The urinary iodine (UI) concentration (μg l− 1) is not interchangeable with 24 h UI excretion (μg per 24 h). The two values are interchangeable only if the volume of urine passed in 24 h is one litre. The average volume of urine passed by an adult is approximately 1.5 l per 24 h. Therefore, the median UI excretion given as μg per 24 h will be 50% higher than the median iodine excretion given as μg l− 1.
The concentration of iodine in a spot or casual urine sample cannot be used to diagnose iodine deficiency in an individual. The UI concentration may vary up to threefold in an individual during a day. This means that it is necessary to collect repeated urine samples from an individual over a period of time and estimate the median or average, in order to evaluate their iodine status.
A moderate fall in the concentration of serum free T4 during pregnancy is not a sign of maternal iodine deficiency. Even in iodine-replete women, a 10–20% fall in serum free T4 is observed in late pregnancy.
An increase in the concentration of serum thyroglobulin (Tg) during pregnancy is not a sign of a maternal iodine deficiency.Even in iodine-replete women, the serum Tg concentration may increase during pregnancy. This is probably caused by the greater thyroid secretory activity of pregnant women.
A higher concentration of thyroid-stimulating hormone (TSH) and Tg in cord blood than in maternal blood is not a sign of iodine deficiency in the mother or neonate. This is a normal phenomenon, not related to iodine deficiency.
Thyroid function in a full-term foetus, a neonate or a small child is not more sensitive to a mild iodine deficiency than in the mother. Prospective intervention studies and cross-sectional studies show no evidence for such a difference.
An adequate intake of iodine is essential for thyroid hormone synthesis and consequently for normal development and metabolism. The major determinants of the iodine intake of a population are: the natural iodine in food and waterReference Laurberg, Andersen, Pedersen, Ovesen and Knudsen1; the iodine content of mineral mixtures and food given to domestic animals that provide food for humansReference Phillips2; the use of iodine-containing chemicals by the food industryReference London, Vought and Brown3 and iodine supplements taken by individuals or given to populationsReference Pedersen, Iversen and Laurberg4. In large parts of the world, the natural iodine content of food or water is low and people living in such areas are at risk of iodine deficiency disordersReference Kelly and Snedden5.
Iodine deficiency in a population has a number of harmful consequences for health and economic development; this is reviewed elsewhereReference Hetzel6. The most severe consequence of iodine deficiency is brain damageReference Dunn and Delange7. Sufficient amounts of thyroid hormone are needed for the proper development of the central nervous systemReference Morreale de Escobar, Obregon and Escobar del Rey8, and a woman's requirements for iodine in order to achieve physiological thyroid hormone production are increased during pregnancyReference Glinoer9. Prophylaxis against brain damage caused by iodine deficiency has been the major force behind the tremendous movement in recent decades towards the eradication of iodine deficiencyReference Hetzel, Delange, Dunn, Ling, Mannar and Pandav10. About 70% of the population of the world are now covered to some degree by a public iodine supplementation programme, typically by the fortification of salt. Since the foetus and the young infant are most vulnerable, and since iodine requirements are greater than normal in pregnant and breast-feeding women, there are special concerns about ensuring an adequate iodine intake during these periods.
In pregnancy and also in foetal and neonatal life, thyroid function undergoes a series of interacting physiological changes that complicate the evaluation of iodine statusReference Burrow, Fisher and Larsen11. Some of these changes are occasionally taken for signs of iodine deficiency, even if they are not associated with the iodine intake. When evaluating iodine requirements, physiological alterations should be separated from non-physiological disturbances. This is important because epidemiological studies suggest that a high iodine intake may be associated with more hypothyroidism in a general populationReference Laurberg, Pedersen, Knudsen, Ovesen and Andersen12 and also in women of reproductive ageReference Laurberg13. The optimal iodine intake should be sufficient to prevent iodine deficiency disorders, but not greater.
There are several circumstances in which data on indicators of iodine status and thyroid hormones may be misinterpreted when studying pregnant women and small children.
