Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and their elimination by antioxidant defences, which favours a pro-oxidant state potentially leading to damage( Reference Valko, Leibfritz and Moncol 1 ). In obesity, there is evidence of increased production of free radicals and reactive substances, leading to damage of lipids, proteins and DNA, as well as alterations in cellular functions and signalling. In fact, oxidative stress, along with a low-grade inflammatory state, is thought to represent an important mechanism determining the association between obesity and vascular dysfunction, CVD and altered metabolic states( Reference Codoner-Franch, Valls-Belles and Arilla-Codoner 2 ).
Nitric oxide (NO) is an important regulator of cardiovascular and renal function, synthesised under normal conditions by endothelial and neuronal NO synthase (NOS)( Reference Pacher, Beckman and Liaudet 3 ). The overproduction of NO and other reactive nitrogen species is termed nitrative/nitrosative stress and may also lead to cell damage and changes in signalling( Reference Valko, Leibfritz and Moncol 1 , Reference Pacher, Beckman and Liaudet 3 ). In oxidant and inflammatory states, such as obesity, inducible NOS can produce a great amount of NO, stimulated by inflammatory cytokines, but NO is very sensitive to ROS and combines to generate other reactive compounds. The resulting reduced NO bioavailability is believed to lead to endothelial dysfunction and loss of vasodilatation( Reference Pacher, Beckman and Liaudet 3 ).
In 2011, a review of paediatric studies summarised evidence demonstrating that obese children already present a pro-oxidant milieu and decreased levels of antioxidant enzymes( Reference Codoner-Franch, Valls-Belles and Arilla-Codoner 2 ). More recently, studies in children have shown that 8-isoprostane levels, both in plasma (P-Isop) and urine (U-Isop), besides being positively correlated with measures of obesity( Reference Dennis, Ergul and Gower 4 , Reference Warolin, Coenen and Kantor 5 ), are also associated with blood lipids( Reference Dennis, Ergul and Gower 4 ) and insulin-resistance levels( Reference Dennis, Ergul and Gower 4 ). Moreover, the total antioxidant status (TAS) has also been shown to be decreased in obese children( Reference Faienza, Francavilla and Goffredo 6 ), especially in the presence of other components of the metabolic syndrome( Reference Molnár, Decsi and Koletzko 7 ). However, some studies have found contradictory results, hypothesising that a regulatory increase of the antioxidant system could be in motion, in early phases of obesity, to compensate the higher oxidative stress levels( Reference Eren, Abuhandan and Solmaz 8 ).
Nitrative/nitrosative stress, known to be associated with oxidative stress( Reference Pacher, Beckman and Liaudet 3 , Reference Codoner-Franch, Tavarez-Alonso and Murria-Estal 9 ), has been evaluated in some studies by measuring systemic 3-nitrotyrosine, a marker of tyrosine nitration by peroxynitrite( Reference Pacher, Beckman and Liaudet 3 , Reference Codoner-Franch, Tavarez-Alonso and Murria-Estal 9 ), and also by quantification of markers of NO formation and metabolism, such as plasmatic and urinary nitrates and nitrites (P-NOx or U-NOx)( Reference Codoner-Franch, Tavarez-Alonso and Murria-Estal 9 , Reference Codoñer-Franch, Tavárez-Alonso and Porcar-Almela 10 ), but contradictory results have been reported concerning whether obese children present increased or decreased levels of these markers( Reference Codoner-Franch, Tavarez-Alonso and Murria-Estal 9 – Reference Gruber, Mayer and Mangge 11 ). In two studies of Codoñer-Franch et al., obese children, with an age range from 7 to 14 years, exhibited higher levels of plasma and/urinary NO markers, whereas a reduction in serum nitrate and nitrite concentrations was observed in another study involving adolescents with a mean age of 14 years( Reference Codoner-Franch, Tavarez-Alonso and Murria-Estal 9 – Reference Gruber, Mayer and Mangge 11 ).
The association of oxidative and/or nitrative/nitrosative stress markers with blood pressure levels or vascular dysfunction has rarely been assessed in obese children( Reference Codoner-Franch, Tavarez-Alonso and Murria-Estal 9 , Reference Ostrow, Wu and Aguilar 12 , Reference Loffredo, Martino and Carnevale 13 ). Moreover, oxidative and/or nitrative/nitrosative stress might contribute to the negative impact of obesity on renal function( Reference Pacher, Beckman and Liaudet 3 , Reference Savino, Pelliccia and Chiarelli 14 ), which is believed to start early in childhood( Reference Savino, Pelliccia and Chiarelli 14 ). Therefore, the translation of higher values of oxidative and/or nitrative/nitrosative stress markers into relevant clinical findings needs to be further explored, in order to clarify some of the contradictory evidence reported in young children. Thus, in the present study, we aimed to test the hypothesis that the values of several oxidative stress and NO production/metabolism markers are increased in overweight and obese prepubertal children, and that they correlate with each other and are related to various cardiometabolic risk factors. In addition, we also aimed to assess the association of these markers with renal function.
