Background
Excess body weight (ie, overweight and obesity) is a leading cause of morbidity and mortality (Prospective Studies Collaboration, 2009) as well as a major risk factor for severe illness and death from the novel coronavirus 2019 (Popkin et al., Reference Popkin, Du, Green, Beck, Algaith, Herbst, Alsukait, Alluhidan, Alazemi and Shekar2020). Modest weight loss of ≥5% body weight can help individuals with overweight or obesity to prevent, manage, or reverse weight-related chronic conditions (Anderson & Konz, Reference Anderson and Konz2001; Diabetes Prevention Program Research Group, 2009; Garvey et al., Reference Garvey, Ryan, Look, Gadde, Allison, Peterson, Schwiers, Day and Bowden2012; Rothberg et al., Reference Rothberg, McEwen, Kraftson, Ajluni, Fowler, Nay, Miller, Burant and Herman2017), and reduce individuals’ annual medical care costs by over $2,000 (Cawley et al., Reference Cawley, Meyerhoefer, Biener, Hammer and Wintfeld2015). Due to the human and economic costs of obesity, health policies support the use of evidence-based obesity treatments (eg, weight loss surgery and lifestyle change programs) and intensive behavioral counseling by primary care providers (PCPs) (Kahan & Zvenyach, Reference Kahan and Zvenyach2016). Moreover, clinical practice guidelines encourage PCPs and their practices to play central roles in obesity management (Final Recommendation Statement: Weight Loss to Prevent Obesity-Related Morbidity and Mortality in Adults: Behavioral Interventions - US Preventive Services Task Force, 2003; Jensen et al., Reference Jensen, Ryan, Apovian, Ard, Comuzzie, Donato, Hu, Hubbard, Jakicic, Kushner, Loria, Millen, Nonas, Pi-Sunyer, Stevens, Stevens, Wadden, Wolfe, Yanovski, Jordan, Kendall, Lux, Mentor-Marcel, Morgan, Trisolini, Wnek, Anderson, Halperin, Albert, Bozkurt, Brindis, Curtis, DeMets, Hochman, Kovacs, Ohman, Pressler, Sellke, Shen, Smith and Tomaselli2014), and the patient-centered medical home model provides an ideal context to deliver team-based, coordinated, and longitudinal obesity care (Bernstein et al., Reference Bernstein, Manning and Julian2016).
Despite these initiatives and opportunities, patients with obesity often receive suboptimal weight management in general practice settings due, in part, to provider- and practice-level factors (Tsai & Wadden, Reference Tsai and Wadden2009). Such factors include most PCPs’ lack of specialized training in obesity treatment, brief office visits with competing clinical demands, and strained clinic capacities, which preclude frequent weight-focused visits (Cabana et al., Reference Cabana, Rand, Powe, Wu, Wilson, Abboud and Rubin1999; Foster et al., Reference Foster, Wadden, Makris, Davidson, Sanderson, Allison and Kessler2003; Tsai & Wadden, Reference Tsai and Wadden2009; Salinas et al., Reference Salinas, Glauser, Williamson, Rao and Abdolrasulnia2011; Wadden et al., Reference Wadden, Butryn, Hong and Tsai2014; Ossolinski et al., Reference Ossolinski, Jiwa and McManus2015; Phelan et al., Reference Phelan, Burgess, Yeazel, Hellerstedt, Griffin and van Ryn2015; Mainous et al., Reference Mainous, Tanner and Baker2016; Morris et al., Reference Morris, Chapman, Nelson, Fink, Walker and Cisler2016; Petrin et al., Reference Petrin, Kahan, Turner, Gallagher and Dietz2017; Nhim et al., Reference Nhim, Khan, Gruss, Wozniak, Kirley, Schumacher, Luman and Albright2018). Thus, PCPs commonly recommend general diet and physical activity changes rather than specific obesity treatment and follow-up plans (Bardia et al., Reference Bardia, Holtan, Slezak and Thompson2007; Alexander et al., Reference Alexander, Cox, Boling Turer, Lyna, Østbye, Tulsky, Dolor and Pollak2011; van Dillen et al., Reference van Dillen, van Binsbergen, Koelen and Hiddink2013; Tseng et al., Reference Tseng, Greer, O’Rourke, Yeh, McGuire, Clark and Maruthur2017). Most PCPs, for example, seldom prescribe anti-obesity medications (Saxon et al., Reference Saxon, Iwamoto, Mettenbrink, McCormick, Arterburn, Daley, Oshiro, Koebnick, Horberg, Young and Bessesen2019) or initiate bariatric surgery referrals (Falvo et al., Reference Falvo, Hite Philp and Eid2018; Pearce et al., Reference Pearce, Rychetnik, Wutzke and Wilson2019). Additionally, most general practice settings lack population health management strategies to identify and support individuals who do not meet weight loss goals (Baer et al., Reference Baer, De La Cruz, Rozenblum, Nolido, Orav, Metzler, Block, Halperin, McManus, Aronne, Minero and Bates2020). Yet, such strategies could be an important way to optimize patients’ weight loss outcomes, as individuals who do not achieve early weight loss (eg, within 12 weeks) with one approach