciech2021). Among the rutile, brookite, and anatase phases of TiO2, the anatase crystalline phase has the best photocatalytic activity (Rastgar et al., Reference Rastgar, Zolfaghari, Mortaheb, Sayahi and Naderi2013; Ayoubi-Feiz et al., Reference Ayoubi-Feiz, Aber, Khataee and Alipour2014). The (001) reflection plane of Mnt at 6.1°2θ had the greatest intensity. The raw Mnt has a basal spacing d 001Mnt = 14.5 Å (calculated by the Debye–Scherrer formula) (Mohamed et al., Reference Mohamed, Wojciech, Sylwester and Wojciech2021; Peng et al., Reference Peng, Mingming and Zhonghai2014). For the nanocomposites of Mnt and TiO2, the intensity of the (001) reflection plane in the Mnt tended to decrease gradually when the ratio m Mnt:m TiO2 decreased. The XRD pattern of MT10-1 showed that the (001) reflection plane shifted slightly to the right at 6.8°2θ with basal spacing d 001 MT10-1 = 14 Å and some low-intensity diffraction peaks typical of TiO2. A right shift of the (001) peak was also observed in the XRD pattern of MT5-1, although very small. In addition, when reducing the Mnt ctent in the nanocomposites, the characteristic diffraction peaks for Mnt decreased gradually and were scarcely observed for MT1-1, as TiO2 was dominant in the MT5-1 and MT1-1 samples. This result shows that the Mnt content affected the change in the structure of the nanocomposites. During the denaturation process, TiO2 nanoparticles were inserted into the space between the Mnt sheets or covered the Mnt surface, changing the distance between the Mnt sheets. Based on the analysis above, it was possible to identify the structure of the Mnt/TiO2 corresponding to the house-of-cards structure (Nesmeth et al., Reference Nesmeth, Deskany, Suvgh, Msrek, Klencsar, Vertes and Fendler2003; Phetladda et al., Reference Phetladda, Apisit, Weerapat, Pinit and Weekit2018) and model it as shown in Fig. 5.

Figure 4. XRD patterns of raw Mnt, TiO2, MT10-1, MT5-1, and MT1-1 (ο = montmorillonite, ∇ = quartz, ♦ = TiO2 anatase).

Figure 5. The house-of-cards Mnt/TiO2 nanocomposite.
The FTIR spectra of TiO2, Mnt, MT10-1, MT5-1, and MT1-1 (Fig. 6) in the wavenumber region 400–4000 cm–1 revealed that TiO2 has a peak at wavenumber 480 cm–1, which corresponds to the Ti–O stretching vibration (Aydin et al., Reference Aydin, Alireza, Semra, Canan and Peyman2017; Daesung et al., Reference Daesung, Garima, Minkyu and Sanghoon2019). The raw Mnt had vibrations at wavenumbers 3620 and 3420 cm–1 that are attributed to the -OH symmetric stretching vibrations of water molecules in the Mnt. The absorption bands at 1640 and 1050 cm–1 were attributed to the H-O-H and Si-O stretching vibrations, respectively. Bands at 912 and 785 cm–1 were attributed to the Al-O stretching vibration. In addition, 530 and 472 cm–1 represented the Al-O-Si symmetric stretching vibration and Si-O-Si strain vibration of Si-O-Si, respectively (Nadjia et al., Reference Nadjia, Hocine, Hussein and Bernard2013; Aydin et al., Reference Aydin, Alireza, Mehrangiz and Semra2018). When reducing the Mnt content in the nanocomposites of Mnt and TiO2, there was a vibration competition between the characteristic peaks of TiO2 and Mnt with wavenumbers 480 and 1050 cm–1, respectively. For MT10-1, because the TiO2 content was minimal, only the characteristic vibrations of Mnt can be observed. However, when the Mnt content decreased gradually, a Ti-O vibration in the 400–700 cm–1 region appeared more clearly. In addition, the FTIR spectra of all samples MT10-1, MT5-1, and MT1-1 appeared in the absorption bands at 935 cm–1. This region was attributed to the Ti··O-Si bonding, which indicated that TiO2 particles had been immobilized successfully into the Mnt structure to form the Mnt/TiO2 nanocomposite.

