Introduction
Eastern hoolock Hoolock leuconedys and western hoolock gibbons Hoolock hoolock were considered a single species before 2005. They are now recognized as separate species based on distinctive fur colouration (Mootnick & Groves, Reference Mootnick and Groves2005; Geissmann, Reference Geissmann2007). The eastern species occurs in China, Myanmar and India east of the Chindwin River and the western species in Myanmar, India and Bangladesh west of the Chindwin River (Groves, Reference Groves1967, Reference Groves and Rumbaugh1972; Das et al., Reference Das, Biswas, Bhattacharjee and Mohnot2006).
Several recent studies have described the eastern hoolock gibbon populations outside China. Das et al. (Reference Das, Biswas, Bhattacharjee and Mohnot2006) identified the eastern species for the first time in the Lohit district of Arunachal Pradesh in India. Chetry et al. (Reference Chetry, Chetry, Das, Loma and Panor2008) found an additional 150 groups between Dibang and Lohit in Arunachal Pradesh, India. Brockelman et al. (Reference Brockelman, Naing, Saw, Moe, Linn, Moe, Win, Lappan and Whittaker2009) estimated the population size in Mahamyaing Wildlife Sanctuary in Myanmar to be c. 5,900. Walker et al. (Reference Walker, Molur, Brockelman, Das, Islam, Geissmann, Fan, Mittermeier, Wallis, Rylands, Ganzhorn, Oates and Williamson2009) estimated that the Hukaung Valley Reserve in northern Myanmar could support tens of thousands of eastern hoolock gibbons, potentially the largest population in the world.
No recent data are available on the current distribution, status and population size of H. leuconedys in China. Early studies found that the eastern hoolock gibbon was distributed in nine counties along the west bank of the Salween River: Lushui, Baoshan, Tengchong, Longling, Lianghe, Yingjiang, Longchuan, Luxi and Ruili (Fig. 1; Li & Lin, Reference Li and Lin1983; Tan, Reference Tan1985; Yang et al., Reference Yang, Zhang and Li1985, Reference Yang, Zhang and Li1987; Ma & Wang, Reference Ma and Wang1986, Reference Ma and Wang1988; Fooden et al., Reference Fooden, Quan and Luo1987). A census carried out during 1992–1994 recorded 36–67 groups in only four counties: Baoshan, Tengchong, Yingjiang and Longchuan (Lan et al., Reference Lan, Ma and Han1995).