are unlikely to lose weight without additional support or a completely different treatment (Elfhag & Rössner, Reference Elfhag and Rössner2010; Handjieva-Darlenska et al., Reference Handjieva-Darlenska, Handjiev, Larsen, van Baak, Jebb, Papadaki, Pfeiffer, Martinez, Kunesova, Holst, Saris and Astrup2010; Kong et al., Reference Kong, Langlois, Kamga-Ngandé, Gagnon, Brown and Baillargeon2010; Waring et al., Reference Waring, Schneider, Appelhans, Busch, Whited, Rodrigues, Lemon and Pagoto2014; Miller et al., Reference Miller, Nagaraja and Weinhold2015; Unick et al., Reference Unick, Leahey, Kent and Wing2015; Fitzpatrick et al., Reference Fitzpatrick, Appel, Bray, Brooks and Stevens2018; Tronieri et al., Reference Tronieri, Wadden, Chao, Pearl, Alamuddin and Berkowitz2018).
To close knowledge gaps and more effectively help patients lose weight, a growing number of physicians – primarily those specialized in Internal Medicine and Family Medicine – are obtaining certification in obesity medicine through the American Board of Obesity Medicine (ABOM) (Kushner et al., Reference Kushner, Brittan, Cleek, Hes, English, Kahan and Aronne2017). The ABOM was established in 2011 to advance the delivery of evidence-based obesity care. Physicians who complete specific educational requirements and pass a standardized examination become ABOM Diplomates (hereafter referred to as ‘Diplomates’). In 2020, there were 4,152 Diplomates throughout the United States, and 65% were either Family Medicine or Internal Medicine providers (Stats and Data, n.d.). Diplomates are trained to deliver guideline-adherent obesity management care using a broad range of evidence-based resources (eg, anti-obesity pharmacotherapy, nutrition therapy, and bariatric surgery referral) (Reference Gudzune, Wickham, Schmidt and StanfordGudzune et al., 2021). ABOM Diplomates may be a valuable resource in primary care settings, particularly if their expertise can be extended to support other PCPs and their many patients with obesity.
One potential strategy to extend the reach of ABOM Diplomate expertise may be through a team-based, collaborative care approach in which ABOM Diplomates serve as expert consultants to enhance PCPs’ delivery of evidence-based obesity care. Collaborative Care Models (CCMs) are widely used in primary care settings to improve outcomes among patients with other complex chronic conditions through patient self-management support, provider guideline dissemination and education, delivery system redesign such as team-based care, and use of population health management strategies using clinical informatics (Coleman et al., Reference Coleman, Austin, Brach and Wagner2009; Yeoh et al., Reference Yeoh, Wong, Wong, Yam, Poon, Chung, Chong, Fang, Wang, Liang, Cheung, Chan, Zee and Coats2018). Building upon these components, the highly effective CCM for mental health conditions aims to improve outcomes for patients with depression by integrating mental health experts into the primary care team (Press et al., Reference Press, Howe, Schoenbaum, Cavanaugh, Marshall, Baldwin and Conway2017). Specifically, patients work with primary care team members (eg, PCPs, care managers) to develop initial treatment plans. Care managers then use population health management tools proactively to monitor patients’ symptoms (eg, change in depression scores) and facilitate timely intervention such as PCP follow-up or consultation with a mental health provider for patients who may need additional support (Press et al., Reference Press, Howe, Schoenbaum, Cavanaugh, Marshall, Baldwin and Conway2017).
Little is known about the potential weight loss effectiveness of a collaborative care approach to obesity treatment (Ma et al., Reference Ma, Rosas, Lv, Xiao, Snowden, Venditti, Lewis, Goldhaber-Fiebert and Lavori2019; Lv et al., Reference Lv, Xiao, Majd, Lavori, Smyth, Rosas, Venditti, Snowden, Lewis, Ward, Lesser, Williams, Azar and Ma2020). Prior work testing a collaborative care approach for patients with depression and obesity demonstrated modest weight loss at 12 months, though the majority of participants did not achieve clinically significant weight loss (Ma et al., Reference Ma, Rosas, Lv, Xiao, Snowden, Venditti, Lewis, Goldhaber-Fiebert and Lavori2019; Pagoto et al., Reference Pagoto, Schneider, Whited, Oleski, Merriam, Appelhans, Ma, Olendzki, Waring, Busch, Lemon, Ockene and Crawford2013). Suboptimal weight loss may have been due, in part, to use of a one-size-fits-all lifestyle change program rather than personalized obesity treatment and follow-up plans (Lv et al., Reference Lv, Xiao, Majd, Lavori, Smyth, Rosas, Venditti, Snowden, Lewis, Ward, Lesser, Williams, Azar and Ma2020).