The UI concentration (μg l−1) is not interchangeable with 24 h UI excretion (μg per 24 h)
The original recommendations for iodine intake were mainly developed from data on the association between the prevalence of goitre in groups of people and their average UI excretion. The results from a large survey conducted in Central America in the late 1960s shown in Fig. 1 reveal how a mild iodine deficiency (average UI excretion of 50–99 μg day− 1) was characterised by endemic goitre in some areas, moderate iodine deficiency (25–49 μg day− 1) by endemic goitre in many areas and severe iodine deficiency ( < 25 μg day− 1) by endemic goitre in all areasReference Ascoli and Arroyave14.
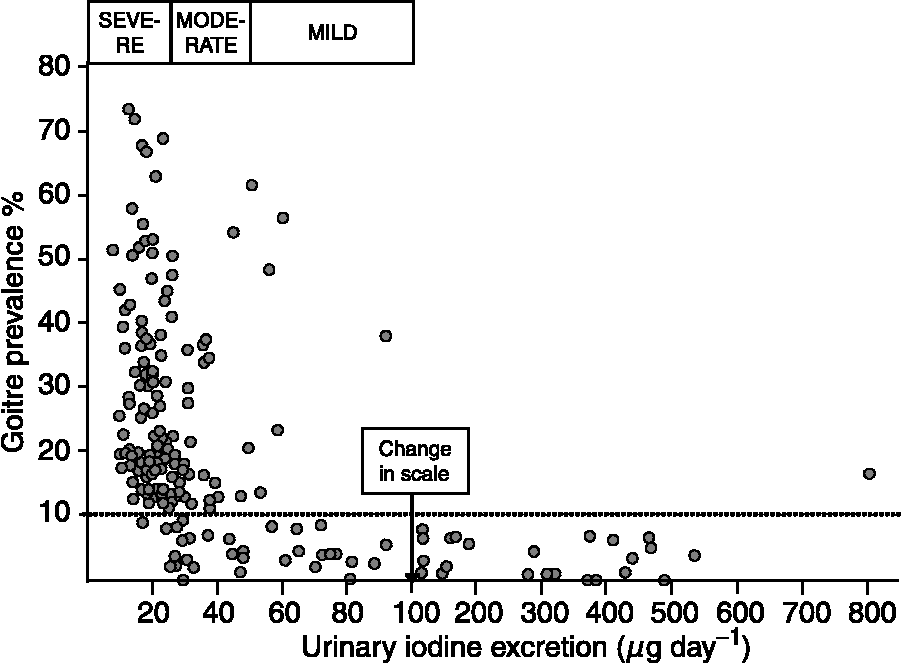
Fig. 1 The average urinary iodine (UI) excretion (μg day− 1) and the prevalence of goitre by clinical examination in people in 186 localities in Central America between 1965 and 1967Reference Ascoli and Arroyave14. In each locality, members of approximately 20 randomly selected families were investigated. A total of 21 611 people from 3712 families were investigated for goitre, and the concentrations of iodine and creatinine were measured in a late morning spot urine sample in 3181 randomly chosen participants. The daily iodine excretion was estimated from iodine and creatinine concentrations using an equation correcting for body weight, and age- and sex-dependent differences in 24 h urinary creatinine excretionReference Arroyave, Méndez and Ascoli39. The boxes represent the range in UI excretion that corresponds to a severe, moderate or mild iodine deficiency. The dotted line was added in the original publication to indicate the definition of endemic goitre (goitre prevalence of more than 10%) at the time of investigation. Redrawn from Ascoli and ArroyaveReference Ascoli and Arroyave14 with permission.
Since collecting all urine passed for 24 h is cumbersome to do and may be incomplete, the iodine concentration in spot sample of urine expressed as microgram of iodine per gram of creatinine is often usedReference Patrito, Marocco and Costa15. If on average, the 24 h urinary creatinine excreted by an individual in the population under study is close to 1 g, this would give a value nearly identical to 24 h UI excretion. However, creatinine excretion may deviate substantially from 1 g per 24 h in some population groupsReference Remer and Manz16, Reference Bourdoux17. In particular, it may be lower than 1 g in protein-deficient populations and in children and may be higher in young men.
For these reasons, the iodine/creatinine ratio came into discredit and was replaced by the simple concentration of iodine in urine18. This corresponds to 24 h UI excretion if the volume of urine produced by the group under study is 1 l day− 1, as it may be in schoolchildren. However, in adolescents and adults, the average urine volume is more likely 1.5 l per 24 h, and therefore, an iodine concentration of 100 μg l− 1 corresponds to an iodine excretion of approximately 150 μg per 24 h.