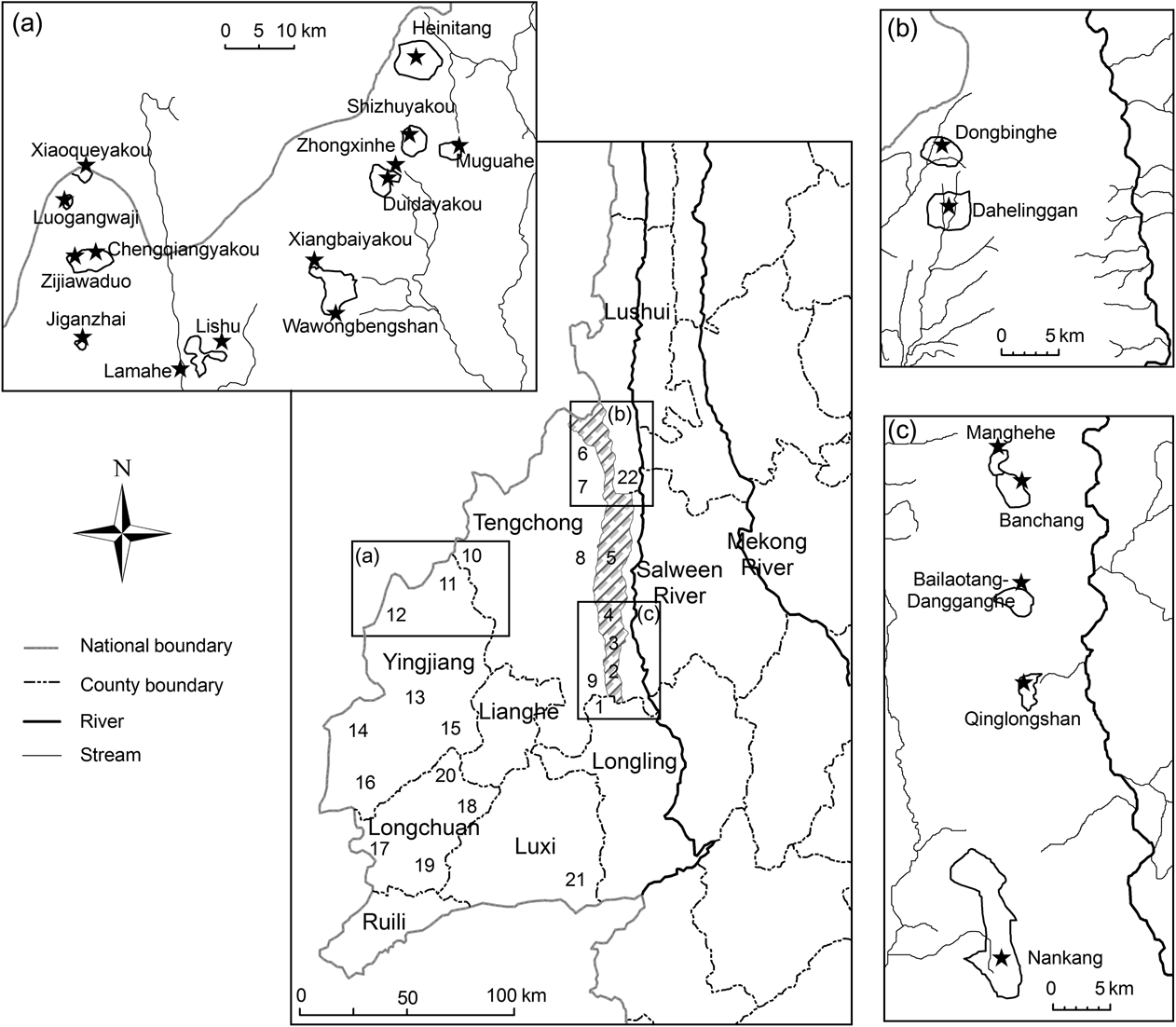
Fig. 1 Historical and current distribution area of Hoolock leuconedys west of the Salween River in Yunnan, China: 1, Nankang; 2, Bawan; 3, Saige; 4, Baihualin; 5, Mangkuan; 6, Zizhi; 7, Datang; 8, Jietou; 9, Dahaoping; 10, Houqiao; 11, Zhina; 12, Sudian; 13, Menglong; 14, Xima; 15, Jiucheng; 16, Jiemao; 17, Husa; 18, Wangzishu; 19. Bangwa; 20, Fuguo; 21, Zhongshan; 22, Shangjiang. Localities 1–9 are within Gaoligongshan Nature Reserve.
The eastern hoolock gibbon is categorized as Vulnerable on the IUCN Red List (Brockelman & Geissmann, Reference Brockelman and Geissmann2008) and as a Class I species under the Chinese animal conservation laws. Despite this, in China, only the population inside Gaoligongshan Nature Reserve is legally protected. In the study reported here we resurveyed the eastern hoolock gibbon in China, assessing its current population size and distribution, and examined the factors threatening the species.
Methods
Although a number of researchers have written about the distribution of the hoolock gibbon in China (Li & Lin, Reference Li and Lin1983; Tan, Reference Tan1985; Yang et al., Reference Yang, Zhang and Li1985, Reference Yang, Zhang and Li1987; Ma & Wang, Reference Ma and Wang1986, Reference Ma and Wang1988; Fooden et al., Reference Fooden, Quan and Luo1987; Lan et al., Reference Lan, Ma and Han1995), only Yang et al. (Reference Yang, Zhang and Li1985) and Lan et al. (Reference Lan, Ma and Han1995) reported detailed location information. We determined our survey area by combining the historical information from Yang et al. (Reference Yang, Zhang and Li1985), Lan et al. (Reference Lan, Ma and Han1995) and information obtained from interviews.
In October and November 2008 we conducted interviews with local people, especially nature reserve rangers and old hunters, in the historical range of gibbons in China. During the interviews we asked for information about the current and historical distribution, hunting, local extinction and any relevant environmental catastrophes in recent history, both natural and anthropogenic. Additional informal interviews were also conducted opportunistically during the field surveys.