Our team aims to develop and pilot test an innovative care model – the Weight Navigation Program (WNP) – to integrate Diplomates into primary care settings, enhance delivery of personalized obesity treatment, and successfully help more patients to achieve ≥5% body weight loss (versus traditional care models) while reducing burden on individual PCPs. The aim of this paper is to describe the design, rationale, and planned evaluation for the WNP. We hypothesize that the program will be feasible and acceptable among patients and providers. We also hypothesize that patients who engage in the WNP will achieve greater weight loss than a contemporaneous matched cohort of patients from another primary care clinic with similar sociodemographic characteristics.
Methods
This study was approved by the University of Michigan Institutional Review Board. Funding to support this clinical-research initiative was provided by the University of Michigan’s Elizabeth Weiser Caswell Diabetes Institute (CDI), Michigan Center for Diabetes and Translational Research Pilot and Feasibility Grant Program (D.H.G; 5 P30DK092926-09), and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (D.H.G; 1 K23 DK123416-01A1). Additionally, the Division of Metabolism, Endocrine, and Diabetes (MEND) provided clinic space and support staff, and the Department of Family Medicine supported one faculty member’s half-day per week commitment to this pilot program.
WNP team members and stakeholders
The Weight Navigation Program (WNP) team consists of an Endocrinologist (AK; Program Director), a Family Medicine physician and ABOM Diplomate (AO; Medical Director), an Internal Medicine physician-researcher and ABOM Diplomate (DHG; Research Director), a clinical project manager (CD), a research project manager, a clinic scheduler, and a medical assistant. The Program Director interfaces with institutional stakeholders, including representatives from the programs shown in Table 1, coordinates personnel training, and directs program implementation. The Medical Director interfaces with PCP colleagues, leads informational talks about the WNP and referral criteria, and provides clinical care for WNP patients; she is referred to as the Weight Navigator (WN) hereafter. The Research Director leads efforts to refine and evaluate the effects of the WNP.
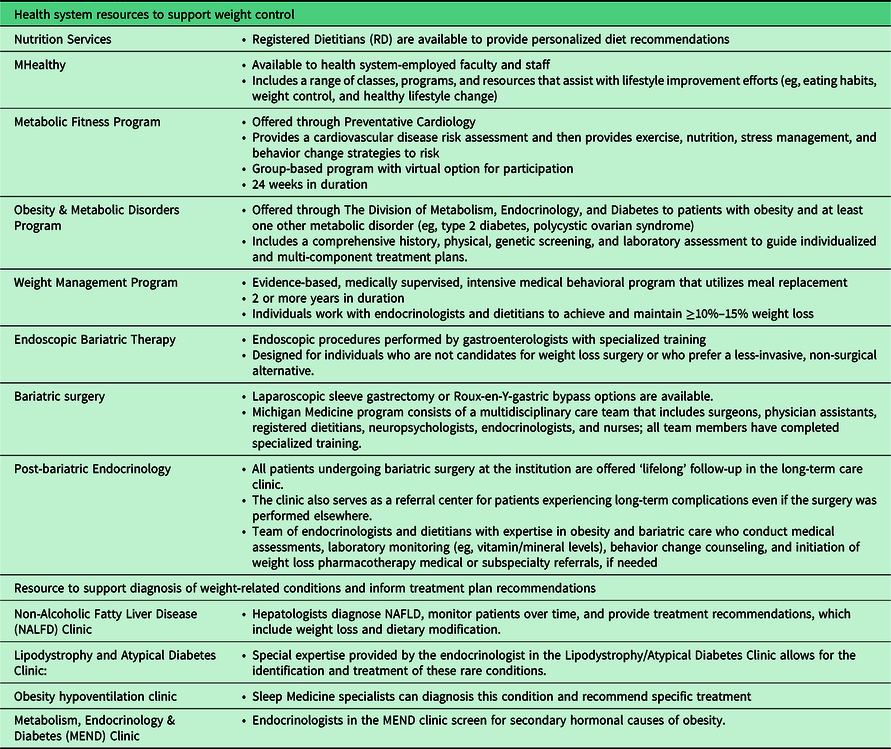
Table 1. A summary of locally available weight management resources
Key representatives from the weight management and evaluation subspecialty groups shown in Table 1 facilitated a brief training experience for the Weight Navigator to gain knowledge in the full spectrum of health system and community weight management resources. Over the course of 3 months, the Weight Navigator spent time with team members from the various groups to learn the details of each program’s eligibility criteria, goals, and operations, thus enabling her to better guide patients’ informed treatment choices.
