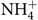The intestinal microbiota is a complex community composed of several micro-organisms(Reference Cani1,Reference Rinninella, Raoul and Cintoni2) that acts on functions such as modulation of the intestinal epithelium, strengthening of intestinal integrity(Reference Natividad and Verdu3,Reference Khosravi and Mazmanian4) , prevention of pathogenic colonisation(Reference Rinninella, Raoul and Cintoni2,Reference Doré, Simrén and Buttle5,Reference Bäumler and Sperandio6) , energy gathering and storage(Reference Den Besten, Van Eunen and Groen7), regulation of immunity(Reference Doré, Simrén and Buttle5,Reference Gensollen, Iyer and Kasper8) , synthesis, extraction and absorption of metabolites and nutrients, digestion regulation and signalling of cell pathways(Reference Rinninella, Raoul and Cintoni2).
The composition of the microbiota varies amongst individuals due to intrinsic and extrinsic factors such as different life stages, genetics, lifestyle, eating habits, ethnicity, frequency of physical activity, culture and environmental habits(Reference Rinninella, Raoul and Cintoni2,Reference Schmidt, Raes and Bork9) . In order to modulate the microbiota, strategies such as the insertion of probiotics and/or prebiotics into products can be adopted.
Probiotics generally promote health benefits for their consumers by competing with other potentially putrefying or pathogenic bacteria for adhesion sites and nutrients(Reference Gibson and Roberfroid10–Reference Hemaiswarya, Raja and Ravikumar12). In addition, these micro-organisms can modulate the immune system(Reference O’Flaherty, Saulnier and Pot13,Reference Gerritsen, Smidt and Rijkers14) , are able to improve the nutritional value of food and nutrient bioavailability and may help to decrease intestinal inflammatory processes and blood pressure(Reference Ranadheera, Baines and Adams15–Reference Saad, Delattre and Urdaci17). However, these effects are specific to each host and depend on the strain selected, making it impossible to attribute the same health benefits to all bacteria classified as probiotics(Reference Kotarski and Savage18–Reference Nader-Macías and Tomás20).
The main products chosen by industry for probiotic delivery are yogurt and fermented milks(Reference Cruz, Antunes and Sousa21), but other non-dairy and dairy foods such as ice creams and even supplements have been tested to diversify the consumption of probiotic products(Reference Cruz, Antunes and Sousa21,Reference Hill, Guarner and Reid22) . Although ice cream is an occasional foodstuff(23), it is one of the products that, besides being nutritious, has great potential as a functional vehicle for the addition of bioactive, probiotic and prebiotic compounds, due to its neutral pH and low storage temperature(Reference Cruz, Antunes and Sousa21). Dietary supplements represent another product commonly used for probiotic delivery with a view to supplementing the diet (oral ingestion)(24).
In vivo experiments are ideal and more representative to evaluate the administration of pro- and prebiotics, although the cost, time and ethics may be a limiting factor(Reference Van de Wiele, Boon and Possemiers25). Alternatively, the use of in vitro models may be able to simulate the microbiological and physiological processes of the gastrointestinal tract and, when associated with molecular analyses, may facilitate the understanding of the functioning of different systems or pathways, as well as complementing in vivo studies, with the advantage of the possible control of several parameters(Reference Van de Wiele, Boon and Possemiers25,Reference Marzorati, Qin and Hildebrand26) .
Amongst the different models, in vitro fermentative models are excellent tools to assess how the microbiota and gastrointestinal environment can be altered by distinct variables (diet, drugs, pathogens and toxic compounds)(Reference Verhoeckx, Cotter, López-Expósito, Verhoeckx, Cotter and López-Expósito27). The models can basically be divided into Batch Fermentation Models, such as the VTT One Compartment Fermentation Model(Reference Aura, Maukonen, Verhoeckx, Cotter and López-Expósito28), and Dynamic Fermentation Models, represented by the TNO In Vitro Model of the Colon(Reference Venema, Verhoeckx, Cotter and López-Expósito29), the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®)(Reference Van de Wiele, Van den Abbeele, Ossieur, Verhoeckx, Cotter and López-Expósito30) and the Computer-Controlled Multicompartmental Dynamic Model of the Gastrointestinal System SIMGI (SIMulatorGastro-Intestinal)(Reference Barroso, Cueva, Peláez, Verhoeckx, Cotter and López-Expósito31).
The SHIME® is one of the dynamic models which is useful for studying the composition and function of the intestinal microbiota. The human digestion is simulated in an environment in which the numbers and proportions of the different micro-organisms and the conditions such as temperature, pH, inoculum and retention time are all similar to those of the human organism(Reference Marzorati, Qin and Hildebrand26,Reference Possemiers, Marzorati and Verstraete32) .
The aim of the present study was to evaluate an ice cream and a commercial dietary supplement as vehicles for probiotic delivery and compare them using the SHIME®, analysing the modulation of the microbiota, SCFA and the production of ammonia.
Experimental methods
Dietary supplement
The commercial dietary supplement Bidrilac® (Chr-Hansen) was used, containing the micro-organisms Lactobacillus acidophilus LA-5 and Bifidobacterium animalis subsp. lactis BB-12, plus inulin, mannitol, silicon dioxide and vanilla flavour. The sachet was purchased from a local pharmacy, dissolved in 100 ml of water and used shortly after dissolution. Each sachet (1 g) contained 9 log colony-forming units (CFU)/g of LA-5, 10 log10 CFU/g of BB-12 and 0·22 g of inulin(Reference Rodrigues, Silva and Simabuco33).