Figure 6. FTIR spectra of TiO2, raw Mnt, MT10-1, MT5-1, and MT1-1.
The UV-Vis diffuse reflectance spectra (Fig. 7) of TiO2, Mnt, MT10-1, MT5-1, and MT1-1 showed that the absorption edges of Mnt and TiO2 nanoparticles appeared at the wavelengths of 380 and 395 nm, respectively. When the Mnt content in the nanocomposite is large (MT10-1), the adsorption edge of MT10-1 is almost the same as that of Mnt. On the contrary, if the Mnt content in the nanocomposites (MT5-1 and MT1-1) is reduced, the adsorption edge of these nanocomposites behaves similarly to that of TiO2 nanoparticles. In other words, a blue shift of the absorption edges toward the ultraviolet region was found with increasing m Mnt:m TiO2 ratio due to the quantum size effect. In nanostructures, the discrete energy levels become more pronounced, and the energy band structure is altered. The energy levels become quantized, and the electronic properties of the material change. This results in changes in the material’s electronic and optical properties, such as the band gap energy. The band gap energy values of TiO2, Mnt, MT10-1, MT5-1, and MT1-1 (Fig. 8) are calculated based on Eqn 3 as 3.12, 2.75, 2.83, 2.97, and 3.07 eV, respectively. This indicates an increase in the optical band gap energy of Mnt by increasing the immobilization of TiO2 nanoparticles in the Mnt interspaces:
where
![]() $ {E}_{\mathrm{g}} $
is the band gap energy (eV), and λ is the absorption edge (nm).
$ {E}_{\mathrm{g}} $
is the band gap energy (eV), and λ is the absorption edge (nm).

Figure 7. UV-Vis diffuse reflectance spectra of TiO2, Mnt, MT10-1, MT5-1, and MT1-1.

Figure 8. The band gap extrapolation lines of TiO2, Mnt, MT10-1, MT5-1, and MT1-1.
Adsorption experiment
10 mg of each material (TiO2, Mnt, MT10-1, MT5-1, and MT1-1) was added to 100 mL of 10 ppm RhB solution and stirred under non-irradiation conditions (dark conditions) for 210 min. The variation of the C/C 0 concentration ratio over time (Fig. 9) shows that TiO2 had no adsorption capacity (C/C 0 = 0.95, H =5 %), while Mnt had the best RhB adsorption (C/C 0 = 0.38, H = 62%). The RhB adsorption efficiency decreased gradually with the decrease in the ratio m Mnt:m TiO2. These results indicated that reducing the Mnt content will reduce the adsorption of dye molecules of nanocomposites. The adsorption capacity vs time of TiO2, Mnt, MT10-1, MT5-1, and MT1-1 was calculated according to Eqn 4 showing that the maximum adsorption capacity in 210 min of Mnt, MT10-1, MT5-1, and MT1-1 was 74.8, 59.7, 41.2, and 18.3 mg g–1, respectively. After 60 min, the RhB concentration from the adsorption process did not change significantly. Therefore, the adsorption–desorption equilibration time of Mnt and nanocomposites was 60 min:
where q is the adsorption capacity (mg g–1); V is the volume of solution (L); m is the amount of adsorbent (g); C o is the concentration of the initial solution (mg L–1); and Ct is the concentration of the solution at time t (mg L–1).

Figure 9. RhB adsorption in a dark environment by TiO2, Mnt, MT10-1, MT5-1, and MT1-1.
Photocatalytic experiment
To evaluate the photocatalytic degradation of RhB, 10 mg of each material (TiO2, Mnt, MT10-1, MT5-1, and MT1-1) was added to 100 mL of 10 ppm RhB solution and stirred under 15 W UVC irradiation for 210 min. Before the UVC excitation source was switched on, the solution was stirred for 60 min to reach adsorption–desorption equilibrium. The change in concentration of the C/C 0 ratio vs time (Fig. 10) suggested that the 254 nm UVC light source has excitation energy (E UVC≈4.9 eV) greater than the band gap of TiO2 (E g≈3.2 eV) but also not enough energy to degrade RhB (C/C 0=0.85, H≈15%). TiO2 was known to be a typical photocatalytic metal oxide but had a very low photodegradation efficiency (C/C 0 = 0.8, H≈20%). The reason was that the recombination time between e+ and h- occurs too fast (recombination≈10–11 to 10–12 s) (Chung et al., Reference Chung, Yong and Abdul2011); so it did not generate many free radicals such as •OH or •O2 to participate in the degradation of organic dyes. Although Mnt adsorbed RhB molecules for the first 60 min when stirred in the dark (C/C 0 = 0.4), the change in RhB concentration in the solution was insignificant after UVC irradiation (C/C 0 = 0.3). This result demonstrated that Mnt was not photocatalytic. This small change in concentration may be due to the continuous adsorption–desorption of RhB by Mnt during the 210 min irradiation period.