Study design
This is a clinical quality improvement project with an embedded single-arm pilot study. Specifically, patients with obesity who desire to lose weight will be referred by their PCP to the WNP. Patients scheduled for a WNP appointment will be offered the opportunity to participate in research; we will aim to recruit 30 participants to the research arm of the study Research activities include (1) surveys at 0, 6, and 12 months; (2) optional semi-structured interview participation at 0 and 6 months; and (3) use of a text messaging program for remote weight monitoring.
Study setting
Michigan Medicine is a large, academic medical center that includes 14 adult primary care clinics throughout southeast Michigan that serve approximately 240 000 patients. The pilot Weight Navigation Program will be initiated at a single Michigan Medicine primary care site. Michigan Medicine also offers diverse resources for the evaluation and management of obesity and obesity-related chronic conditions (Table 1). Despite the availability of these programs, unpublished survey data of PCPs in our health system (n = 107) demonstrate that most providers refer patients with obesity to a dietitian (n = 89, 83%) while only a minority utilize other resources such as medical weight management (n = 23, 21.5%) or weight loss surgery (n = 20, 18.7%).
Participants WNP eligibility
Primary care patients with a body mass index (BMI) ≥ 30 kg/m2 and at least one weight-related chronic condition (eg, type 2 diabetes, hypertension, hyperlipidemia, non-alcoholic fatty liver disease, obstructive sleep apnea) may be referred by their PCP to the WNP. WNP exclusion criteria include primary care received at a location other than the pilot clinic site and pregnancy or breastfeeding. Patients may be scheduled for a WNP appointment regardless of insurance type. However, patients’ eligibility and out-of-pocket costs for obesity treatments may differ based on insurance status; this will be taken into consideration by the Weight Navigator when working with patients to develop personalized treatment plans.
Pilot research program eligibility
Eligibility criteria included the following: (1) scheduled for a WNP; (2) willingness to complete surveys at 0, 6, and 12 months; (3) willingness and ability to self-report weight data by text message; and (4) willingness to receive outreach from the study team in response to text messaging data. Exclusion criteria are the inability to read or write English.
Pilot research program eligibility
Eligibility criteria included the following: (1) scheduled for a WNP; (2) willingness to complete surveys at 0, 6, and 12 months; (3) willingness and ability to self-report weight data by text message; and (4) willingness to receive outreach from the study team in response to text messaging data. Exclusion criteria are the inability to read or write English.
Recruitment
Patients will be referred to the WNP by PCPs. All patients scheduled for a WNP appointment will be eligible for participation in a single-arm, 12-month pilot study. We anticipate that the Weight Navigator will see approximately 4 patients per 4-h clinic session once per week. Accounting for vacations, holidays, and canceled appointments, we estimate that the Weight Navigator will see approximately 150 patients during the first year of the program.
All patients scheduled for a WNP appointment will be eligible for participation in a single-arm, 12-month pilot research study. A research project manager will contact scheduled patients by telephone at least 5 days prior to their appointment date to invite them to participate in research and describe the study processes. Individuals who desire to participate in research will complete an online informed consent document and the baseline survey prior to the WNP appointment using the RedCap survey platform (REDCap, n.d.). Recruitment will continue until we reach our target of 30 participants.
Intervention
The WNP model draws on principles of the collaborative care model (CCM), (Woltmann et al., Reference Woltmann, Grogan-Kaylor, Perron, Georges, Kilbourne and Bauer2012; Miller et al., Reference Miller, Grogan-Kaylor, Perron, Kilbourne, Woltmann and Bauer2013; Press et al., Reference Press, Howe, Schoenbaum, Cavanaugh, Marshall, Baldwin and Conway2017) personalized medicine (Yanovski & Yanovski, Reference Yanovski and Yanovski2018), and population health management(Neuwirth et al., Reference Neuwirth, Schmittdiel, Tallman and Bellows2007) to enhance the delivery of team-based, patient-centered, outcome-driven obesity care. Figure 1 shows a conceptual overview of the WNP, which is described in detail below.