Ice cream production
The probiotic ice cream was produced using the Cuisinart Commercial Quality Compressor Ice Cream & Gelato Maker, located in the School of Applied Sciences (Unicamp). The ice cream was manufactured to contain the same viable counts of the probiotics found in the commercial dietary supplement and also the same quantity of inulin, in order to enable a reliable comparison between them. The ingredients and quantities used to prepare 100 g of ice cream were: 25 g of whole milk powder (Nestlé), 0·73 g of inulin (GR of vegetable origin, Beneo-Orafti), 7 g of sucrose (Caravelas), 1 g of vanilla extract (Dr Oetker), 20 g of dairy cream (Shefa), 1 g of emulsifier (Emustab, Duas Rodas) and the probiotic cultures. The same probiotic strains found in the commercial supplement (L. acidophilus LA-5 and B. animalis subsp. lactis BB-12) were purchased from Chr-Hansen as a commercial direct vat set to produce the ice cream. These probiotics (0·2 g of BB-12 and 0·16 g of LA-5) were previously activated in milk(Reference Rodrigues, Silva and Simabuco33,Reference Magariños, Selaive and Costa34) and added manually at the end of the ice cream making process.
The total amount administered for both supplementation forms was 3·08 g of inulin and 10 log CFU of L. acidophilus and 11 log CFU of B. animalis. When the prebiotic and probiotic were delivered by dairy food, a total of 420 g of ice cream was administered.
Physicochemical analysis of the ice cream
The ice cream was characterised by its total solid content, titratable acidity, pH and proximate composition. All analyses were carried out in triplicate.
The total solids, titratable acidity and pH were determined following the methodology described by IAL(35) and the proximate composition according to the AOAC methods. The moisture content was determined in a drying oven at 105°C for 6 to 8 h and the ash content in a muffle furnace at 550°C for 8 h. The protein content was determined using the micro-Kjeldahl method, where the crude protein was calculated by multiplying the N content by the factor 6·38 for dairy products(36). The lipid content was quantified using the Mojonnier method(37) and the total carbohydrates calculated by difference.
Simulated digestion in the dynamic colonic model
The SHIME® was used to simulate the human digestion process. The SHIME® reactor is computer-controlled and consists of five closed compartments representing the stomach, small intestine, ascending colon, transverse colon and descending colon(Reference Molly, Woestyne and De Smet38). The simulator located at the School of Pharmaceutical Sciences (Food Science and Nutrition Department, Unesp, Araraquara, Brazil) was adapted for this study, where the transverse and descending colons were replaced by the triplicate of the ascending colon, aiming to obtain replicates of the experiment for a statistical comparison of the data.
The volumetric capacity, pH, temperature (37°C) and retention time (24 h) were controlled(Reference Possemiers, Verthé and Uyttendaele39), and the last three compartments were stirred with a magnetic stirrer throughout the whole process. Anaerobiosis of the system was achieved by the addition of N, and the pH value corrected in each vessel using hydrochloric acid or sodium hydroxide accordingly, to be in the range from 5·6 to 5·9(Reference Molly, Woestyne and De Smet38,Reference Possemiers, Verthé and Uyttendaele39) .
The compartments were colonised with faeces from eight healthy male volunteers from the Army Cadet Preparatory School (EsPCEx), aged between 18 and 22 years old (Research Ethics Committee, No. 2.845.537). According to the procedures presented by Duque etal.(Reference Duque, Monteiro and Adorno40), as adapted from Possemiers etal.(Reference Possemiers, Marzorati and Verstraete32), the samples were homogenised (all the collected samples containing different amounts were mixed) and 40 g diluted in 200 ml of phosphate buffer (pH 6·5), composed of 7·08 g/l of monosodium phosphate (Synth), 5·98 g/l of disodium phosphate (Synth) and 1 g/l of sodium thioglycolate (Merck). This mixture was then homogenised in a stomacher and centrifuged at 3000 g for 15 min. The supernatant was collected, and 10 ml was added to each of the last three compartments together with 500 ml of sterile feed medium, which is a carbohydrate-based medium with an important role in the environmental adaptation and inoculum growth, with the formation of a stable and representative community(Reference Molly, Woestyne and De Smet38).
The feed medium used in SHIME® was prepared in distilled water, consisting of 3 g/l starch (Maisena, Unilever Brazil), 2 g/l pectin (Sigma-Aldrich), 4 g/l of gastric mucin type II swine (Sigma-Aldrich), 1 g/l xylan (Megazyme), 1 g/l peptone (Kasvi), 1 g/l of arabinogalactan (Sigma-Aldrich), 0·4 g/l glucose (Synth), 3 g/l yeast extract (Kasvi) and 0·5 g/l of l-cysteine (Sigma-Aldrich)(Reference Possemiers, Verthé and Uyttendaele39). In order to simulate the duodenum conditions, an artificial pancreatic juice composed of 12·5 g/l sodium bicarbonate (LS Chemicals), 6 g/l Ox Bile (Sigma-Aldrich) and 0·9 g/l pancreatin (Sigma-Aldrich) was added to the second reactor(Reference Possemiers, Verthé and Uyttendaele39).
Experimental protocol
The experimental period in the SHIME® reactor lasted 7 weeks. For the microbiota stabilisation period (control), the feed medium (240 ml) and pancreatic juice (60 ml) were inserted into the system and allowed to stabilise for 14 d(Reference Possemiers, Verthé and Uyttendaele39,Reference van de Wiele, Boon and Possemiers41) . After 2 weeks of stabilisation, the first treatment was administered and allowed to develop for 14 d. The treatment consisted of 1 g of dietary supplement dissolved in 100 ml of sterile filtered water and 140 ml of feed medium. Between treatments, a 7-d washout period was applied, where only the feed medium (240 ml) and pancreatic juice (60 ml) were added. After the washout, the second treatment (14 d) started, with the addition of 30 g/d of the probiotic ice cream (corresponding to half of the recommended portion for daily consumption, according to RDC 359, Brazil(23)), 70 ml of sterile filtered water and 140 ml of feed medium. The experiment was carried out in biological triplicate.
Metabolites production
After the simulated digestion, the samples from the colon compartment (n 6) were collected and stored at –20°C and the ammonium ions (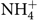 $\text{NH}_4^ + $) quantified according to Bianchi etal.(Reference Bianchi, Rossi and Sakamoto42) using a specific ion meter (model 710A, Orion) coupled to a selective ammonia ion electrode (model 95-12, Orion). Briefly, the electrode was calibrated with different standards (10, 100 and 1000 parts per million) (Thermo - Orion), and then 0·2 ml of solution added to 10 ml of each sample for adjustment of the ionic strength in order to make the readings. The readings were carried out in triplicate at a temperature of 25°C with constant stirring. The results were divided by the molar mass of the ammonium ion (18·04) and expressed in mmol/l.