Methods
Study design and sample
We studied children aged 8–9 years who have been followed-up since birth in a previously established cohort study (Generation XXI)( Reference Larsen, Kamper-Jorgensen and Adamson 15 ). The children included in this cohort are believed to be representative of the population of Northern Portugal, as a very broad catchment area was included and the participation proportion was high (92 % of the mothers invited accepted to participate). From the original cohort (n 8647), 4590 children attended a face-to-face follow-up visit at 7 years of age, including anthropometric evaluation and blood sample collection, thus being eligible for the ObiKid project – a specific project aiming to clarify the impact of childhood obesity and associated co-morbidities on the kidney( Reference Correia-Costa, Afonso and Schaefer 16 ). At a significance level of 0·05 and considering a power of 85 % to detect a difference of at least 8 ml/min per 1·73 m2 in the value of estimated glomerular filtration rate (eGFR) between normal weight and overweight/obese children, and assuming standard deviations of 24 and 22 ml/min per 1·73 m2 in each group, respectively( Reference Duzova, Yalçinkaya and Baskin 17 ), we estimated that a minimum sample size of 300 children (150 in each group of normal weight and overweight/obese children) would be needed. Assuming that about 35 % would be excluded because of refusal to participate, exclusion criteria or incomplete information, 463 children were preselected to be consecutively screened according to the date of their 7-year-old evaluation: sixteen could not be contacted, thirty-two refused to participate, twenty-three, although willing to participate, were unable to schedule the study visits during the recruitment period and sixty-eight met exclusion criteria (four chronic diseases (genetic, renal or metabolic), one chronic usage of medication (affecting blood pressure or glucose or lipid metabolism), fifty-one with residence >30 km away from the study site and six pairs of twins). We enrolled 324 participants, between August 2013 and August 2014; however, for the present analysis, we additionally excluded eleven children because of incomplete evaluation, such as absence of blood or urine samples for oxidative stress and NO production markers determination. Children included in the final analysis (n 313) were fairly representative of the eligible children with respect to length at birth, distribution in small-for-gestational age or large-for-gestational age classes, the child’s sex, parents’ number of schooling years, and children’s weight, height and systolic or diastolic blood pressure at the follow-up visit at 7 years of age.
Data collection and variables definition
The study visits took place at the Department of Clinical Epidemiology, Predictive Medicine and Public Health, Faculty of Medicine, University of Porto. Anthropometric and general physical examinations were performed according to standard procedures and as previously reported(18). Waist circumference was indexed to height (waist:height ratio (WHtR, cm/m)). BMI was calculated, and BMI-for-age values were classified according to the WHO reference data for BMI z-score into the following categories: normal weight (≤+1 sd, including only one child with thinness), overweight (>1 sd and ≤+2 sd) and obesity (>2 sd)( Reference de Onis, Onyango and Borghi 19 ). Body fat percentage was assessed by foot-to-foot bioelectrical impedance analysis (model TBF-300; Tanita).
Ambulatory blood pressure monitoring (ABPM) for 24 h was performed on all children with a portable, non-invasive, oscillometric blood pressure recorder (model 90 207; Spacelabs Healthcare). The non-dominant arm was used in all children with a cuff size appropriate to the child’s arm circumference. Blood pressure measurements were taken automatically at 20-min intervals during the daytime and at 30-min intervals during the night-time. A minimum monitoring duration of 24 h with gaps of <2 h was required for acceptance; five exams were excluded of the ABPM analysis because of insufficient readings. All readings were used to calculate mean 24 h, day and night mean arterial pressure (MAP) and systolic and diastolic blood pressures by SpaceLabs software. Sustained hypertension( Reference Flynn, Daniels and Hayman 20 ) was defined as an average systolic and/or diastolic blood pressure measurement ≥95th percentile, during the day or the night on ABPM, according to the reference values( Reference Flynn, Daniels and Hayman 20 ), a systolic or diastolic blood pressure load ≥25 %, during the day or the night, and an office systolic and/or diastolic blood pressure ≥95th percentile, according to the American Academy of Pediatrics criteria( 21 ). When office blood pressure values were below the 95th percentile, but the remaining criteria were verified, the children were classified as presenting masked hypertension( Reference Flynn, Daniels and Hayman 20 ). The absence of dipping was considered as a fall in the MAP during night-time of <10 % of the corresponding daytime MAP. Carotid–femoral pulse wave velocity (PWV) analysis was performed by a trained cardiopneumology technician with a portable device (Micro Medical PulseTrace PWV PT4000; Kent); digital volume pulse waveform had to fill two-thirds of the display with little or no noise and artifact to be considered, and three measurements of PWV were obtained and averaged for the analysis.