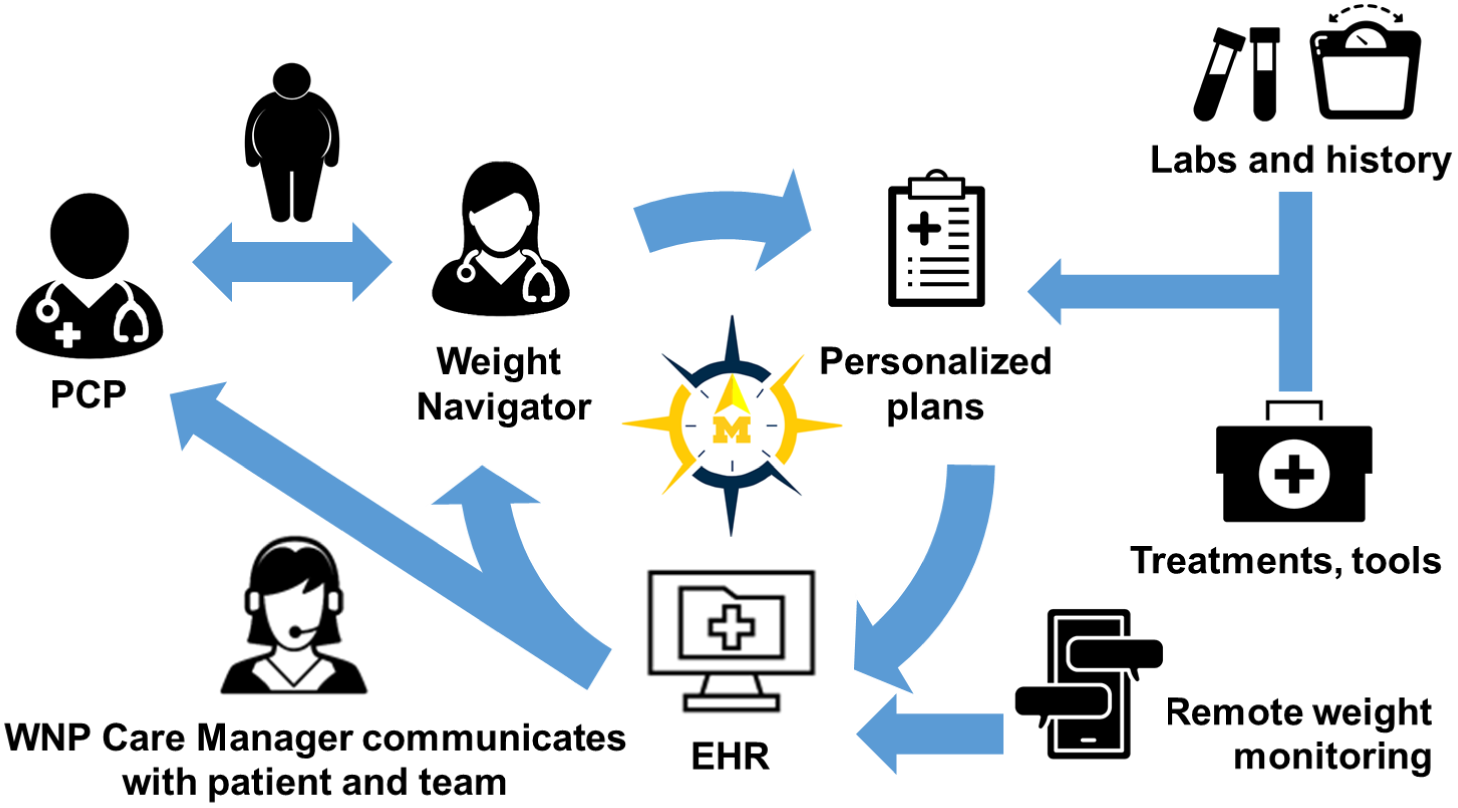
Figure 1. Weight Navigation Program (WNP) design. Patients with obesity and ≥1 weight-related condition who desire to lose weight are referred by their primary care providers (PCPs) to the WNP. The Weight Navigator and patient develop a personalized obesity treatment plan using existing health system, community, and pharmacotherapeutic resources. The plan is communicated to PCPs via the Electronic Health Record (EHR). Patients self-report weight data using an EHR-based text messaging platform. The WNP team is notified of patients’ weight changes according to pre-specific thresholds. The WNP Care Manager initiates tailored outreach to support patients over time, address potential barriers, and facilitate changes to the treatment plan, if needed, to optimize patients’ outcomes.
Eligible patients who desire to lose weight may be referred to the WNP by their PCP. Referral orders will be reviewed by a clinical scheduler who will confirm patient eligibility, contact patients to schedule an appointment, and send patients a pre-visit weight history questionnaire via the patient Electronic Health Record (EHR) portal in advance of their appointment; weight history questionnaire topics are shown in Table 2. To ensure the Weight Navigator has access to relevant clinical data at the time of the WNP encounter, the referral order will prompt PCPs to obtain a comprehensive metabolic panel, complete blood count, hemoglobin HA1c, fasting glucose, fasting lipid panel, and thyroid function tests.