The discrepancy between the UI concentration and 24 h iodine excretion has been shown in a number of studies. Figure 2 illustrates this point and shows that the concentration of iodine in a casual sample was, on an average, 60–65% of the amount excreted in 24 hReference Rasmussen, Ovesen and Christiansen19. In the study illustrated in Fig. 2, reliable estimates of the 24 h iodine excretion were obtained from the iodine and creatinine concentrations measured in a non-fasting urine sample collected in the morning and adjusted using data on the average 24 h urinary creatinine excretion in a similar cohort of people, as suggested by Knudsen et al. Reference Knudsen, Christiansen, Brandt-Christensen, Nygaard and Perrild20 The values of UI excretion in microgram per day depicted in Fig. 1 are derived from the iodine and creatinine concentrations measured in a spot sample of urine using a somewhat similar principleReference Ascoli and Arroyave14.
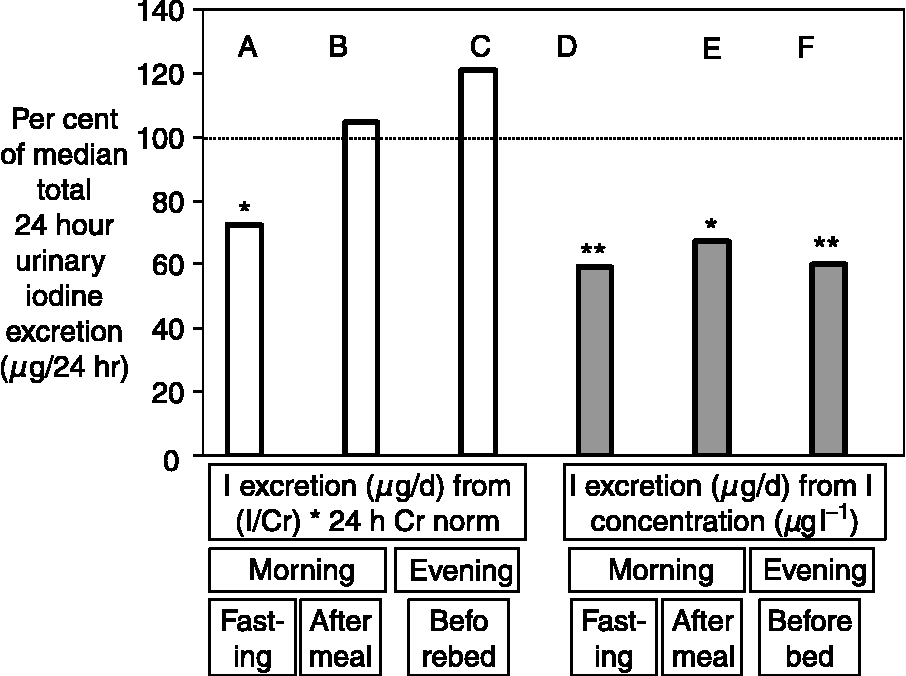
Fig. 2 Comparison of various methods used to estimate 24 h urinary iodine (UI) excretion using a single urine sample collected from healthy adults (n = 21). The columns show the median estimated 24 h UI excretion, obtained from a single urine sample by measurements and calculations as indicated, expressed as a percentage of the median amount of iodine directly measured in the 24 h urine collected on the same day. The estimates in the columns A–C were obtained using the equation: 24 h iodine excretion (μg per 24 h) = (iodine concentration (μg l− 1)/creatinine concentration (g l− 1)) × (24 h creatinine excretion for group (g per 24 h)), whereas the estimates in columns D–F (shaded) were obtained from the simple assumption that: 24 h iodine excretion (μg per 24 h) = iodine concentrations in the sample of urine (μg l− 1). The UI excretion (μg per 24 h) was considerably underestimated from the iodine concentration in a casual sample at all times of the day (D–F) (*P = 0.006, **P = 0.001). When the creatinine concentration was used to correct the iodine content, only the iodine excretion estimated from a fasting morning urine sample (A) was significantly different from the actual iodine content of the 24 h collection. The normal 24 h creatinine excretion for people of the same sex and age used for calculation (‘24 h Cr norm’) were the average values taken from a population study. The data are from reference 19.