Laboratory procedures
A venous blood sample was collected after an overnight fast of at least 8 h and analysed for creatinine, cystatin C, uric acid, glucose, insulin, lipids, high-sensitivity C-reactive protein (hs-CRP), IL-6, myeloperoxidase (MPO), P-Isop, P-NOx and TAS. Insulin resistance was determined using the homoeostasis model assessment index (HOMA-IR). All participants collected a 24-h urine sample, which was analysed for creatinine, U-Isop, urinary hydrogen peroxide (U-H2O2) and U-NOx. All the parents received information on the correct methods of 24-h urine collection and, upon sample delivery, compliance was rechecked by a brief questionnaire. The samples were considered valid if urinary creatinine was within the range of 11·3–28·0 mg/kg per d (according to age- and sex-specific reference values( Reference Remer, Neubert and Maser-Gluth 22 )) and if the urinary volume was over 300 ml; on the basis of these criteria, fifteen urine samples were excluded from the analysis.
All the standard laboratory analyses were performed in the Clinical Pathology Department of Centro Hospitalar São João, Porto, Portugal. Serum creatinine assay was based on the Jaffé compensated traceable to an isotope dilution MS method (Olympus AU 5400 automated analyzer; Beckman-Coulter). Serum cystatin C was assayed using a particle-enhanced immunonephelometric assay (N latex Cystatin C; Siemens). Serum uric acid was evaluated by a kinetic uricase–peroxidase assay in an automated analyzer (Olympus AU 5400 automated analyzer). The Zappitelli combined formula was used to estimate eGFR( Reference Zappitelli, Parvex and Joseph 23 ). hs-CRP was tested by immunonephelometric assay with CardioPhase hs-CRP (Siemens Healthcare Diagnostics®), and IL-6 was tested by immunoassay (Cobas Integra 700 Autoanalyzer; Roche). Urinary creatinine was determined by a standard automated clinical chemistry analyzer (Olympus AU 5400 automated analyzer). All the plasma, serum and urine samples used for the determination of inflammatory and oxidative stress biomarkers were stored at −80°C until assayed. MPO and oxidative stress and NO production/metabolism markers were assessed at the Department of Pharmacology and Therapeutics of the Faculty of Medicine, University of Porto, using commercial kits and by following the manufacturer’s specific instructions. In brief, serum MPO was quantified by an immunoenzymatic assay (BioCheck, MPO Enzyme Immunoassay Test Kit; Oxis International Inc.). Plasma TAS was evaluated by a spectrophotometric assay (Antioxidant Assay Kit; Cayman Chemical Company) that measures the combined antioxidant activities of water- and lipid-soluble antioxidants, including vitamins, glutathione, uric acid, bilirubin, albumin, etc. This assay depends on the ability of the antioxidants present in the sample to inhibit the absorbance of the radical cations of 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid). The antioxidant capacity of the sample is compared with that of Trolox, a water-soluble tocopherol analogue, and is expressed as mm Trolox equivalents. Free isoprostanes were quantified in plasma (P-Isop) containing the preservatives butylated hydroxy toluene (BHT, 0·005 %, w/v) and indomethacin (10 μm), which were added before storage. Solid-phase extraction was performed before the measurement of P-Isop by a competitive enzyme immunoassay (15-Isoprostane F2t ELISA Kit; Oxford Biomedical Research, Inc.). U-Isop were quantified by a competitive enzyme immunoassay (Urinary Isoprostane ELISA Kit; Oxford Biomedical Research, Inc.) in non-extracted urine containing BHT (0·005 %, w/v) added before storage and incubated with β-glucuronidase before the assay, as a significant amount of isoprostanes is excreted in urine conjugated with glucuronide( Reference Milne, Dai and Roberts 24 ). Total nitrates and nitrites (NOx) were evaluated in P-NOx and U-NOx by a colourimetric assay (Nitrate/Nitrite Colorimetric Assay Kit; Cayman Chemical Company). Plasma samples were ultrafiltered before assay using 30-kDa filters. Urinary excretion of H2O2 (U-H2O2) was evaluated by a microplate fluorimetric assay (Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit; Molecular Probes, AlfaGene).
The intra- and inter-assay CV (%) for oxidative stress and proinflammatory biomarkers determined at the Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Porto, were as follows: 7·23 and 9·76 % (TAS); 3·58 and 13·32 % (U-H2O2); 5·76 and 9·54 % (U-Isop); 2·49 and 9·62 % (P-Isop); 5·06 and 9·31 % (U-NOx); 3·21 and 6·26 % (P-NOx); and 2·35 and 9·38 % (MPO).
The intra-assay CV (for lower and higher concentrations) of standard blood analyses performed at the Clinical Pathology Department of Centro Hospitalar São João were determined by the manufacturers of the kits: 1·06–1·13 % (total cholesterol); 1·92–1·33 % (HDL-cholesterol); 1·76–1·46 % (TAG); 1·71–1·12 % (uric acid); 5·76–1·50 % (hs-CRP); 3·10–1·10 % (IL-6); 1·25–1·11 % (glucose); 2·48–1·31 % (creatinine); 2·6–2·5 % (insulin); and 3·5–4·2 % (cystatin C).