Table 2. Weight history questionnaire topics

During a 1-h appointment, the Weight Navigator will review patients’ medical history, weight history, co-morbidities, and laboratory data. The Weight Navigator may identify potential obesogenic conditions (eg, hypothyroidism), obesogenic medications (eg, insulin), obesity-related conditions (eg, non-alcoholic fatty liver disease), and other co-morbidities that may hinder weight loss (eg, untreated depression). Such observations and management recommendations will be communicated to patients during the visit and to their PCPs via the EHR. Clinical assessment and review of laboratory data will also allow the Weight Navigator to determine whether additional evaluation is necessary (eg, secondary hormonal workup).
The Weight Navigator and patient will review available health system, community, and pharmacologic obesity treatments and together develop a plan that is responsive to the patient’s individual experiences, current preferences, and financial constraints. The Weight Navigation will place initial treatment referrals. In the visit encounter note, the Weight Navigator will also detail other treatment modalities that the PCP may consider in the future, if needed. Moreover, if a patient desires to try an anti-obesity medication, the Weight Navigator will make a treatment recommendation and detail the medication titration schedule in the visit note. The Weight Navigator will also provide information to patients and their PCPs regarding the potential out-of-pocket medication costs and availability of drug discount programs.
Visits will be billed using the 99 215 Evaluation and Management (E/M) code for a 60-min visit. The 99 417 E/M code may be added if the provider spends an additional 15 min on same-day documentation.
To optimize patients’ achievement of ≥5% body weight loss, we will test a population health management strategy to support individuals over time and specifically identify and support early weight loss non-responders (eg, those that do not achieve ≥3% body weight loss within 12 months). Research participants will be invited to self-report weight data via the Michigan Patient Outreach Texting Application (MPOTA), a text messaging platform integrated with Michigan Medicine’s EHR (EPIC) that allows for the remote collection of patient-reported data. We worked with Michigan Medicine’s Virtual Care Department to adapt MPOTA for Weight Management (MPOTA-WM).
MPOTA-WM participants will be asked to self-weigh at least once per week and report their weight data via text message. Participants without access to a home scale will be provided with one by the study team. Participants will be asked to use the same scale throughout the duration of the study. Participants will self-select the day(s) of the week and time of the day to receive the following text message: ‘Hello, this is your Weight Navigation Program Research Team. What was your weight in pounds today? Reply STOP to opt out of receiving messages from our team’. The WNP team will receive inbox notifications based on the following parameters, which have been adapted from prior literature (Baer et al., Reference Baer, De La Cruz, Rozenblum, Nolido, Orav, Metzler, Block, Halperin, McManus, Aronne, Minero and Bates2020): (1) weight gain of ≥ 3 pounds at any point; (2) weight loss of varying thresholds (eg, 3%, 5%, and 10% body weight loss); (3) non-response to MPOTA for weight management messages for 14 days; and (4) participants’ ‘STOP’ responses, indicating that they are discontinuing their participation in the text messaging program. The research project manager will initiate tailored outreach; those losing weight, for example, will receive a supportive EHR portal message while those gaining weight will receive a phone call to discuss barriers to weight management, individuals’ preferences and needs, and other available weight management options, as detailed in the WNP encounter note. Communication will be documented in the EHR and patients’ primary care, WNP, and/or weight management subspecialty providers will be alerted, as appropriate.
Outcome measures
We have 4 outcome categories: (1) feasibility and acceptability of the WNP; (2) clinical impact of the WNP; (3) feasibility, acceptability, and preliminary effectiveness of adding remote weight monitoring by text messaging to the WNP; and (4) feasibility and acceptability of research processes including recruitment and data collection.
Feasibility and acceptability of the WNP
Consistent with recommendations for conducting pilot studies (Eldridge et al., Reference Eldridge, Chan, Campbell, Bond, Hopewell, Thabane and Lancaster2016), our primary outcomes will be measures of feasibility (ie, extent to which an innovation can be successfully used in a given setting) and acceptability (ie, stakeholder perception that an innovation is satisfactory). Measures of WNP feasibility will include rates of (1) referral (ie, number of patients referred to the WNP divided by the number of patients eligible for the program, as determined by data extraction from the EHR) and (2) uptake (ie, number of patients referred to the program divided by the number of individuals who complete a WNP appointment).
We will assess WNP intervention acceptability through semi-structured interviews with a purposive sample of WNP research program participants to explore individuals’ experiences with the program and solicit feedback on opportunities for improvement. We will also survey primary care providers and patients regarding their satisfaction with the program and solicit feedback on opportunities for improvement.