In practical terms, the shift from using a UI excretion of 100 μg per 24 h to a UI concentration of 100 μg l− 1, as the low threshold indicating a sufficient iodine intake, resulted in an increase in the recommended iodine intake for many groups of people without any real evidence that this was necessary to avoid iodine deficiency disorders. The impact of this was considerable. For example, Fonzo et al. Reference Fonzo, Germano, Gallone and Migliardi21 studied UI excretion in over 3800 young men in Piedmonte and the Aosta Valley, a formerly severely iodine-deficient area in Italy. The median UI concentration was 101.8 μg l− 1 and the conclusion was that iodine intake may still be of borderline sufficiency. But the median 24 h UI excretion in these young men would probably be approximately 150 μg, which corresponds to the recommended daily iodine intake using the old system.
The concentration of iodine in a spot sample of urine is rarely identical to 24 h UI excretion. The UI excretion of groups of healthy adolescents and adults measured as μg per 24 h is often equal to UI measured as μg l− 1 × 1.5. When UI excretion is used to evaluate iodine intake, a correction should also be made for the amount of iodine excreted through other routes, mostly in faeces, which is approximately 10% of intake.
The concentration of iodine in a spot or casual urine sample cannot be used to diagnose iodine deficiency in an individual
Such misinterpretation may be illustrated by a recent study of iodine deficiency in Spanish schoolchildrenReference Serra-Prat, Diaz, Verde, Gost, Serra and Puig Domingo22. A cross-sectional study of 987 four-year-old children gave a mean UI concentration of 214 μg l− 1 (median 189 μg l− 1), which is not low. Nevertheless, it was concluded that 7.8% of the children had iodine deficiency, because 7.8% of urinary samples had an iodine concentration of < 100 μg per lReference Serra-Prat, Diaz, Verde, Gost, Serra and Puig Domingo22.
The concentration of iodine in casual samples of urine may vary up to threefold in an individual during a single dayReference Rasmussen, Ovesen and Christiansen19, and in a group of people, the distribution of average iodine concentrations in several samples from the same subjects is much narrower than the distribution of values from single spot samples from the same peopleReference Andersen, Pedersen, Pedersen and Laurberg23. Only the median or average can be used to classify iodine intake. If iodine deficiency is to be diagnosed in an individual, a series of samples taken over a period of time should be collected and analysed. Alternatively, a group of individuals may be studied and median values from single sampling used for evaluation of the entire group.
A moderate fall in the serum concentration of free T4 during pregnancy is not a sign of maternal iodine deficiency
When a reliable method such as equilibrium dialysis is used to make measurements, the serum concentration of free T4 is 10–20% lower than normal in late pregnancyReference Weeke, Dybkjær, Granlie, Eskjær Jensen, Kjærulff, Laurberg and Magnusson24. This decrease is not ameliorated by giving iodine supplements to mothersReference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen25, but iodine-deficient women may show an even greater fall in their free T4 concentrationReference Glinoer9. Thus, a low free T4 concentration in late pregnancy may be a sign of a low iodine intake, but not necessarily so.
An increase in the serum concentration of Tg during pregnancy is not a sign of a maternal iodine deficiency
In population studies, the serum Tg concentration is a good marker of iodine deficiencyReference Knudsen, Pedersen, Joergensen, Perrild, Ovesen and Laurberg26, but a high serum concentration of Tg is not a specific sign of iodine deficiency. The release of Tg from the thyroid may be altered in a number of thyroid disease states, and even if the iodine intake is sufficient, stimulation of thyroid hormone secretion will lead to an increase in the serum concentration of TgReference Feldt-Rasmussen27. During a normal pregnancy, there is a considerable increase in requirements for thyroid hormone and therefore also in thyroid secretory activityReference Alexander, Marqusee, Lawrence, Jarolim, Fischer and Larsen28.