Ethics
The ObiKid study was approved by the Ethics Committee of Centro Hospitalar São João, Entidade Pública Empresarial (E.P.E.) and Faculty of Medicine, University of Porto, and complies with the Helsinki Declaration, the guidelines for the ethical conduct of medical research involving children( Reference McIntosh, Bates and Brykczynska 25 ) and the current national legislation. Written informed consent from parents (or their legal substitute) and verbal assent from children were obtained regarding information and biological samples gathering.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 20.0. The distribution of oxidative stress and NO production/metabolism markers by classes of BMI (normal weight, overweight and obesity) is shown in box plot graphs and compared using Kruskal–Wallis and Dunn’s post hoc tests. Spearman’s correlations were used to test bivariate associations between oxidative stress and NO production/metabolism markers and between those and obesity indices, cardiovascular risk factors and renal function. Linear multivariate regression models were fitted to identify variables independently associated with oxidative/NO production markers (only for oxidative/NO production markers presenting significantly different values in overweight and obese children). The oxidative stress and NO production/metabolism markers had an asymmetric distribution, and therefore those included in the regression models as dependent variables were logarithmised (base 10), allowing us to obtain a normal distribution. Variables with significant correlations were included in the linear regression models, except for those expected to introduce collinearity – for example, among anthropometric variables, BMI z-score was preferred. All models were adjusted for sex and age (months) as well as for eGFR, as urinary excretion of biomarkers might be affected by different values of glomerular filtration. Regarding the remaining variables, a backward, stepwise approach was used to fit the final models. P values were considered statistically significant if <0·05.
Although for the majority of parameters we had a final number of 313 blood samples and 298 urine samples (after the exclusion of fifteen non-valid urine samples), there were some missing values in the following biomarkers due to insufficient volume of samples or reagents to perform sample processing, dilution tests and to run the assays in duplicate: P-Isop (n 288), U-Isop (n 297), U-H2O2 (n 295), P-TAS (n 312) and serum MPO (n 309).
Results
General characteristics and cardiovascular and biochemical parameters by classes of BMI
Obese children presented higher night-time MAP and PWV values, higher insulin resistance and higher concentrations of TAG, non-HDL-cholesterol and uric acid (Table 1). Overweight and obese children also had significantly higher hs-CRP and MPO concentrations and lower eGFR values (Table 1).
Table 1 General characteristics of the study subjects by BMI z-score classes (Mean values and standard deviations; numbers and percentages; medians and 25th, 75th percentiles)

WHtR, waist:height ratio; MAP, mean arterial pressure; PWV, pulse wave velocity; HOMA-IR, homoeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; MPO, myeloperoxidase; eGFR, estimated glomerular filtration rate by Zappitelli combined formula.
* Sustained hypertension and masked hypertension were defined according to the revised American Heart Association criteria for ambulatory blood pressure monitoring interpretation( Reference Flynn, Daniels and Hayman 20 ).
† The sample size for MPO was 161 normal weight, eighty-eight overweight and sixty obese children.
Biomarkers of oxidative stress and nitric oxide production/metabolism across BMI classes
In Fig. 1, the distribution of oxidative stress and NO synthesis markers by classes of BMI is depicted. The plasma values of TAS were not significantly different across BMI classes.

Fig. 1 Distribution of oxidative stress and nitric oxide (NO) production/metabolism markers by classes of BMI (normal weight, overweight and obesity). The normal weight, overweight and obese group classification is according to the WHO classification for BMI z-score values( Reference de Onis, Onyango and Borghi 19 ). The oxidative stress markers data are expressed as medians and 25th, 75th percentiles. Median values between groups were compared with Kruskal–Wallis tests; pairwise significant differences according to Dunn’s tests are presented. Panels (a)–(f) depict the distribution of oxidative stress and NO production/metabolism markers by classes of BMI: (a) total antioxidant status (TAS, mm Trolox equivalents) (163 normal weight; eighty-nine overweight and sixty obese); (b) urinary hydrogen peroxide (U-H2O2, nmol/d) (154 normal weight; eighty-four overweight and fifty-seven obese); (c) plasma isoprostanes (P-Isop, ng/ml) (151 normal weight; seventy-eight overweight and fifty-nine obese); (d) urinary isoprostanes (U-Isop, ng/d) (154 normal weight; eighty-six overweight and fifty-seven obese); (e) plasma nitrates and nitrites (P-NOx, nmol/ml) (163 normal weight; eighty-nine overweight and sixty-one obese); (f) urinary nitrates and nitrites (U-NOx, µmol/d) (154 normal weight; eighty-seven overweight and fifty-seven obese). * P<0·05, *** P<0·001.
The median values of U-Isop were higher in the obese group. No significant difference was found in P-NOx concentration across BMI groups, but significantly higher values of U-NOx were found in overweight and obese children. No differences were observed in U-H2O2 median values between the normal weight, overweight and obese groups.