Clinical impact of the WNP
Change in weight
We will abstract weight and height data from the EHR for all WNP patients who complete a clinic visit within the first 12 months of clinical program operations. We will evaluate average weight change among WNP patients during the 12 months following their WNP appointment. We will also identify a contemporaneous cohort of patients with obesity matched by gender and approximate age (within 10 years) to WNP patients. The matched cohort will be selected from another Michigan Medicine primary care clinic located less than 1 mile from the pilot site clinic and serving a similar patient population. We will compare between-group weight change among WNP patients and the matched cohort. In addition to evaluating mean weight change, we will also evaluate achievement of ≥5% body weight loss.
Referrals to health system weight management resources
We will compare the number of WNP referrals to health system weight management resources to the number of referrals by PCPs of patients in the matched cohort.
Patients’ engagement with health system weight management resources
We will compare the rate of engagement in health system weight management resources among WNP patients as compared to patients in the matched cohort.
Prescriptions for anti-obesity medications
We will compare the number of WNP prescriptions for anti-obesity medications to the number of prescriptions by PCPs of patients in the matched cohort.
Patients’ use of anti-obesity medications
We will compare the number of refills for anti-obesity medications among WNP patients to patients in the matched cohort.
Change in survey measures
Research program participants will be asked to complete surveys at baseline, 6 months, and 12 months; we will determine changes in measures at 6 and 12 months compared to baseline. Survey measures include motivation to lose weight (Kullgren, Reference Kullgren2016), perceived competence to lose weight (Williams et al., Reference Williams, Freedman and Deci1998), social support (Sarason et al., Reference Sarason, Sarason, Shearin and Pierce1987), and eating behaviors (de Lauzon et al., Reference de Lauzon, Romon, Deschamps, Lafay, Borys, Karlsson, Ducimetière and Charles2004). At baseline, we will ask participants to report sociodemographic characteristics.
Feasibility, acceptability, and preliminary effectiveness of adding remote weight monitoring by text messaging to the WNP
MPOTA-WM uptake
Determined by the number of individuals who consent to research divided by the number of individuals who self-report at least 1 weight measure by text message.
MPOTA-WM engagement
Determined by the number of self-reported weights divided by the total number of possible reporting days.
Qualitative experience with MPOTA-WM
During interviews with research program participants, we will also explore individuals’ experiences with MPOTA-WM and solicit feedback on opportunities to improve the text messaging program.
Change in weight
We will evaluate change in weight from baseline to 12 months of follow-up among MPOTA-WM users. To account for potential differences between clinic and home scales, the first weight entered by text message will serve as participants’ baseline weight for remote weight monitoring and this will be compared with follow-up MPOTA-WM data.
Feasibility and acceptability of research processes including recruitment and data collection
Rate of research recruitment
Determined by the number of individuals who consent to research divided by the total number of individuals invited to participate in research divided by the number of days in the recruitment period.
Research retention
Determined by the number of research participants who complete surveys at 6 and 12 months divided by the total number of research participants.
Data analyses
Quantitative data analysis
Measures of central tendency (eg, proportions, means, standard deviations) will be used for all descriptive analyses. We will calculate mean 3-month and 6-month weight changes from baseline. We will also calculate the number of participants that achieve ≥5% body weight loss at 6 and 12 months. We will compare between-group changes (ie, all WNP participants versus WNP research participants and all WNP participants versus matched cohort) in weight using a difference-in-difference analytic approach. Among WNP research participants, we will also calculate mean weight change based on MPOTA-WM data. We will conduct all analyses using Stata 15.
Qualitative data analysis
Semi-structured interviews will be recorded and transcribed verbatim. Interviews will be imported into qualitative analysis software, Dedoose ( n.d.), and analyzed using template qualitative analysis (Brooks et al., Reference Brooks, McCluskey, Turley and King2015). The initial codebook will be developed to reflect interview questions, and additional codes will be subsequently generated to reflect new concepts that emerge from the data. Two trained coders will independently review and code each transcript and then meet to resolve coding differences and iteratively revise the codebook.
Integrated analysis
Consistent with a mixed-methods sequential explanatory design (Ivankova, Reference Ivankova2006), we will integrate (ie, connect) the quantitative and qualitative findings in the final stage of data analysis. In this way, we will interpret our quantitative data in the context of qualitative participant experience.
Discussion
We describe the design, rationale, and study protocol for a quality improvement initiative with an embedded single-arm pilot study. The WNP aims to overcome the known challenges of providing evidence-based obesity management in general practice settings. This model consists of three components that have demonstrated effectiveness when used independently for other primary care improvement efforts: (1) team-based collaborative care; (2) personalized weight management plans; and (3) population health management to optimize patients’ outcomes.