Using the information obtained during the interviews we conducted field surveys between 17 March and 17 April 2009 and during 8–15 August 2009. Groups of hoolock gibbons heard by rangers or staff of the nature reserve or forestry bureau 1 month before or 1 week after the survey periods are also included in the results. We recorded the species’ presence by monitoring its distinctive calls from 38 listening posts on ridge tops. This method was developed by Brockelman & Srikosamatara (Reference Brockelman and Srikosamatara1993) and is widely used in gibbon surveys (Johnson et al., Reference Johnson, Singh, Duangdala and Hedemark2005; Buckley et al., Reference Buckley, Nekaris and Husson2006; Das et al., Reference Das, Biswas, Bhattacharjee and Mohnot2006; Jiang et al., Reference Jiang, Luo, Zhao, Li and Liu2006; Phoonjampa & Brockelman, 2006; Brockelman et al., Reference Brockelman, Naing, Saw, Moe, Linn, Moe, Win, Lappan and Whittaker2009). At least two surveyors monitored gibbon vocal activity from sunrise, c. 06.30, to 12.00 for a minimum of 4 consecutive days (Table 1). These listening posts covered all potential ranges. During each survey the geographical coordinates of listening posts, direction and estimated distance to the calling gibbons, starting and stopping time of calling bouts, and number of singing individuals per group were recorded when possible. We used triangulation to estimate the location of each singing group. We also tried to observe gibbons directly and record their group composition when they called near the listening posts. During 10–20 March 2008 we conducted an intensive line transect survey focused on primates in Nanking Park, and the data obtained for hoolock gibbons in that survey are also included here.
Table 1 Locations, survey date, survey details and number of groups and individuals of Hoolock leuconedys heard and/or seen in the species' range west of the Salween River in Yunnan (Fig. 1) in 2008–2009.

1 Localities in italics lie within Gaoligongshan Nature Reserve
2 Other groups heard by rangers or staff of nature reserve or forestry bureau 1 month before or 1 week after the survey
3 Surveyed by line transects
Results
We heard 32–34 groups and five solitary individuals of H. leuconedys (Table 1). Another 8–9 groups were recorded by rangers or staff from the nature reserve or forestry bureau (Table 1). The total recorded population was therefore 40–43 groups and five solitary individuals. By multiplying the mean group size obtained from this survey, 3.9, by the number of groups, we estimate the total population to be < 200 individuals.
We located the species in 17 forest patches in nine townships over three counties (Fig. 1, Table 1). Each forest patch contained up to five groups, although five patches contained only one group (Table 1). Within Gaoligongshan Nature Reserve, although the forest between subpopulations were seemingly of adequate quality for H. leuconedys (with the exception of Bangchang and Manghehe), the patches were separated from each other by > 3 km (Fig. 1). The population appears to have become separated into three isolated subpopulations: Sudian–Zhina–Houqiao along the China–Myanmar border, Datang and Zizhi on the west slope of Gaoligongshan, and the east slope of Gaoligongshan (Fig. 1). We found gibbons at altitudes of 1,600–2,600 m, representing the highest altitude recorded for either western or eastern hoolock gibbons.
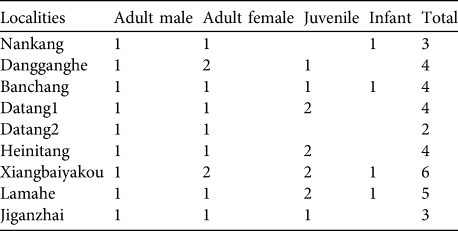
We observed nine groups in detail, in eight locations. Each group consisted of one single adult male, 1–2 adult females, 1–2 juveniles and 0–1 infants (Table 2). Mean group size was 3.9.
Table 2 Composition of nine groups of H. leuconedys observed in detail.
Commercial logging has been banned since 1998 in most of China, including the area of this study. However, it still occurs at a small scale. Commercial logging, both historical and current, has resulted in habitat destruction, degradation and fragmentation, and is the main threat to gibbons living outside Gaoligongshan Nature Reserve. Five small isolated forest patches outside the Reserve support only one group (Table 1). According to our interviews with local people commercial logging was the main reason for gibbon extirpation in Mulonghe in Sudian, which held the largest gibbon population in 1994 (Lan et al., Reference Lan, Ma and Han1995), and was possibly the main reason for the large population decline in Heinitang in Houqiao.