$\text{NH}_4^ + $) quantified according to Bianchi etal.(Reference Bianchi, Rossi and Sakamoto42) using a specific ion meter (model 710A, Orion) coupled to a selective ammonia ion electrode (model 95-12, Orion). Briefly, the electrode was calibrated with different standards (10, 100 and 1000 parts per million) (Thermo - Orion), and then 0·2 ml of solution added to 10 ml of each sample for adjustment of the ionic strength in order to make the readings. The readings were carried out in triplicate at a temperature of 25°C with constant stirring. The results were divided by the molar mass of the ammonium ion (18·04) and expressed in mmol/l.
The SCFA were analysed according to the protocol adopted by Duque etal.(Reference Duque, Monteiro and Adorno40), with modifications. The samples (n 3, second week of the colon reactors) were centrifuged (14 000 g, 5 min), and 2 ml of the supernatant was stored for analysis. Analytical curves were constructed from stock solutions of the acids of interest (acetic, propionic and butyric). The samples were filtered through Millex® filters (0·45 μm) into flasks and then injected into an Agilent HP-6890 gas chromatograph equipped with an Agilent model HP-5975 mass-selective detector. A DB-WAX capillary column (60 m × 0·25 mm × 0·25 μm) was used under the following conditions: injector temperature = 220°C, column = 35°C, 2°C/min, 38°C; 10°C/min, 75°C; 35°C/min, 120°C (1 min); 10°C/min, 170°C (2 min); 40°C/min, 170°C (2 min), and detector = 250°C. Helium was used as the carrier gas at a flow rate of 1 ml/min.
Real-time PCR
The composition of the colonic intestinal microbiota was confirmed by real-time quantitative PCR. Samples were collected once a week, centrifuged (14 000 g, 5 min) and the pellet freeze-dried. The DNA of the samples was extracted using a QIAamp™ PowerFecal™ DNA Kit (QIAGEN Group) and quantified by nanodrop, adding to a mix composed of Power SYBR® Green PCR Master Mix (Thermo Fisher Scientific), primers (forward and reverse) and ultrapure water free of endonucleases (DEPC-treated water). The readings were made using a StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific).
Specific primers for L. acidophilus and B. animalis were used for amplification of the DNA and used to prepare a standard curve composed of the DNA extracted from isolated cultures of the bacterial strains(Reference Possemiers, Bolca and Grootaert43). The primer sequences (Thermo Fisher Scientific) used for the quantification of L. acidophilus were forward: 5′-CTTTGACTCAGGCAATTGCTCGTGAAGGTATG-3′ and reverse: 5′-CAACTTCTTTAGATGCTGAAGAAACAGCAGCTACG-3′(Reference Herbel, Lauzat and von Nickisch-Rosenegk44), and for B. animalis forward: 5′-CACCAATGCGGAAGACCAG-3′ and reverse: 5′-GTTGTTGAGAATCAGCGTGG-3′(Reference Junick and Blaut45).
Analysis of the microbiota diversity
The diversity of the intestinal microbiota was analysed by next-generation sequencing (Neoprospecta Microbiome Technologies) where specific primers amplified the V3–V4 region of the 16S ribosomal RNA (rRNA), 341F and 806R of 1 ng of DNA(Reference Wang and Qian46,Reference Caporaso, Lauber and Walters47) .
The PCR were carried out in triplicate using a Platinum Taq (Invitrogen USA) under the following conditions: 95°C for 5 min, twenty-five cycles at 95°C for 45 s, 55°C for 30 s and 72°C per 45 s, with a final extension of 72°C for 2 min for the first PCR (PCR 1). For the second PCR (PCR 2), the conditions were 95°C for 5 min, ten cycles at 95°C for 45 s, 66°C for 30 s and 72°C for 45 s, with a final extension of 72°C for 2 min. After the last PCR, the samples were cleaned with AMPure beads (Beckman Coulter)(Reference Christoff, Sereia and Boberg48).
The sequencing libraries were prepared according to company technology, and the sequencing was carried out using the MiSeq platform (Illumina).
Bioinformatical analysis
FASTQC was used to carry out the initial quality control of the sequences and subsequently filtered with Trimmomatic (0·36). A total of 911 717 raw sequences were submitted to quality control, and after filtering, 822 120 sequences reads were obtained from all the samples (control, supplement, washout and ice cream) according to online Supplementary Table S1 (BioProject – RPJNA573727). UCHIME2 was used to remove the chimeras(Reference Edgar, Haas and Clemente49). The remaining sequences were processed using the Quantitative Insights into Microbial Ecology (QIIME version 1.9.0) software(Reference Caporaso, Kuczynski and Stombaugh50) and then grouped into operational taxonomic units with 97 % identity by the QIIMEʼs UCLUST method(Reference Edgar51). The bacterial 16S rRNA database, Greengenes (13_5 release)(Reference McDonald, Price and Goodrich52) and RDPII classifier(Reference Wang, Garrity and Tiedje53) were selected to access the taxonomic annotation and PyNast used to align key sequences(Reference Caporaso, Bittinger and Bushman54). α-Diversity was estimated using QIIME to generate rarefaction curves, Goodʼs coverage, Chao1 richness(Reference Chao and Bunge55) and Shannon diversity(Reference Shannon56), and UniFrac was used to calculate the β-diversity metrics (weighted and unweighted measurements)(Reference Hamady, Lozupone and Knight57,Reference Lozupone and Knight58) .
Statistical analysis
The results were computed, submitted to a normality test and reported as mean values with their standard errors, submitted to a one-way ANOVA, followed by the Tukey multiple comparisons test, with a significance level of 5 % (P < 0·05). The metabolites SCFA and ammonia, and the probiotic species L. acidophilus and B. animalis by real-time quantitative PCR analysis were obtained using the statistical software Prism 7.0 (Software MacKiev© 1994–2016), and all data from relative abundance were performed using XLSTAT statistical and data analysis solution version 2019.4.2 (Addinsoft).