Limitations and opportunities
This program has several potential limitations. First, it will be implemented at a single site and thus may not be generalizable to all practice settings. However, we believe the WNP model offers a conceptual and practical framework that may be successfully adapted to diverse primary settings where at least one physician is certified in obesity medicine. Second, cost barriers may impede patients’ use of certain weight management resources. Fortunately, there is growing insurance coverage for weight management services (Jannah et al., Reference Jannah, Hild, Gallagher and Dietz2018), drug discount programs support the use of some anti-obesity medications (Prescription Prices, Coupons & Pharmacy Information – GoodRx, n.d.), and most health plans cover nutrition counseling services. Moreover, we will utilize low-cost, community-based resources such as Diabetes Prevention Programs when developing personalized obesity treatment plans. Fourth, some components of the current WNP model (eg, MPOTA-WM outreach) require research funding, which may limit implementation in general practice settings. However, to the extent possible, the WNP model uses billable services, and we will aim to identify opportunities to integrate MPOTA-WM into routine clinical care. Moreover, remote monitoring tools are now common features of many EHR systems, reimbursement guidelines encourage use of such tools (Final Policy, Payment, and Quality Provisions Changes to the Medicare Physician Fee Schedule for Calendar Year 2021 | CMS, (n.d.), and additional policy changes on the horizon may enable more providers to play active roles supporting patients with obesity (Cassidy, Reference Cassidy2019).
Future directions
We plan to evaluate the pilot WNP using mixed quantitative and qualitative data collection methods. These data will enable us to evaluate the program’s feasibility, acceptability, and preliminary weight loss effectiveness. Moreover, we will use qualitative data obtained during interviews with patients to identify the features of the program that work well and those that could be improved. These data will enable us to refine the program and test its effectiveness in diverse practice settings. We anticipate evaluating the refined program in a future large-scale pragmatic trial using an effectiveness-implementation hybrid type 1 design to evaluate the program’s weight loss effectiveness at 12 and 24 months while also gathering information on implementation processes and determinants (Curran et al., Reference Curran, Bauer, Mittman, Pyne and Stetler2012).
Conclusions
Obesity is a complex chronic condition that demands evidence-based, personalized, and longitudinal care. To deliver such care in general practice, the WNP leverages ABOM Diplomate expertise, health system and community weight management resources, and EHR-based population health management tools. Moreover, to the extent possible, the WNP uses existing weight management resources and billable services to help patients develop personalized weight management plans. Thus, the model may provide an effective, sustainable, and scalable opportunity to improve obesity management in primary care settings.
Acknowledgements
We would like to thank Michigan Medicine’s Department of Family Medicine and Division of Endocrinology, Metabolism, and Diabetes for their support of this program. We would also like to thank Cheryl Hershey and Spring Stonebraker for their roles in project management.
Author contributions
DHG wrote the original draft of the manuscript. All authors contributed to the conceptualization of the Weight Navigation Program. All authors revised the manuscript and approved the final draft.
Financial support
Funding to support this clinical-research initiative was provided by the University of Michigan’s Elizabeth Weiser Caswell Diabetes Institute, Michigan Center for Diabetes and Translational Research Pilot and Feasibility Grant Program (D.H.G; 5 P30DK092926-09), and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (D.H.G; 1 K23 DK123416-01A1).
Conflicts of interest
Drs Othman, Gabison, Piatt, Heisler, Kilbourne, and Kraftson report no Conflicts of Interest.
Dorene Markel and Chris Dallas report no Conflicts of Interest.
Dr Griauzde has received grant funding from the US National Institutes of Health. She has also received consulting fees from the National Kidney Foundation of Michigan.
Dr Oshman has stock holdings in Johnson and Johnson, Merck, Abbvie, Eli Lilly and Abbott, unrelated to this study.
Dr Richardson has received grant funding from the US National Institutes of Health, Blue Cross Blue Shield of Michigan, Apple, Dexcom, and Twine Consulting LLC.
Dr Kullgren has received grant funding from the US National Institutes of Health, the US Department of Veterans Affairs, the Robert Wood Johnson Foundation, the Donaghue Foundation, the Healthwell Foundation, and the State of Michigan Department of Military and Veterans Affairs. He has also received consulting fees from SeeChange Health, HealthMine, and the Kaiser Permanente Washington Health Research Institute; and honoraria from the Robert Wood Johnson Foundation, AbilTo, Inc., the Kansas City Area Life Sciences Institute, the American Diabetes Association, and the Luxembourg National Research Fund.
Support was also provided by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation (University of Michigan) and with the Helsinki Declaration of 1975, as revised in 2008.