Figure 10. Photocatalytic activities of RhB by TiO2, Mnt, MT10-1, MT5-1, and MT1-1.
In particular, the photocatalytic effect by nanocomposites of Mnt and TiO2 increased significantly. MT10-1 had the greatest photodegradation efficiency (C/C
o = 0.085, H≈91.5%), MT5-1 and MT1-1 have C/C
o of 0.3 and 0.4 (H = 70 and 60%), respectively. The lower the mass ratio m
Mnt:m
TiO2, the lower the photocatalytic efficiency. This showed that Mnt influenced the photocatalytic degradation of organic dye molecules. When a semiconductor material is exposed to light, electrons are excited from the valence band to the conduction band, leaving behind positively charged holes in the valence band. These electron-hole pairs are highly reactive and can participate in redox reactions. Because of its good adsorption properties, Mnt acted as an adsorption center to capture RhB molecules in clay mineral layers and suppressed the TiO2 electron-hole pair recombination by trapping photogenerated e- into empty d orbitals of the metal in Mnt (Fangfei et al., Reference Fangfei, Yinshan, Maosheng, Mengmeng, Bing and Xuehong2009; Ling et al., Reference Ling, Yuan, Huang, Guo, Yang and Yu2011; Asma et al., Reference Asma, Muhammad, Ali, Faiza, Maaz, Qasim and Muhammad2021; Anh et al., Reference Anh, Thi Duy Hanh, Kuan-Yew and Swee-Yong2022). Therefore, the •OH or •O2- reactive species generated from the photocatalytic reaction of TiO2 will easily react with the RhB molecules and decompose them into intermediates (Eqns 15 and 16). Oxygen molecules in the air can be reduced by electrons from the conduction band to form superoxide radical anion (•
![]() $ {\mathrm{O}}_2^{-} $
). This species is a potent oxidizing agent. Hydroxyl radicals (•OH) are generated through the reaction of photogenerated holes with water or hydroxide ions. They are highly reactive and can oxidize organic pollutants. The reduction of oxygen molecules by electrons from the conduction band can also lead to the formation of hydrogen peroxide (H2O2). This species can act as an oxidant in photocatalytic reactions. Hydroperoxyl radical (HO₂•) can be formed through the reaction of superoxide radicals with water. It is involved in various oxidation processes. The positive holes left behind in the valence band can participate directly in redox reactions with adsorbed species on the catalyst surface. Photo-oxidation in the valence band and photo-reduction in the conduction band can occur step by step according to the following equations (Eqns 4–16) (Gao et al., Reference Gao, Xin, Fang, Xin, Zhou, Yan and Jun2008; Ewa et al., Reference Ewa2021; Kelechi et al., Reference Kelechi, Kennedy, Kalu and Nnamdi2021):
$ {\mathrm{O}}_2^{-} $
). This species is a potent oxidizing agent. Hydroxyl radicals (•OH) are generated through the reaction of photogenerated holes with water or hydroxide ions. They are highly reactive and can oxidize organic pollutants. The reduction of oxygen molecules by electrons from the conduction band can also lead to the formation of hydrogen peroxide (H2O2). This species can act as an oxidant in photocatalytic reactions. Hydroperoxyl radical (HO₂•) can be formed through the reaction of superoxide radicals with water. It is involved in various oxidation processes. The positive holes left behind in the valence band can participate directly in redox reactions with adsorbed species on the catalyst surface. Photo-oxidation in the valence band and photo-reduction in the conduction band can occur step by step according to the following equations (Eqns 4–16) (Gao et al., Reference Gao, Xin, Fang, Xin, Zhou, Yan and Jun2008; Ewa et al., Reference Ewa2021; Kelechi et al., Reference Kelechi, Kennedy, Kalu and Nnamdi2021):
Photo-oxidation in the valence band (VB):
Photo-reduction in the conduction band (CB):
To further elucidate the RhB degradation photocatalytic ability of MT10-1 nanocomposite, the TiO2 content was increased from 10 to 30 mg to compare with the photocatalytic efficiency of MT10-1 (Fig. 11). Although the TiO2 content in MT10-1 was only ~0.9 mg, the photocatalytic efficiency (H MT10-1=91.5%, C/C 0=0.085) was greater than the photocatalytic efficiency of 10 mg TiO2 (H 10mgTiO2=20%) or 20 mg TiO2 (H 20mgTiO2=62%) or even 30 mg TiO2 (H 30mgTiO2=68%). This indicated the essential role played by Mnt in the nanocomposite. In the presence of Mnt, only a small amount of TiO2 was sufficient for TiO2 to exhibit photocatalytic activity.