Correlations between biomarkers of oxidative stress and nitric oxide production/metabolism
As shown in Table 2, TAS was negatively correlated with U-Isop. P-NOx was positively correlated with U-NOx, but P-Isop and U-Isop presented no correlation. U-H2O2 was positively correlated with U-Isop and U-NOx.
Table 2 Spearman’s correlations between oxidative stress and nitric oxide production/metabolism markersFootnote †
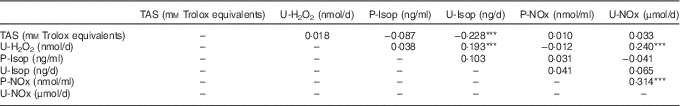
TAS, total antioxidant status; U-H2O2, urinary hydrogen peroxide; P-Isop, plasma isoprostanes; U-Isop, urinary isoprostanes; P-NOx, plasma nitrates and nitrites; U-NOx, urinary nitrates and nitrites.
*** P<0·001.
† The final sample size for each parameter was 312 (TAS), 295 (U-H2O2), 288 (P-Isop), 297 (U-Isop), 313 (P-NOx) and 298 (U-NOx).
Correlations of obesity indices, metabolic parameters and inflammatory markers with biomarkers of oxidative stress and nitric oxide production/metabolism
Both BMI z-score and WHtR were positively associated with P-Isop, U-Isop and U-NOx. Body fat mass was negatively correlated with U-H2O2 and positively correlated with U-Isop (Table 3).
Table 3 Spearman’s correlations of oxidative stress and nitric oxide production/metabolism markers with anthropometry and cardiovascular and biochemical parametersFootnote †

TAS, total antioxidant status; U-H2O2, urinary hydrogen peroxide; P-Isop, plasma isoprostanes; U-Isop, urinary isoprostanes; P-NOx, plasma nitrates and nitrites; U-NOx, urinary nitrates and nitrites; WHtR, waist:height ratio; MAP, mean arterial pressure; PWV, pulse wave velocity; HOMA-IR, homoeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; MPO, myeloperoxidase; eGFR, estimated glomerular filtration rate by Zappitelli combined formula.
* P<0·05, ** P<0·01, *** P<0·001.
† The final sample size was 313 with the exceptions of MPO (309), TAS (312), U-H2O2 (295), P-Isop (288), U-Isop (297) and U-NOx (298).
Total cholesterol and non-HDL-cholesterol levels were negatively correlated with U-NOx, and TAG levels were positively correlated with U-Isop. The HOMA-IR values were positively correlated with U-Isop. Uric acid concentrations were positively correlated with P-NOx (Table 3).
MPO concentrations were negatively correlated with TAS values. Both U-Isop and P-Isop values were positively correlated with MPO, and U-Isop was also positively correlated with hs-CRP (Table 3).
Correlations of cardiovascular and renal function indices with biomarkers of oxidative stress and nitric oxide production/metabolism
PWV was positively correlated with TAS. Daytime and night-time MAP values were not correlated with oxidative stress or NO production/metabolism markers. Renal function (eGFR) was negatively correlated with P-NOx, but no association was found with U-NOx or oxidative stress markers (Table 3).
Multivariate regression models for urinary isoprostanes and urinary nitrates and nitrites
In the multivariate models fitted (Table 4), HOMA-IR and U-H2O2 were associated with higher U-Isop values, independently of the BMI and eGFR (r 2 0·27). Total cholesterol and U-H2O2 values were associated with U-NOx, independently of the BMI, eGFR and P-NOx concentration (r 2 0·17).
Table 4 Multivariate linear regression models for urinary isoprostanes and urinary nitrates and nitritesFootnote * (Adjusted linear regression coefficients (β) and 95 % confidence intervals, estimated by linear regression models with log U-Isop or log U-NOx as the dependent variable (adjusted for all variables in the table and additionally for sex, age (months) and eGFR by Zappitelli combined formula))
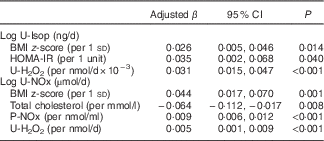
Log U-Isop, urinary isoprostanes (logarithm base 10); Log U-NOx, urinary nitrates and nitrites (logarithm base 10); HOMA-IR, homoeostasis model assessment of insulin resistance; U-H2O2, urinary hydrogen peroxide; P-NOx, plasma nitrates and nitrites.
* The final sample size was 297 for U-Isop, 298 for U-NOx, 295 for U-H2O2 and 313 for P-NOx, BMI z-score, HOMA-IR and total cholesterol.
Influence of plasma nitrates and nitrites on renal function in normal weight and overweight/obese children
In Fig. 2, the distribution of eGFR by tertiles of P-NOx concentration, in normal weight and in overweight/obese children, is shown. Although all children had normal renal function, in overweight/obese children, the eGFR values decreased across tertiles of P-NOx (median: 139·3 (25th, 75th percentile 128·0, 146·5), 128·0 (25th, 75th percentile 121·5, 140·4), 129·5 (25th, 75th percentile 119·4, 138·3), age- and sex-adjusted P for linear trend=0·003).