Results and discussion
Physicochemical analysis of the ice cream
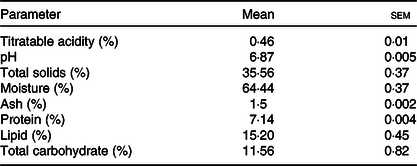
According to Table 1, the composition of the ice cream manufactured met the minimum criteria for the total solids (28 %), lipid (3·0 %) and protein (2·5 %) contents as recommended by the Brazilian Health Regulatory Agency(59).
Table 1. Titratable acidity, pH value, total solids and proximate composition of the ice cream (n 3)
(Mean values with their standard errors)
Production of metabolites
Table 2 shows the metabolites produced (mmol/l) in the ascending colon during the in vitro experiment with different matrices.
Table 2. SCFA and ammonia levels obtained in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) reactor with the administration of the dietary supplement and ice cream*
(Mean values with their standard errors)
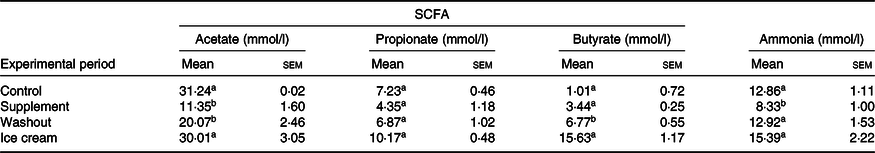
a,b Mean values within a column with unlike superscript letters were significantly different by the Tukey test (P < 0·05).
* Statistical analysis was carried out in pairs: control and supplement; washout and ice cream (n 3 for SCFA; n 6 for ammonia).
The production of SCFA was greater with the administration of probiotic ice cream than with the dietary supplement. With the dietary supplement treatment, there were reductions in the amounts of acetate (P < 0·05) and propionate (P > 0·05) and an increase in the butyrate levels (P > 0·05). With the ice cream treatment, the production of all acids was increased when compared with the corresponding washout period (P < 0·05) and this increase was only not significant for propionate (P > 0·05).
The dietary supplement was able to decrease the amount of acetate and propionate produced and only increased the butyrate production to a certain extent. This result is not common even in in vitro models involving prebiotics and probiotics, where the expected result is an increase of SCFA or no effect at all(Reference Verbeke, Boobis and Chiodini60,Reference Duysburgh, Van den Abbeele and Krishnan61) . However, depending on the fermented fibre selected, some in vitro studies can demonstrate a reduction on specifics SCFA(Reference Wang, Wichienchot and He62), such as the work presented by Yang etal.(Reference Yang, Martínez and Walter63) where the propionate amount was decreased after inulin and pectin treatments. Situations where there is a reduction on SCFA normally involve specific diseases such as inflammatory bowel disease(Reference Huda-Faujan, Abdulamir and Fatimah64) and allergy(Reference Böttcher, Nordin and Sandin65).
Comparing the two products, all the SCFA were produced in larger quantities with the addition of the ice cream as the matrix. As indicated in the literature(Reference Hijova and Chmelarova66), the percentage of acetate was higher than that of the other SCFA, reaching proportions in the ice cream of approximately 54:18:28 and in the supplement 59:23:18 for acetate, propionate and butyrate, respectively.
It is important to emphasise that not only the probiotic strains supplemented are able to produce SCFA but also other micro-organisms belonging to the host gut microbiota. According to a review published by Feng etal.(Reference Feng, Ao and Peng67), acetate is produced mainly via the Wood-Ljungdahl and acetyl-CoA pathways by enteric bacteria such as Bifidobacterium spp., Prevotella spp., Blautia hydrogenotrophica, Lactobacillus spp. and Bacteroides spp.; butyrate is produced via phosphotransbutyrylase/butyrate kinase routes and butyryl-CoA:acetate CoA-transferase routes by several Firmicutes such as Roseburia spp., Faecalibacterium prausnitzii, Eubacterium rectale, Clostridium leptum and Coprococcus catus and propionate is produced via the succinate, propanediol and acrylate pathways by Bacteroidetes and some Firmicutes such as Dialister spp., Phascolarctobacterium succinatutens, Roseburia inulinivorans, Salmonella spp., Veillonella spp. and Ruminococcus obeum. In the present work, it is possible to identify (Fig. 3(b)) some of the bacteria cited above and its distribution on the microbiota analysis through different treatments, for instance, change in the microbiota with ice cream addition increased the genus Dialister, a propionate producer that was also high after the treatment.
Chaikham & Rattanasena(Reference Chaikham and Rattanasena68) observed similar results with probiotic low-fat ice creams (with Lactobacillus casei 01 and L. acidophilus LA5), obtaining an increase in SCFA when compared with their controls (low-fat ice cream and washout periods). The modulating potential of the microbiota was also evaluated with prebiotics, as shown in the study by Van de Wiele etal.(Reference Van de Wiele, Boon and Possemiers25), which aimed to test the action of chicory inulin. The presence of inulin was able to increase the production of SCFA by 44 % in 5 weeks of treatment, mainly of propionate and butyrate. Inulin has a bifidogenic effect, and this increase can be explained by the additional biomass of Bifidobacteria that are able to degrade inulin, producing more SCFA(Reference Van de Wiele, Boon and Possemiers25). In the present work, the same amount of inulin was used to manufacture the probiotic ice cream as was found in the dietary supplement (0·22 g per 1 g). The amount required to claim an ice cream as a synbiotic food is 2·5 g of inulin per serving portion. To avoid research bias between the ice cream and the dietary supplement, due to the quantity of inulin, the authors decided to calculate on the basis of 0·22 g of inulin per 30 g ice cream portion, which was the amount administered daily to the SHIME® model. Thus, the ice cream manufactured in this research can be considered probiotic, but not synbiotic, due the low quantity of prebiotic added.