Figure 11. Photocatalytic activity of MT10-1 compared with those of commercial TiO2 present in various amounts.
The surface textures of TiO2, Mnt, MT10-1, MT5-1, and MT1-1 were determined by the N2 adsorption–desorption isotherms (Fig. 12 ). The BET analysis of TiO2 nanoparticles shows a surface area of 20.5 m2 g–1, indicating a moderate extent of surface exposure for reactions or adsorption. The pore diameter of 5.6 Å suggests very small, possibly microporous structures, while a pore volume of 0.080 cm3 g–1 reflects a relatively low porosity. All the samples (Mnt and nanocomposites of Mnt) showed the hysteresis loops of type IV isotherm-desorption curves. Type IV isotherms originate from the capillary condensation in the mesopores and the interaction of molecules in the condensed state. In addition, the hysteresis loops were H4 types according to the IUPAC (the International Union of Pure and Applied Chemistry) classification. They were commonly found in microporous and medium mesopore materials. This indicated that Mnt and nanocomposites of MnT and TiO2 had open slit-shaped pores of plate-like particles. The specific surface areas of Mnt and MT10-1 were obtained from the BET measurement, and the pore size and the pore volume of Mnt and MT10-1 were calculated by the BJH method and are summarized in Table 1. Among them, the hysteresis loop of Mnt and MT10-1 has the most obvious openness. The BET surface area of MT10-1 (116.8 m2 g–1) was significantly enhanced compared with that of raw Mnt (83.2 m2 g–1) because of more open and less ordered pore structures (Phetladda et al., Reference Phetladda, Apisit, Weerapat, Pinit and Weekit2018). At low pressure, the adsorption of both Mnt and MT10-1 increased slowly, reflecting the adsorption of N2 molecules on the inner surface of the mesoporous single-to-multilayer system. The jump in adsorption quantity at p/p 0=0.42–0.53 continued to increase at higher pressures, indicating a mesoporous system. In contrast, the hysteresis loops of MT5-1 and MT1-1 show that both nanocomposites have a poorly mesoporous structure. The layers of Mnt appear to be disordered due to the presence of a large amount of intercalated TiO2.

Figure 12. N2 adsorption–desorption isotherms of Mnt, MT10-1, MT5-1, and MT1-1.
Table 1. Textural characteristics of TiO2, Mnt, MT10-1, MT5-1, and MT1-1

The pore-size distribution is depicted in Fig. 13. The average pore widths of Mnt, MT10-1, MT5-1, and MT1-1 are 4.1, 3.5, 3.2, and 1.02, respectively (table inset). This shows that TiO2 ions reduced the pore size when they intercalated in the montmorillonite layers. The more TiO2 concentration increases, the more the pore size decreases. This result is consistent with the house-of-cards structural model.

Figure 13. Pore-size distribution curves of Mnt, MT10-1, MT5-1, and MT1-1.
The photocatalytic stability of MT10-1 for RhB degradation was tested by running three consecutive experiments under the same conditions (Fig. 14). Photocatalysts after each experiment were centrifuged, washed, dried, and finely ground for use in subsequent experiments. After three consecutive runs, the accumulation of photocatalyst products and RhB molecules on the photocatalyst surface reduced the active sites, resulting in a decrease in photocatalytic degradation efficiency of 17%.