In Denmark, we found a higher serum Tg concentration in pregnant women than in controlsReference Pedersen, Børlum, Hansen, Johannesen, Knudsen and Laurberg29. To evaluate if this difference was caused by a low iodine intake alone or if it was due to an increase in thyroid secretory activity associated with pregnancy, we compared the concentration of Tg in serum of pregnant and non-pregnant women in areas with different iodine intakesReference Laurberg, Bjarnadottir, Pedersen, Børlum and Hreidarsson30. As illustrated in Fig. 3, the concentration of Tg in serum was higher in the area of low iodine intake, but pregnant woman had higher serum Tg concentration than control women in all areas. The findings suggest that the increase in serum Tg concentration during pregnancy is primarily caused by greater thyroid secretory activity and that it is not a sign of iodine deficiency. This is supported by a recent longitudinal study of serum Tg concentration in pregnant women living in Sweden: the concentration was approximately 33% higher in late pregnancy than 1-year post-partum Reference Soldin, Tractenberg, Hollowell, Jonklaas, Janicic and Soldin31.
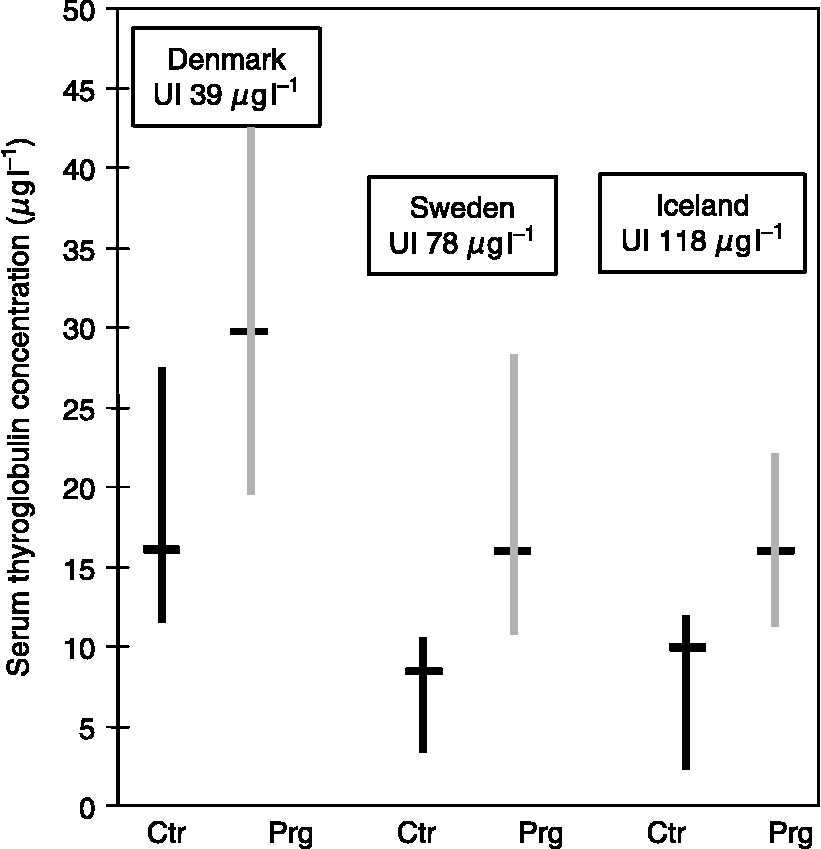
Fig. 3 The serum thyroglobulin concentration (median with 95% confidence interval) of pregnant women (Prg) and non-pregnant controls (Ctr) in three places with different iodine intakes (East-Jutland, Denmark; North Sweden; and Iceland)Reference Laurberg, Bjarnadottir, Pedersen, Børlum and Hreidarsson30. Serum was obtained from 20 Prg admitted for delivery at full term and after an uncomplicated pregnancy; Ctr were 20 non-pregnant healthy hospital employees of a similar age. None of the women took iodine-containing supplements. The median urinary iodine concentrations in spot urine samples from the Prg are shown in boxes above the bars.
A higher concentration of TSH and Tg in cord blood than in maternal blood is not a sign of iodine deficiency in the mother or neonate
In a recent study performed in the Sudan, the median concentrations of TSH and Tg in cord blood serum were 2–3 times higher than in the mother's bloodReference Eltom, Eltom, Idris and Gebre-Medhin32. The authors concluded: ‘The study suggests that in areas with mild iodine deficiency, neonates may be at the limit of decompensation as evidenced by their enhanced TSH and Tg levels’. It is however normal to find a considerably higher concentration of TSH and Tg in cord blood than in maternal bloodReference Morreale de Escobar, Obregon and Escobar del Rey8–Reference Burrow, Fisher and Larsen11. Moreover, this difference is not ameliorated by iodine supplementationReference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen25.