Hunting is also a threat. Although the management and patrolling of Gaoligongshan Nature Reserve has improved recently, some illegal hunting still occurs there. We heard gunshots in Qionglongshan and Banchang during our survey. The rangers involved in the survey reported that at least three gibbons have been killed by hunters in the past 15 years. We presume that the decline of the gibbon in Bawan and extirpation in Jietou and Dahaoping were caused by hunting because the forests in these areas are still intact and apparently suitable for gibbons. Lan et al. (Reference Lan, Ma and Han1995) reported that the Lisu people in Sudian and Heinitang do not have a tradition of killing gibbons. In our interviews we were told of only a few reported cases of people shooting gibbons in these areas. This might be the main reason why gibbons have survived in the small unprotected forest patches where they are easy to detect and shoot. Because local people still hunt other primates the gibbon was the only primate we found in most of the patches.
Agricultural encroachment, especially for tsaoko cardamom Fructus tsaoko planting, is another threat. Tsaoko cardamom is a culinary and medicinal herb and its collection is the main livelihood for local people in this region. Cardamom plantations occur throughout the gibbons’ habitat at altitudes of 1,800–2,200 m outside Gaoligongshan Nature Reserve. To plant cardamom, trees are felled in moist valleys to reduce canopy density to 50–70% and small trees and lianas are cleared. In addition to cardamom plantations the Lisu practice rotational agriculture in Sudian. They cut down primary forest for plantations every year. This has also resulted in destruction and fragmentation of the species habitat.
Discussion
Estimates of 41–48 groups of H. leuconedys in 1985 (Yang et al., Reference Yang, Zhang and Li1985), 36–67 in 1994 (Lan et al., Reference Lan, Ma and Han1995) and 40–43 in our 2008–2009 survey suggest that the species’ population is stable in China (Table 3). However, our surveys were more intensive than previous studies and it is possible that the earlier research underestimated population size. There are indications that the gibbon population may be declining, including the fact that populations in Nankang, Bawan, Houqiao, Datang and Sudian have declined by ≥ 50% since 1994. Furthermore, nine subpopulations, in Mankuang, Jietou, Dahaoping, Menglong, Xima, Jiucheng, Jiemao, Husa and Anding, have been extirpated in the past 2 decades (Table 3).
Table 3 Number of family groups of H. leuconedys recorded in 1985, 1994 and 2009 in each township with known populations in China. A blank indicates the site was not visited in that year.
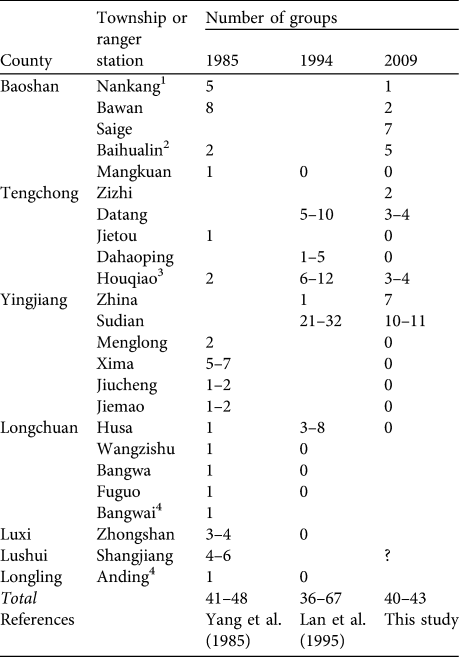
1 Information for Nankang in 1985 was obtained from interviewees during the present study
2 Baihualin includes Ganding in Yang et al. (Reference Yang, Zhang and Li1985)
3 Houqiao is the new name of Guyong in Lan et al. (Reference Lan, Ma and Han1995)
4 We could not confirm the locations of Bangwai and Anding and they are therefore not shown on Fig. 1
Hoolock gibbons usually live in groups containing one adult pair (Islam & Feeroz, Reference Islam and Feeroz1992) and our study confirmed this. Seven out of nine groups observed comprised one adult pair and 0–3 offspring and another two groups comprised one adult male and two adult females. However, a multi-female group has also been recorded in H. hoolock (Ahsan, Reference Ahsan1995).