Figure 14. Photocatalytic activity of MT10-1 for three consecutive runs.
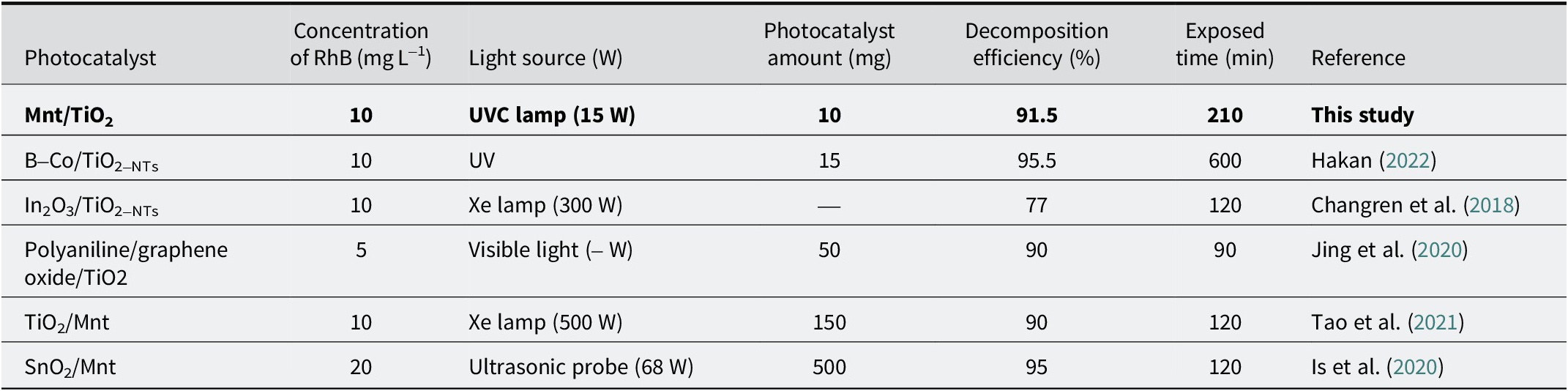
We compared this with other photocatalysts based on TiO2 or Mnt from previous studies, in Table 2. Although each study had different preparation methods with various substances based on parameters to evaluate the effectiveness, e.g. initial RhB concentration, photocatalyst content, irradiation source power, and time exposed, the RhB degradation efficiency of MT10-1 in the present study was significantly better. The results found that the less TiO2, the better the photocatalytic efficiency, and the best ratio determined in this study was m Mnt:m TiO2=10:1. This mass ratio indicated an important contribution of Mnt to the RhB degradation of porous heterostructures based on Mnt and TiO2.
Table 2. Comparing the photocatalytic efficiency of TiO2-based photocatalysts for the degradation of rhodamine B with earlier studies
Kinetics model and intermediate products
The kinetic model of the photocatalytic degradation of RhB from Mnt and the nanocomposites of Mnt and TiO2 were analyzed by the Langmuir–Hinshelwood apparent first-order kinetics equation. Figure 15 shows a plot of linear lines between ln(C 0/C) vs time t corresponding to Mnt, TiO2, MT10-1, MT5-1, and MT1-1, demonstrating that the photodegradation was consistent with the Langmuir–Hinshelwood first-order kinetic model and this result was consistent with many previous studies (Hannatu et al., Reference Hannatu, Mansor, Mohd, Nor, Aminu and Tawfik2017; Ali et al., Reference Ali, Hajir, Javad, Mohammad and Mehrorang2021). Table 3 summarizes the kinetics of RhB degradation over time of Mnt, TiO2, MT10-1, MT5-1, and MT1-1. The results showed that the kinematic equations had a linear form with high coefficients of determination, R 2. MT10-1 had the highest K value compared with Mnt, TiO2, MT5-1, and MT1-1, demonstrating that MT10-1 had the greatest photodegradation efficiency.

Figure 15. Fitting curves of the kinetic model for Mnt, TiO2, MT10-1, MT5-1, and MT1-1.
Table 3. Kinetic equations, reaction rate constants (K), and regression coefficients (R 2) of photocatalytic degradation of RhB for Mnt, TiO2, MT10-1, MT5-1, and MT1-1