Thyroid function in a full-term foetus, a neonate or in a small child is not more sensitive to a mild iodine deficiency than in the mother
As discussed above, the most severe consequences of a thyroid hormone deficiency caused by a low iodine intake are observed in the foetus and during the first years of life. During this period, the iodine stores of the thyroid are small relative to daily thyroid hormone production, and undoubtedly, a sudden cessation of iodine supply would lead to a much faster decrease in thyroid hormone secretion in the neonate than in the mother. However, there is little evidence that thyroid hormone secretion is more impaired in the foetus or neonate than in the mother in localities where there is a mild iodine deficiency.
In two randomised prospective studies of pregnant women with mild to moderate iodine deficiency, iodine supplements led to a lower serum TSH concentration in the women in late pregnancy, but iodine had no significant effect on the concentration of TSH in cord bloodReference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen25, Reference Glinoer, De Nayer, Delange, Lemone, Toppet, Spehl, Grun, Kinthaert and Lejeune33. In an observational study of women living in an area with mild to moderate iodine deficiency, we found that mothers supplemented with iodine had a lower TSH concentration in serum at full term than non-supplemented control mothers. On the other hand, when the mother had been taking iodine supplements, the TSH concentration in cord blood serum was higher than in cord blood serum of controlsReference Nohr and Laurberg34. In a study performed in Sydney, Australia, McElduff et al. found a positive correlation between the maternal UI concentration during pregnancy and the neonatal serum TSH concentrationReference McElduff, McElduff, Gunton, Hams, Wiley and Wilcken35. The median concentration of iodine in the urine of mothers (n = 84) was 109 μg l− 1. Similarly, the same researchers found a positive correlation between the concentrations of TSH in neonates and the concentration of iodine in breast-milkReference Chan, Hams, Wiley, Wilcken and McElduff36. Even a recent large Spanish study, in which the authors suggested that iodine deficiency was the cause of low intelligence, found a positive correlation between the concentration of iodine in urine and serum TSH. The median UI concentration in the children was 90 μg per lReference Santiago-Fernandez, Torres-Barahona, Muela-Martinez, Rojo-Martinez, Garcia-Fuentes, Garriga, Leon and Soriguer37.
Iodine exerts profound regulatory effects on many processes in the thyroid gland, which includes inhibition of thyroid hormone secretion after excessive iodine intake. As indicated, some studies suggest that the foetal and neonatal thyroid is more sensitive to the inhibitory effect of iodine than the maternal thyroid and that slight inhibition may occur even at a relatively low iodine intake. However, the interaction between the pituitary and thyroid glands is complex at around the time of birth, and certainly there is no indication that a slightly high serum TSH concentration in cord blood indicates a risk of any kind. In neonates with a slightly high concentration of TSH in serum, there was no decrease in cord serum free T4 concentrationReference Nohr and Laurberg34. Moreover, maternal thyroid function is probably more important for brain development in utero than foetal thyroid functionReference Morreale de Escobar, Obregon and Escobar del Rey8.
It is important that the supply of iodine is adequate for both the mother and the child, but there is no evidence that a mild iodine deficiency is more harmful for the thyroid of the neonate than for the thyroid of the mother. Along the same lines, a prospective randomised study of 121 preterm infants given a preterm formula containing a standard concentration of iodine (68 μg l− 1) or an increased concentration (272 μg l− 1) showed no difference in thyroid function and clinical outcomesReference Rogahn, Ryan, Wells, Fraser, Squire, Wild, Hughes and Amegavie38.
Conclusion
It is very important to avoid iodine deficiency during pregnancy and in the first years of life in order to prevent brain damage. It is however also important to know how to evaluate both iodine intake and the signs of iodine deficiency in pregnant women and small children. The effects of iodine on the thyroid are complex and, at present, it is not advisable to increase iodine intake to an amount above that necessary to prevent iodine deficiency disorders.