Fig. 2 Distribution of estimated glomerular filtration rate by Zappitelli combined formula (eGFR) by tertiles of plasma nitrates and nitrites (P-NOx) concentrations. The tertiles of P-NOx (≤10·76; 10·77–14·27; >14·27) were defined on the basis of all enrolled participants (n 313). The eGFR data are expressed as medians and 25th, 75th percentiles. The value of P for linear trend was estimated by linear regression analysis of eGFR values on each marker, adjusting for age (months) and sex. Panels (a) and (b) depict the distribution of eGFR by tertiles of P-NOx in normal weight (a), P for trend=0·078 and in overweight/obese children (b), P for trend=0·003.
Discussion
The results of our study show that obese children presented significantly higher values of U-Isop and U-NOx. It is noteworthy that U-Isop were associated with several cardiometabolic risk factors such as HOMA-IR, MPO, hs-CRP and TAG. In addition, in overweight and obese children, we found a strong negative association between eGFR values and P-NOx concentrations.
The direct measurement of free radicals and reactive molecules is difficult to achieve, and several methods have been developed to indirectly infer the levels of oxidative stress, both through the measurement of antioxidant capacity and through the quantification of oxidatively damaged biomolecules. Our results showed that the plasma antioxidant capacity did not differ across BMI classes. Unaltered plasma TAS has also been described in other studies related to childhood obesity. In obese children without the metabolic syndrome, plasma TAS values were similar to those found in the control group( Reference Molnár, Decsi and Koletzko 7 , Reference Eren, Abuhandan and Solmaz 8 ). In addition, in a study evaluating children with type 2 diabetes mellitus, as well as age-, sex- and BMI-matched controls (obese group) and unmatched controls, TAS values were not different between the three groups( Reference Stringer, Sellers and Burr 26 ). Nonetheless, several methods of TAS determination exist that are not necessarily correlated with one another( Reference Codoner-Franch, Valls-Belles and Arilla-Codoner 2 ), and contradictory findings concerning the values of this parameter in association with obesity have been reported( Reference Faienza, Francavilla and Goffredo 6 , Reference Eren, Abuhandan and Solmaz 8 ). Despite the absence of differences in plasma antioxidant capacity across BMI classes, we found that TAS was inversely correlated with markers of pro-oxidant/proinflammatory status, such as U-Isop and serum MPO. Indeed, under conditions of elevated ROS production, the need for neutralisation of these species implies a higher consumption of systemic antioxidants( Reference Sousa, Afonso and Carvalho 27 ).
Quantification of lipid peroxidation markers constitutes the most used approach for the evaluation of oxidative stress. Isoprostanes are primarily derived from the free radical-catalysed peroxidation of arachidonic acid and represent a reliable biomarker of endogenous lipid peroxidation( Reference Milne, Dai and Roberts 24 , Reference Sousa, Afonso and Carvalho 27 ). In our study, we measured free isoprostanes in plasma and urine. It is known that both free and esterified (bound) isoprostanes can be measured in plasma or serum samples( Reference Liu, Morrow and Yin 28 ). We opted to quantify only free isoprostanes in plasma, because in many samples it was not possible to obtain a complete separation of phases during the procedure recommended for the hydrolysis of esterified isoprostanes and further analysis of total isoprostanes. Nevertheless, other authors have not found advantage in the measurement of total isoprostanes rather than free isoprostanes in plasma( Reference Liu, Morrow and Yin 28 ). We reported that P-Isop and U-Isop levels were positively correlated with measures of obesity, which is in line with previous studies in children( Reference Codoner-Franch, Valls-Belles and Arilla-Codoner 2 , Reference Dennis, Ergul and Gower 4 , Reference Warolin, Coenen and Kantor 5 , Reference Ostrow, Wu and Aguilar 12 , Reference Loffredo, Martino and Carnevale 13 , Reference Savino, Pelliccia and Giannini 29 ). Similar to some studies( Reference Dennis, Ergul and Gower 4 , Reference Savino, Pelliccia and Giannini 29 ), we also found that isoprostanes were associated with insulin resistance. Of note, our findings also indicate that this association is independent of BMI. This is in line with a recent study where the authors also found increased lipid peroxidation in association with insulin resistance, independently of adiposity, thus hypothesising that the degree of insulin resistance is the major determinant of the oxidative stress found in the obese, which may further impair pancreatic function and amplify the metabolic and cardiovascular complications( Reference Codoñer-Franch, Navarro-Ruiz and Fernández-Ferri 30 ).