Similar to the results obtained with dietary supplement administration in the present study, the research presented by Bianchi etal.(Reference Bianchi, Rossi and Sakamoto42) aimed to evaluate four fermented formulations based on aqueous extracts of soya and quinoa, using the SHIME® reactor. Of the four formulations tested: placebo, prebiotic (fructo-oligosaccharide), probiotic (L. casei Lc-01) and synbiotic, none was able to significantly increase the SCFA levels. These fatty acids are rapidly used up as a source of energy by the resident microbiota or colonocytes, soon after their formation, not allowing for significant accumulation(Reference Bianchi, Rossi and Sakamoto42,Reference Topping and Clifton69,Reference Macfarlane and Macfarlane70) .
The same result is not observed in in vitro models where there is no colonocytes. In this type of research, for example, butyrate will be the end product from carbohydrates and proteins fermentation and will not be absorbed by gut mucosa (90–95 %) as normally it would in vivo, being quantified in its totality(Reference McNeil, Cummings and James71–Reference Venegas, De la Fuente and Landskron73). In addition, different factors, such as the presence of inorganic terminal electron receptors, individual preference of each species and the fermentation strategy of the micro-organism, amongst others, can interfere with the fatty acid formation process(Reference Bianchi, Rossi and Sakamoto42,Reference Macfarlane, Gibson and Gibson74) .
The increase in the amount of SCFA is a desired effect, especially for intestinal health since these acids can bring benefits such as the inhibition of pathogenic micro-organisms and increase in mineral absorption by reducing the luminal pH, stimulation of cellular proliferation in the epithelial tissue, increase of mucin production, modulation of metabolic activity such as intestinal homoeostasis, as well as the prevention and treatment of the metabolic syndrome, some types of cancer and intestinal disorders(Reference Hu, Chen and Xu75–Reference Ríos-Covián, Ruas-Madiedo and Margolles78).
Another metabolite of interest produced by the microbiota during fermentation is ammonia. The dietary supplement was able to significantly (P < 0·05) reduce ammonia production when compared with the control period. In the second treatment, the ice cream increased the amount of ammonia produced, although not significantly (P > 0·05). The levels recorded in the washout period were compatible with those of the control (P > 0·05), proving that the reduction in the metabolite concentration was due to the administration of the supplement.
The ammonia reduction with administration of functional compounds such as probiotics and prebiotics has been demonstrated on other studies(Reference Windey, De Preter and Verbeke79–Reference Terpend, Possemiers and Daguet81). The decrease following the addition of the supplement can be the result of changes in the microbiota, where depending on nutrient availability, they may shift their metabolism favouring the saccharolytic over proteolytic fermentation routes(Reference Allsopp, Possemiers and Campbell80,Reference Terpend, Possemiers and Daguet81) .
On the other hand, the discrete increase in the ammonia levels when ice cream was administered can be explained by the amount of protein present in the food matrix, an important fact since the degradation of this macronutrient will generate ammonia and other derivatives(Reference Montalto, D’Onofrio and Gallo82,Reference Scott, Gratz and Sheridan83) . An experiment carried out with rats showed that increased milk protein intake was able to raise the amount of proteolytic metabolites in the colon(Reference Andriamihaja, Davila and Eklou-Lawson84). Some proteins can bind to other nutrients such as sugar, making them less digestible, but still fermentable by resident colon bacteria, which is a factor that can modify the host microbiota(Reference Dominika, Arjan and Karyn85).
Besides, the liberation of ammonium ions may be associated with the higher metabolic activity of some Bifidobacterium and Lactobacillus species which participate in deamination processes, according to Scott etal.(Reference Scott, Gratz and Sheridan83).
Increased ammonia levels should be avoided, since depending on the amount, this metabolite can be toxic, being able to alter the cellular morphology of the colonocytes and act as a promoter of carcinogenesis in the intestinal tissue, increasing the risk of cancer development(Reference Montalto, D’Onofrio and Gallo82,Reference Davila, Blachier and Gotteland86) . In addition, when in the bloodstream, it may be linked to hepatic encephalopathy, as well as neurotoxic effects(Reference Watanabe, Sakai and Sato87–Reference Hamer, De Preter and Windey89).
Real-time quantitative PCR
For the real-time quantitative PCR analysis, the concentrations of L. acidophilus and B. animalis were higher after administration of the strains in the SHIME system (P < 0·05). Interestingly, regardless of the treatment used, the species remained in the same log (105 for L. acidophilus and 106 for B. animalis). The lack of detection during the control period showed that the species were not originally from the host microbiota. Fig. 1 shows the real-time quantitative PCR analysis for quantification of the species L. acidophilus and B. animalis.
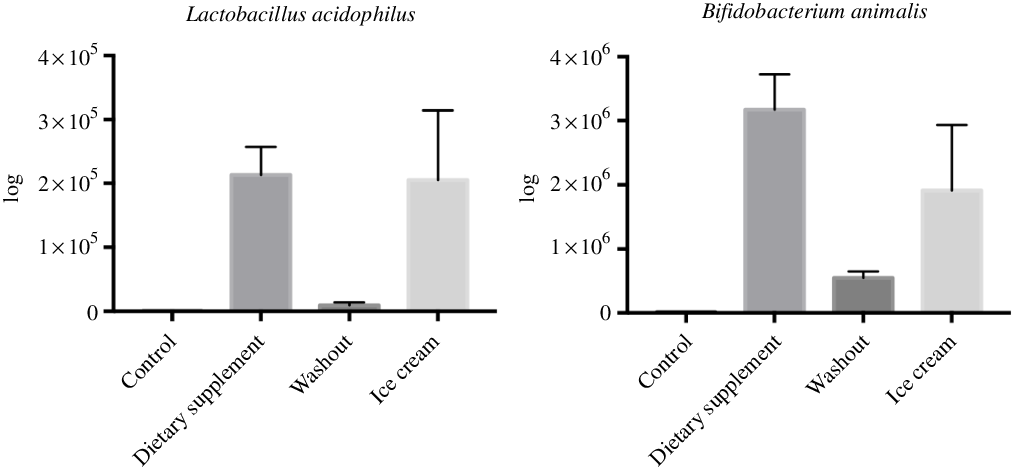
Fig. 1. Amounts of Lactobacillus acidophilus and Bifidobacterium animalis in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) reactor after administration of the dietary supplement and ice cream. Values are means (n 6), with their standard errors represented by vertical bars. The statistical analysis was carried out in pairs, that is, control and supplement; washout and ice cream.