The reproduction of gibbons both within and outside Gaoligongshan Nature Reserve seems to be healthy. Eight of nine groups had juveniles or infants (Table 2). The mean group size (3.9) is within the range of the mean group sizes of western hoolock gibbons in India and in Bangladesh (Das et al., Reference Das, Biswas, Bhattacherjee, Mohnot, Lappan and Whittaker2009), and is greater than that of eastern hoolock gibbons in Mahamyaing Wildlife Sanctuary in Myanmar (Brockelman et al., Reference Brockelman, Naing, Saw, Moe, Linn, Moe, Win, Lappan and Whittaker2009).
Although we found hunting of hoolock gibbons to be rare, even low levels of hunting can have a serious impact on small populations of gibbons (Fan & Jiang, Reference Fan and Jiang2007). Seal (Reference Seal, Tunhikorn, Brockelman and Tilson1994) suggested that a 3% annual harvest could drive a gibbon population to local extinction. Although hunting has been banned, a total cessation of hunting activities can be difficult to achieve in these poor areas where there are limited livelihood opportunities. Conservation awareness education about the conservation status of gibbons and improving the livelihood options for local people are essential for conservation of this gibbon and its habitat. We found that most local people did not know that the hoolock gibbon is a threatened species or that it is listed as a Class I species under Chinese animal conservation laws, which accords it the highest protection available. Besides education, stricter enforcement of existing laws and increased frequency of patrols by nature reserve staff and local forestry bureaus could decrease the impact of hunting on the gibbon population.
Another potential threat to H. leuconedys is its small effective population size and possibly a lack of gene flow. Gibbons usually only disperse over short distances (Brockelman et al., Reference Brockelman, Reichard, Treesucon and Raemaekers1998; Lappan, Reference Lappan2007). A severely fragmented population can suffer from a suite of problems, such as low availability of suitable mates, loss of genetic variability, inbreeding depression and other cumulative effects of population fragmentation (Jiang et al., Reference Jiang, Luo, Zhao, Li and Liu2006). Long-term population monitoring is needed to clarify if hoolock gibbons disperse between scattered forest patches. Translocations can be a good way to save small isolated populations that are at risk of extirpation. The forest inside Gaoligongshan Nature Reserve appears adequate to support a large number of gibbons but the gibbon population within the Reserve is small. It may be advisable to translocate isolated gibbons into the Reserve.
Tsaoko cardamom plantations reduce the density of the hoolock gibbon’s food trees and may cause gibbons to use more energy in foraging because of the discontinuous canopy. These plantations can also decrease long-term forest regeneration because of the clearing of small trees. However, cardamom plantations have the potential to have a positive effect on gibbon conservation. Because cardamom needs to be planted under a canopy, local people preserve forests near their villages for planting. In interviews local people admitted that they might have cut down all of the trees in the area if the forest had not been suitable for cardamom plantation. These preserved forests provide refuge for gibbons. Future comparative studies focused on the behaviour of the hoolock gibbon in forests with and without cardamom plantations could provide important insights for the conservation of the species.
Based on our findings we recommend that the habitat in Houqiao, Zhina and Sudian, along the China–Myanmar border, which holds half of the total population of H. leuconedys in China should be protected. Yingjiang Forestry Bureau is planning to expand Tongbiguan Nature Reserve to cover this area and Fauna & Flora International China has secured funding for a transboundary conservation project for the hoolock gibbon along the border with Myanmar.
Acknowledgements
This study was supported by a Rufford Small Grant, Dali University and the Provincial Natural Science Foundation of Yunnan (2008CD142; KY436940). Yunnan Provincial Forestry Bureau, Gaoligongshan National Nature Reserve and Yingjiang Forestry Bureau gave permission for the survey. We thank the two anonymous reviewers and Dr Heidi Bissell for her valuable comments and for editing the article, and all survey participants.
Biographical sketches
Fan Peng-Fei studies the behavioural ecology and conservation biology of gibbons in China, including Nomascus concolor and Nomascus nasutus. He is currently studying H. leuconedys in Gaoligongshan Nature Reserve. Xiao Wen studies the diversity and conservation of primates in Yunnan. Huo Sheng studies H. leuconedys in Nankang, Gaoligongshan Nature Reserve. Ai Huai-Sen, Wang Tian-Can and Lin Ru-Tao are officers of Gaoligongshan Nature Reserve and work on gibbon conservation.