The photocatalytic degradation not only discolored RhB but also broke the bonds in the RhB molecule into smaller molecules and was identified through the LCMS method (Fig. 16). After 210 min of excitation by UVC radiation, the final solution was analyzed, and seven by-products were identified comprising the proposed scheme of RhB degradation (Fig. 17). The results showed that the initial organic dye (RhB 443m/z) (Tao et al., Reference Tao, Xinyu and Shuyi2021) in the final solution was detected at just a weak intensity. First, the N-demethylation process formed nitrogen-centered reactive radicals. The by-product of this process was formed from the cleavage of the xanthene ring in the RhB molecular structure by one (or several) ethyl group, including N,N,N-diethyl-N′-ethyl rhodamine (415m/z); N,N-diethyl-rhodamine (388m/z) (Changren et al., Reference Changren, Zijun, Cong, Xiaoqing, Guoqing and Huageng2018); N′-monomethyl-ethyl rhodamine (360m/z) (Hegazey et al., Reference Hegazey, Abdelrahman and Kotp2020); and N′-ethyl rhodamine (317m/z) (Sharmaa et al., Reference Sharmaa, Dionysiou, Sharm, Kumar, Al-Muhtaseb, Naushad and Stadler2019). Second, the dye chromophore structure of RhB was cleaved. During this process, the carbon centered in the RhB molecules was attacked by active species such as h+, •OH, and •
![]() $ {\mathrm{O}}_2^{-} $
and oxidized them to the subsequent low-weight by-products (9-phenyl- 3H-xanthene (257m/z) and benzoic acid (123m/z)) (Tao et al., Reference Tao, Xinyu and Shuyi2021). Finally, the ring-opening process broke the ring and formed a smaller compound, namely malonic acid (115m/z) (Ali et al., Reference Ali, Amir, Moslem, Sahand and Babak2018). Malonic acid is an organic acid and can undergo biodegradation through biological processes. When released into the environment, malonic acid can be metabolized by various microbial organisms that possess the necessary enzymes to break down its chemical structure. These micro-organisms use malonic acid as a carbon and energy source during their metabolic activities, leading to its degradation into carbon dioxide (CO2) and water (H2O) or other simple and non-harmful by-products. This biodegradation process helps to reduce the concentration of malonic acid in the environment and keeps the natural ecosystem stable.
$ {\mathrm{O}}_2^{-} $
and oxidized them to the subsequent low-weight by-products (9-phenyl- 3H-xanthene (257m/z) and benzoic acid (123m/z)) (Tao et al., Reference Tao, Xinyu and Shuyi2021). Finally, the ring-opening process broke the ring and formed a smaller compound, namely malonic acid (115m/z) (Ali et al., Reference Ali, Amir, Moslem, Sahand and Babak2018). Malonic acid is an organic acid and can undergo biodegradation through biological processes. When released into the environment, malonic acid can be metabolized by various microbial organisms that possess the necessary enzymes to break down its chemical structure. These micro-organisms use malonic acid as a carbon and energy source during their metabolic activities, leading to its degradation into carbon dioxide (CO2) and water (H2O) or other simple and non-harmful by-products. This biodegradation process helps to reduce the concentration of malonic acid in the environment and keeps the natural ecosystem stable.

Figure 16. By-products of RhB degradation by MT10-1 were identified using LCMS.

Figure 17. The proposed pathway of rhodamine B degradation under UVC light irradiation.
Conclusions
In the present study, Mnt/TiO2 nanocomposites with different mass ratios were synthesized by simple wet stirring and no high temperature with TiO2 nanoparticles immobilized on the surface and randomly inserted into the Mnt to form the house-of-cards structure. Investigating the influence of the ratio m Mnt:m TiO2 showed that the nanocomposite with the ratio m Mnt:m TiO2 = 10:1 had the highest RhB photocatalytic efficiency (HMT10-1 = 91.5%). Due to its good adsorption properties, Mnt acts as an adsorption site and prevents recombination. The lifetime of free holes is extended to enhance the production of •OH or •O2– radicals to degrade RhB. The study shows the critical role of Mnt in improving the photocatalytic efficiency of Mnt–TiO2 nanocomposites and the potential to become an advanced material in the treatment of polluted water.
Author contribution
Bang Tam Thi Dao: Conceptualization, Methodology, experiments, Writing- Reviewing, and Editing. Thu Loan Thi Ha: Conceptualization, Methodology, experiments, Writing- Reviewing, and Editing. Trung Do Nguyen: characterizations and operation, Writing- Reviewing, and Editing. Hon Nhien Le: Characterizations and operation, Writing, Reviewing and Editing. Tien Trung Vu: Visualization, Investigation; Huu Truong Nguyen: Visualization, Investigation; Chi Nhan Ha-Thuc: Supervision, Writing Reviewing, and Editing.
Acknowledgements
This work was also supported by Laboratory of Fundamental Materials Science - Faculty of Materials Science and Technology from the University of Science, VNU-HCM.
Financial support
This research is funded by Vietnam National University, Ho Chi Minh City (VNU-HCM) under grant number 562-2022-18-01.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
No data available with manuscript.