Similar results were also observed by Moens etal.(Reference Moens, Van den Abbeele and Basit72) when administering a food supplement in the SHIME® with mucosal compartments. They aimed to assess the influence of the probiotics (L. acidophilus, L. plantarum, L. rhamnosus and Enterococcus faecium) present in Symprove™ on the microbiota of three healthy humans during a 3-week period. As a result, some of the bacteria (L. acidophilus and L. rhamnosus) were not detected during the control period but could be identified after the initial administration as from 1 to 2 weeks. Rochet etal.(Reference Rochet, Rigottier-Gois and Ledaire90) also compared the administration of B. animalis DN-173 010 in a fermented product and in the freeze-dried form, in a randomised study with healthy adults. They observed that for the freeze-dried B. animalis, DN-173 010 cells were able to survive the digestion as well as the same strain in the fermented product and that the enzymatic activities were similar.
A previous phase of the research carried out by the present authors, compared the same dietary supplement and two dietary matrices (ice cream and fermented milk) to evaluate the viability and metabolic activity (by flow cytometry) after simulated digestion using a static model(Reference Rodrigues, Silva and Simabuco33) and observed higher viable cell counts, higher metabolic activity and less cellular damage when probiotics were added to the food matrices, as compared with the dietary supplement.
Microbiota diversity analysis
The identification of the microbiota in each experimental period was analysed by next-generation sequencing. Table 3 shows the richness and diversity of the samples during the simulated digestion process.
Table 3. Number of Chao1 and Shannon indexes obtained for all the samples*
(Mean values with their standard errors)
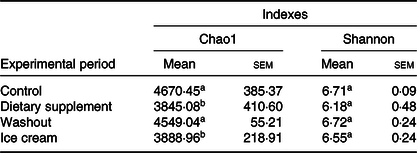
a,b Mean values within a column with unlike superscript letters were significantly different by the Tukey test (P < 0·05).
* Statistical analysis was carried out in pairs: control and supplement; washout and ice cream (n 6).
According to Table 3, for both treatments (dietary supplement and ice cream), a non-significant reduction in the Shannon index was observed, suggesting no impairment of the microbial diversity. In conclusion, the products did not impair the microbial diversity. On the other hand, the total number of micro-organisms (richness) found in the triplicate of the ascending colon using the SHIME model was statistically reduced (P < 0·05 for the dietary supplement and ice cream), as evidenced by the Chao index. This effect may have been due to the ‘core microbiome’ changes caused by both treatments.
During the whole experiment, four main phyla were identified: Actinobacteria, Bacteroidetes, Firmicutes and Proteobacteria (data not shown). According to Breban(Reference Breban91), the predominant phyla in the intestinal microbiota are Bacteroidetes and Firmicutes, a result confirmed by the present study during the control, supplement treatment and washout periods. It was observed that the treatments were able to modify the microbiota, since different phylum arrangements were registered before and after each period. Administration of the supplement showed a tendency of decreasing Actinobacteria (from 25·4 to 16·9 %) (P > 0·05) and a tendency of increasing the relative abundance of Firmicutes (from 34·5 to 42·5 %) (P > 0·05). On the other hand, with the addition of ice cream, there was an increase of approximately 50 % in the amount of Proteobacteria (from 5·4 to 9·9 %) (P = 0·09) and significant reduction in Bacteroidetes (from 27·8 to 19·8 %) (P < 0·05).
A different outcome was observed with another milk matrix. Unno etal.(Reference Unno, Choi and Hur92) investigated the consumption of a probiotic-fermented milk (ABCT-BH starter culture containing L. acidophilus CSG, Lactobacillus brevis HY7401, Bifidobacterium longum HY8001, L. casei HY2782 and Streptococcus thermophilus, plus a dietary fibre mix and lactulose) for 3 weeks and observed an increase in Bacteroidetes and reduction in Firmicutes. The potential mechanisms of these changing outcomes caused by the administration of probiotics and prebiotics are a reduction in the pH of the lumen, bacteriocin production and nutrient competition, amongst others, that can favour the growth of specific micro-organisms(Reference Gerritsen, Smidt and Rijkers14).
The administration of the probiotic products caused a pattern change in the percentage of relative abundance of the microbial orders during the different experimental periods (Fig. 2). A similarity between the control and washout periods was found during the experiment, with statistical predominance only for Bacteroidales and Clostridiales orders (online Supplementary Table S2). Comparing all the experimental periods, a statistical improvement (P < 0·05) of Coriobacteriales, Burkholderiales and Selenomonadales for the ice cream treatment was observed (online Supplementary Table S2 and Fig. 2). However, the relative abundance of microbiological orders was not statistically different due to dietary supplement administration. High abundance of Coriobacteriales was observed by Rettedal etal.(Reference Rettedal, Altermann and Roy93) after dairy drink treatment using rats model, a result similar to the presently found. The increase in Selenomonadales is probably due to the higher abundance of the genus Dialister observed.