Isoprostanes are not only markers of oxidative stress but also exert several actions relevant to the pathogenesis of vascular dysfunction, as they are potent vasoconstrictors in most vascular beds, induce platelet aggregation, and enhance the adhesion of neutrophils and monocytes to endothelial cells, thus contributing to atherosclerosis( Reference Milne, Dai and Roberts 24 ). These effects and the positive association of U-Isop with HOMA-IR, proinflammatory markers and TAG observed in our study suggest that the increased oxidative stress found in obese children might already be causing injury to the arterial wall, even in the absence of established hypertension. Furthermore, U-Isop may represent an early marker of cardiometabolic dysfunction.
Obese children exhibit worse profiles of carotid intima-media thickness, PWV and flow-mediated dilatation, reflecting vascular damage( Reference Meyer, Kundt and Steiner 31 , Reference Sakuragi, Abhayaratna and Gravenmaker 32 ), and several clinical and experimental obesity-related studies have found that oxidative stress is significantly associated with changes in these vascular parameters( Reference Giannini, de Giorgis and Scarinci 33 – Reference McNeilly, McClean and Murphy 36 ). Interestingly, in our sample, we found an unexpected positive correlation between PWV and TAS values. This finding might indicate that an increase in PWV triggers the activation of antioxidant defences. As alterations in PWV appear to be consistently associated with arterial diameter( Reference Fok, Jiang and Clapp 37 ), we hypothesise that a decrease in vasodilator tone might be a stimulus for the activation of nuclear factor E2-related factor 2, a key transcription factor that regulates the expression of antioxidant genes and appears to play a major role in the regulation of vascular function( Reference Marczak, Marzec and Zeldin 38 , Reference Chen, Lu and Chen 39 ).
Regarding the markers of NO production/metabolism, we found that P-NOx and U-NOx were positively correlated with each other, but only the U-NOx values were significantly and positively associated with BMI z-score. Some studies reported lower concentrations of NOx in obese children as reflecting a decreased NO bioavailability( Reference Gruber, Mayer and Mangge 11 ), whereas other authors reported findings similar to ours, with obese children presenting higher U-NOx values( Reference Codoñer-Franch, Tavárez-Alonso and Porcar-Almela 10 ). Although it is not possible to differentiate between NO production from constitutive or inducible NOS isoforms, we believe that the higher values of U-NOx observed in overweight and obese children of our study reflect a higher excretion of systemic NO metabolites due to increased NO biosynthesis by the inducible NOS. Indeed, several studies in experimental models of obesity have reported an up-regulation of inducible NOS expression and decreased or unaltered endothelial NOS. Obese Zucker rats exhibited increased mRNA and protein expressions of vascular inducible NOS and unaltered expression of vascular endothelial NOS, compared with the group of lean Zucker rats( Reference Justo, Candiracci and Dantas 40 ). Moreover, the administration of a high-fat diet to C57BL/6j mice induced the mRNA expression of inducible NOS but not of the other two isoforms in white adipose tissue and skeletal muscle( Reference Tsuchiya, Sakai and Suzuki 41 ). Furthermore, endothelial NOS protein expression was shown to be decreased in the adipose tissue from db/db mice and high-fat diet-fed, wild-type C57BL/6j mice( Reference Sansbury, Cummins and Tang 42 ).
Of note, in our study, P-NOx was positively correlated with uric acid, a product of xanthine oxidase, which suggests that both inducible NOS and the pro-oxidant xanthine oxidase are activated in parallel, exacerbating the systemic proinflammatory/pro-oxidant status.
Importantly, we detected a significant negative association between P-NOx and eGFR in overweight and obese children. The high amounts of NO and peroxynitrite formed in proinflammatory conditions could inhibit endothelial NOS activity( Reference Pacher, Beckman and Liaudet 3 , Reference Gabbai 43 ), and the reduced endothelial-derived NO production in the kidney vascular tree would result in an imbalance towards higher vasoconstriction( Reference Gabbai 43 ), decreasing eGFR or, at least, preventing hyperfiltration to be evident. Overall, in obesity, the higher values of NO metabolites probably result in more deleterious than beneficial effects on the vascular bed, due to the loss of endothelial NO and its biological effects. A negative correlation between P-NOx and eGFR was also found in adults with normal and mildly impaired renal function( Reference Diaz, Feairheller and Sturgeon 44 ). Nonetheless, very few studies have addressed this association in children. Savino et al. ( Reference Savino, Pelliccia and Schiavone 45 ) reported significantly higher values of both plasmatic and urinary NOx in diabetic children and a positive correlation between these markers and renal resistive indices, hypothesising that increased NO production might contribute to intrarenal haemodynamic abnormalities in diabetic patients. Another study involving children with hypertension and normal controls also reported a negative correlation between P-NOx and eGFR( Reference Goonasekera, Shah and Rees 46 ). We cannot exclude the possibility that a reduced GFR per se might have increased the systemic concentration of NO metabolites. However, if this was the case, then it would be expected that U-NOx values presented a positive correlation with eGFR and/or a negative correlation with P-NOx. Contrary to this hypothesis, in our study, U-NOx values were positively correlated with P-NOx concentrations and were significantly increased in overweight and obese children.