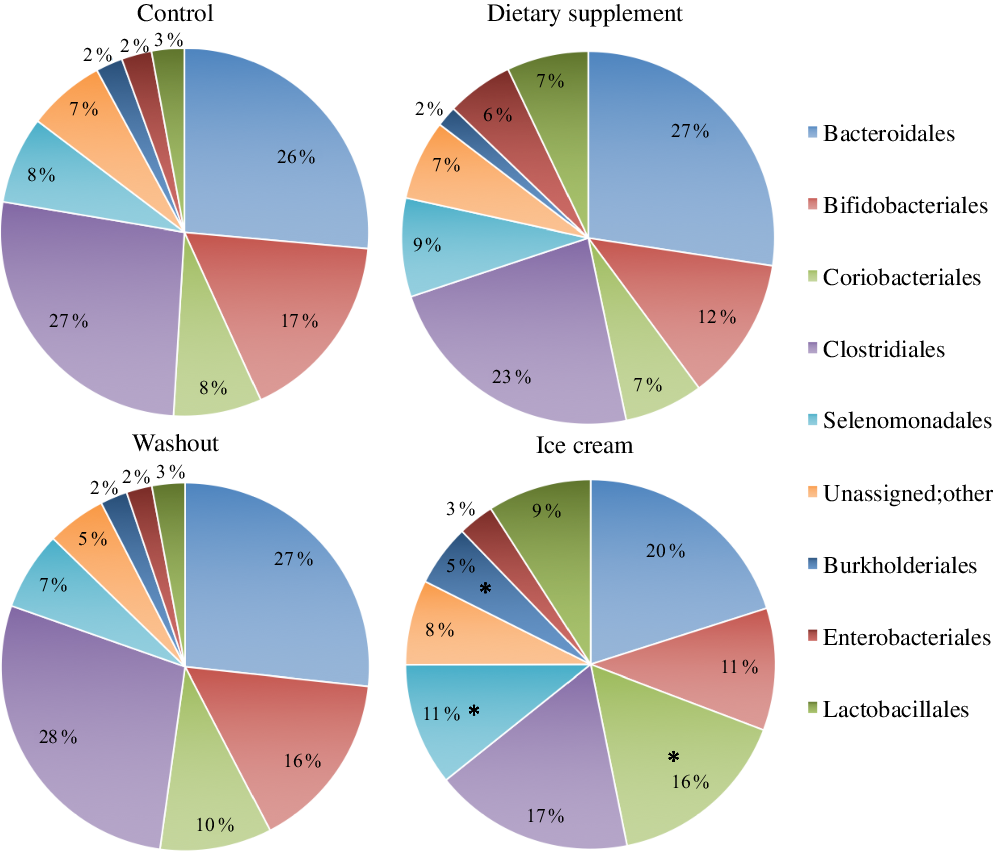
Fig. 2. Bacterial orders present in the microbiota with administration of the dietary supplement and ice cream. The statistical analysis was carried out for the four experimental periods (control, supplement, washout and ice cream). *P < 0·05 (n 6).
A connection between the distribution of the samples and the relative abundance of micro-organisms during the different experimental periods is presented in Fig. 3.
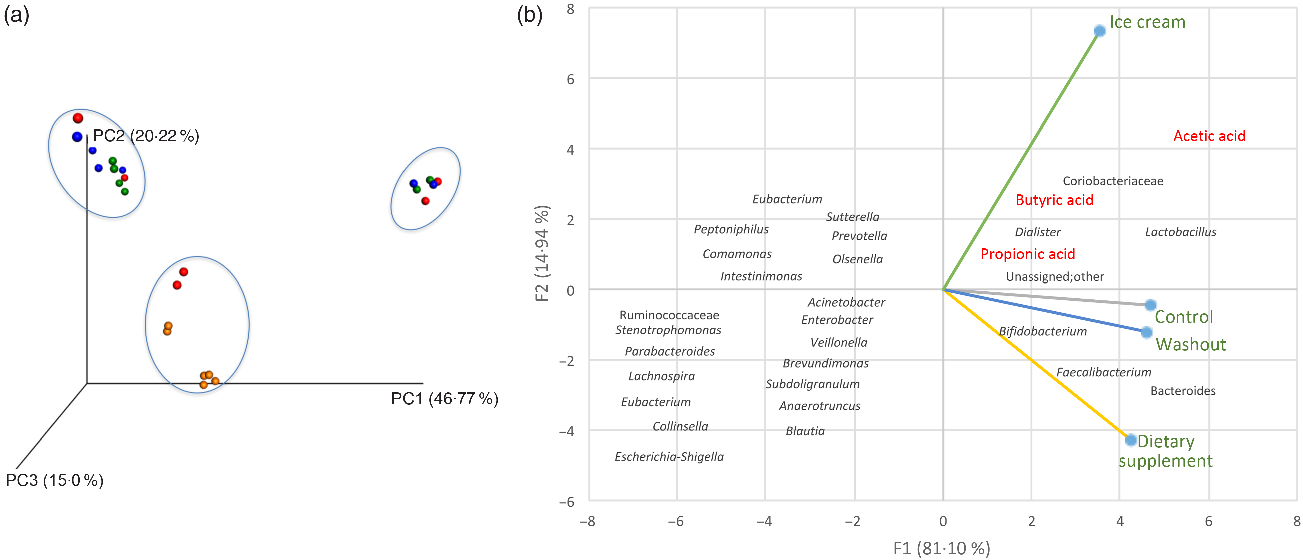
Fig. 3. (a) Principal coordinate analysis of jackknifed unweighted UniFrac distances for the 16S ribosomal RNA (rRNA) gene sequence data; (b) principal component analysis diagram for the abundance of microbial taxa (mostly genus and family level) obtained using 16S rRNA gene sequencing correlated with SCFA production: (1) control ( ); (2) dietary supplement (
); (2) dietary supplement ( ); (3) washout (
); (3) washout ( ); (4) ice cream (
); (4) ice cream ( ). Only operational taxonomic units with abundance values above 1·0 % in all samples are shown (n 6).
). Only operational taxonomic units with abundance values above 1·0 % in all samples are shown (n 6).
The results of the β-diversity analysis with different reactors (A, B and C) can be analysed by the principal coordinate analysis (Fig. 3(a)) and show that the samples corresponding to the treatment with ice cream (orange colour) were isolated from the other experimental periods (control in blue, dietary supplement in red and washout in green), demonstrating a different behaviour of the ice cream samples. This result was confirmed by the principal component analysis (Fig. 3(b)), which was able to explain about 96 % of the data variation between the samples.
The principal component analysis allowed one to observe the similarity between the control and the washout and also that the treatment carried out with the dietary supplement favoured the elevation of beneficial micro-organisms, such as Bifidobacterium spp., Bacteroides spp. and Faecalibacterium spp., the first being one of the probiotic micro-organisms administered and a constituent of the dietary supplement itself, and the last two being considered as next-generation probiotics. Next-generation probiotics, also known as live therapeutic products, are potentially probiotic species or genera not used by the food industry yet(Reference Saarela94). It is interesting to observe that many other SCFA product or bacteria (such as the genus Prevotella, Blautia, Eubacterium and Veillonella) were located at the left side of the principal component analysis graphic (Fig. 3(b)), so they were negatively related to baseline (control), washout and both treatments (dietary supplement and ice cream).