In addition to the proinflammatory mechanisms described, there are other pathways whereby NO production in the kidney might also be affected. We detected an inverse association between U-NOx levels and total cholesterol or non-HDL-cholesterol, which reinforces previous experimental findings of hypercholesterolaemia causing a decrease in NO synthesis in the kidney( Reference Kopkan, Khan and Lis 47 , Reference Attia, Ni and Boer 48 ) and might represent another mechanism contributing to renal impairment in obesity. Other factors such as insulin resistance, diabetes, arterial hypertension, oxidative stress and hyperleptinaemia are also known to affect NO bioavailability and activity and to contribute to renal function decline in obesity( Reference Redon and Lurbe 49 – Reference Bravo, Morse and Borne 53 ).
We did not observe significant changes in urinary excretion of H2O2, a non-radical ROS that has been highlighted as a paracrine mediator of cardiovascular and renal dysfunction( Reference Sousa, Afonso and Carvalho 27 , Reference Sousa, Oliveira and Afonso 54 ). Although we did not find any association between H2O2 and the cardiovascular and renal parameters analysed, H2O2 was positively correlated with other oxidative stress (U-Isop) and NO production markers (U-NOx). Furthermore, we detected an inverse correlation between H2O2 and percentage of body fat mass, which was enhanced in normal weight children and lost in the overweight/obese group, when these groups were analysed separately (data not shown). These results might be related to the concentration of adiponectin, an adipose tissue-derived hormone that reduces ROS values and whose synthesis has been shown to be down-regulated in obesity( Reference Ghantous, Azrak and Hanache 55 ), although this hypothesis requires further testing.
This study has some limitations. First, we did not recommend any dietary restrictions for the 48 h before sample collection, and we did not consider the eating and exercise habits of the children in our analysis. Second, as in every cross-sectional evaluation, the associations found are of interest but should naturally be interpreted with caution, especially regarding causality inferences. We acknowledge that most of the evaluations performed should ideally be repeated throughout the children’s development until adulthood, only then allowing us to clarify thresholds of risk and the clinical relevance of the chronic elevation of oxidative or nitrative/nitrosative stress markers in the long term. Nevertheless, the performance of this cross-sectional broad evaluation of oxidative stress markers and NO metabolism markers in the setting of a birth cohort sample is a major strength of our study, assuring the possibility of further evaluations. The fact that we were able to include a large and homogeneous sample of healthy prepubertal children, with a detailed characterisation of cardiometabolic risk factors, including 24-h ABPM, and renal function, constitutes another important strength of our study.
Conclusions
Our results show that oxidant status and NO are increased in relation to fat accumulation and that oxidative stress and NO biomarkers correlate with each other. Furthermore, increased oxidant status translates into higher cardiometabolic risk, and higher NO concentrations have a negative impact on renal function in overweight and obese children. Nevertheless, it would be important to assure the follow-up of these children, aiming to define markers for early identification of subjects at risk and to clarify the impact of the chronic elevation of these parameters in the long term.
Acknowledgements
The authors gratefully acknowledge the families enrolled in Generation XXI for their kindness, all members of the research team for their enthusiasm and perseverance and the participating hospitals and their staff for their help and support.
This project was supported by the Fundo Europeu de Desenvolvimento Regional (FEDER) funds from Programa Operacional Factores de Competitividade – COMPETE (FCOMP-01-0124-FEDER-028751), by national funds from the Portuguese Foundation for Science and Technology (FCT), Lisbon, Portugal (PTDC/DTP-PIC/0239/2012), and by Calouste Gulbenkian Foundation. L. C.-C. was supported by FCT (grant SFRH/SINTD/95898/2013), T. S. was supported by FCT and Programa Operacional Potencial Humano (POPH)/Fundo Social Europeu (FSE) (EC) (Ciência 2008 and SFRH/BPD/112005) and F. S. was supported by the European Renal Association – European Dialysis and Transplant Association Research Programme, Parma, Italy, and the KfH Foundation for Preventive Medicine, Neu-Isenburg, Germany. The funders COMPETE, FCT, Calouste Gulbenkian Foundation, POPH/FSE (EC), European Renal Association and KfH Foundation for Preventive Medicine had no role in the design, analysis or writing of this article.
L. C.-C., T. S., A. A. and A. A.-T. contributed to conception and design of the study. L. C.-C., T. S., D. C. and J. A. conducted the study. L. C.-C., T. S., D. C. and A. A. were involved in data analysis. L. C.-C. and T. S. wrote the manuscript. All the authors were involved in the interpretation of data and critical revision of the manuscript. A. A. and A. A.-T. supervised the study. L. C.-C., T. S., A. A. and A. A.-T. had primary responsibility for the final content. All the authors read and approved the final version of the manuscript.
There are no conflicts of interest.