The genus Faecalibacterium (increased with dietary supplement) is one of the genera connected to a healthy microbiota(Reference Tap, Mondot and Levenez95,Reference Tap, Derrien and Törnblom96) , and in certain situations, a decrease in the amount of this genus can be connected with some diseases. Studies with F. prausnitzii showed its association with the inflammatory bowel disease, Crohnʼs disease and colorectal colitis(Reference Manichanh, Rigottier-Gois and Bonnaud97–Reference Zhang, Qiu and Zhang99), and also with symptoms of the irritable bowel syndrome(Reference Zhang, Qiu and Zhang99,Reference Pittayanon, Lau and Yuan100) . This species is a butyrate producer, butyrate being the main source of energy for the colonocytes, has anti-inflammatory properties(Reference Sokol, Pigneur and Watterlot98,Reference Lopez-Siles, Duncan and Garcia-Gil101) , and also affects immune regulation and the integrity of the epithelial barrier(Reference Duncan, Hold and Harmsen102,Reference Rivière, Selak and Lantin103) . In susceptible individuals, a decrease in F. prausnitzii and the consequent reduction in butyrate can cause gut inflammation(Reference Fujimoto, Imaeda and Takahashi104). Due to its beneficial properties, mainly on gut-related diseases, studies have been carried out using F. prausnitzii as a possible biomarker to assist diagnostics and prognoses and also as a probiotic for treatment(Reference Lopez-Siles, Duncan and Garcia-Gil101,Reference Fujimoto, Imaeda and Takahashi104,Reference Martín, Miquel and Chain105) .
Bacteroides spp. has been identified in low proportions on different conditions as type 2 diabetes mellitus(Reference Zhang, Shen and Fang106), obesity(Reference Bervoets, Van Hoorenbeeck and Kortleven107), active major depressive disorder(Reference Jiang, Ling and Zhang108) and Alzheimerʼs disease(Reference Zhuang, Shen and Li109). This type of study is important to enlighten different approaches and possible alternative ways of treatments and/or reduction of progress of specific diseases, such as the use of functional compounds as probiotics. O’toole etal.(Reference O’Toole, Marchesi and Hill110) showed in a review that several studies have been made exploring the Bacteroides spp. as next-generation probiotics candidates targeting cancer, intestinal inflammation and heart diseases, among others. Also, Bacteroides fragilis has been used as possible probiotic treatment to behaviour symptoms for autism(Reference Hsiao, McBride and Hsien111).
On the other hand, supplementation of the SHIME® with ice cream (Fig. 3(b)) changed the microbiota pattern when compared with dietary supplement administration, control and washout periods, showing a strong correlation with the family Coriobacteriaceae and the genera Dialister and Lactobacillus. This treatment is more related to the production of the SCFA – acetic, propionic and butyric, confirming the results obtained from the analysis of metabolites produced (Table 2). This finding also agrees with a paper previously published by our research group, where greater metabolic activity was observed for probiotics delivered by dairy matrices(Reference Rodrigues, Silva and Simabuco33).
The genus Dialister has four species, and some of these can be related to diseases (such as possible biomarkers or causes)(Reference Lee, Kim and Gu112,Reference Tito, Cypers and Joossens113) . However, this genus has also been linked to studies regarding its neuroactive potential in the treatment of depression, being considered a potential psychobiotic(Reference Valles-Colomer, Falony and Darzi114). A psychobiotic can be defined as a live micro-organism that, when consumed in adequate amounts, can bring health benefits to individuals with psychiatric illnesses such as stress, depression and anxiety(Reference Dinan, Stanton and Cryan115–Reference Slykerman, Hood and Wickens117).
In the present study, it was observed that a 14-d supplementation with an ice cream containing B. animalis, L. acidophilus and a small quantity of inulin promoted an increase in SCFA production, which can provide several physiological benefits. However, ice cream is a dessert with high energy density (rich in fat and sugar), and even the daily consumption of a functional ice cream should be treated with caution and continue to be an occasional foodstuff.
Conclusion
Both the dietary supplement and ice cream treatments were able to deliver viable probiotic cells. However, ice cream was possibly more effective for gut microbiota modulation because a significant increase of Coriobacteriales, Burkholderiales and Selenomonadales was observed. Moreover, a significant reduction in Bacteroidetes was also evident. Regarding the production of metabolites, dietary supplement was more efficient in reducing the ammonia content and probiotic ice cream was shown to release more acetate, propionate and butyrate fatty acids. Thus, each of the supplementation forms evaluated had specific advantages and could be considered as an alternative to diversify probiotic consumption.
Acknowledgements
The authors thank Jaqueline Petitto (State University of Campinas), Fernanda Campos Freire (São Paulo State University – UNESP) and Sofía Lorena Oddi (Universidad Nacional del Litoral) for their assistance. Also, the authors thank the company Neoprospecta Microbiome Technologies for the next-generation sequencing analysis.
This work was supported by the São Paulo Research Foundation (FAPESP) – Project number 2017/25007-9; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001; and the Fundo de Apoio ao Ensino, à Pesquisa e Extensão (FAEPEX) – Number 519.292. None of the funders had a role in the design, analysis or writing of this article.
V. C. C. R.: masterʼs degree student involved with the design of the study, all the analysis (except the next-generation sequencing of the microbiota and the bioinformatics analysis), analysing the data and writing the article; A. L. R. F. D.: in vitro experimental setup and ammonium ions analysis; L. C. F.: ice cream production and analysis and real-time quantitative PCR analysis; F. M. S.: real-time quantitative PCR and data analysis; A. S.: SCFA analysis; L. C.: bioinformatics analysis; M. F. N.: bioinformatics analysis; K. S.: design of the in vitro experiment and data analysis; A. E. C. A.: design of the study, coordination of the research, analysing of the data and writing the article. All the authors contributed and agreed with the final version of the article.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520000896