Introduction
The world's population is projected to reach 8.5 billion by 2030 and continue to grow around 9.7 billion by 2050 and 10.4 billion by 2100 (https://www.un.org/en/global-issues/population). As the global population expands, it puts additional pressure on agricultural systems to meet the food demand, which increases the need for long-term solutions to these interrelated global problems. Furthermore, the effects of climate change are complicating agriculture and making it more difficult to assure that there will be enough food for everyone. Legume crops play an important role in addressing these issues by providing protein-rich food and facilitating nitrogen fixation in the soil. Among the legumes, lentil (Lens culinaris Medikus) is a crucial crop that contributes to sustainable agriculture on a global scale. Lentil is a diploid (2n = 2x = 14) cool season legume that belongs to the Fabaceae and ranks fifth after common bean (Phaseolus vulgaris L.), dry pea (Pisum sativum L.), chickpea (Cicer arietinum L.) and cowpea (Vigna unguiculata L.) in global pulse production. Lentil cultivation is widespread in several countries, including Canada, India, Nepal, Australia, Turkey, the USA and China. Over the past two decades, there has been a notable increase in global lentil productivity, with average yield rising from 874.5 kg/ha to 1004.3 kg/ha (FAOSTAT, 2022). As per the USDA's nutrient data in 2022, lentil boast a rich nutritional profile, comprising 63.4% carbohydrates, 24.5% protein, 2.7% ash content and less than 2% total fat (Dhull et al., Reference Dhull, Kinabo and Uebersax2022). Furthermore, lentil is recognised for their high dietary fibre content, significant potassium levels, slowly digestible starch and low sodium content, making it a favoured choice among health conscious individuals (Khazaei et al., Reference Khazaei, Subedi, Nickerson, Martínez-Villaluenga, Frias and Vandenberg2019; Dhull et al., Reference Dhull, Kinabo and Uebersax2022). Hence, lentil holds great importance in addressing food and nutritional security challenges worldwide.
In India, lentil ranks second after chickpea as the most significant winter pulse crop. In 2021, India produced 1.49 million tonnes of lentil with an average yield of 859 kg/ha (FAOSTAT, 2022). Indeed, India's current lentil yield is lower than several other countries, largely due to the underperformance of existing cultivars, leading to suboptimal yields. Lentil production is constrained due to both biotic and abiotic stresses (Feleke et al., Reference Feleke, Meseret and Tafes2021). Biotic stresses such as fusarium wilt, ascochyta blight, rust, stemphylium blight, anthracnose, botrytis grey mould and white mould are among the common diseases of lentil (Rodda et al., Reference Rodda, Davidson, Javid, Sudheesh, Blake, Forster and Kaur2017; Rubio Teso et al., Reference Rubio Teso, Lara-Romero, Rubiales, Parra-Quijano and Iriondo2022; Zafeiriou et al., Reference Zafeiriou, Ntoanidou, Baira, Kasiotis, Barmpouni, Machera and Mylona2022). In addition to biotic stresses, lentil crop is vulnerable to various abiotic stresses, such as heat stress, terminal drought and poor soil fertility, which pose significant challenges to their cultivation (Priya et al., Reference Priya, Bansal, Kumar, Dikshit, Kumari, Pandey, Singh, Tripathi, Singh, Kumari and Kumar2021). These environmental factors can adversely affect plant growth, flowering and pod development, leading to reduced yields (Zeroual et al., Reference Zeroual, Baidani and Idrissi2022). Additionally, environmental conditions and genotype interactions further hamper the expression of complex traits, resulting in poor genetic gain (Kumar & Ali, Reference Kumar and Ali2006). Besides biotic and abiotic stresses, the lentil is also challenged by a relatively narrow genetic base. The limited genetic diversity in modern lentil varieties has become a significant concern for improving lentil crop (Holme et al., Reference Holme, Gregersen and Brinch-Pedersen2019; Singh et al., Reference Singh, Kumar, Mehra, Sood, Malhotra, Sinha, Jamwal and Gupta2022). As a result, the full production potential remains unrealized. The effective use of germplasm collections for crop improvement relies on understanding existing genetic variability (Tripathi et al., Reference Tripathi, Kumari, Gore, Mishra, Singh, Mishra, Gayacharan, Dikshit, Singh, Semwal and Mehra2022). In this context, precise phenotypic evaluation for desired traits and their integration into breeding initiatives are key stages in lentil improvement.
In the past, efforts were made to assess the genetic diversity in lentil and studies supported the incorporation of genetic resources from distinct gene pools (Langridge and Waugh, Reference Langridge and Waugh2019). Further, it was also demonstrated that the wild relatives of lentil exhibit significant levels of genetic variation (Singh et al., Reference Singh, Kumar, Mehra, Sood, Malhotra, Sinha, Jamwal and Gupta2022). Recently, a total of 2324 lentil accessions were evaluated for the identification of trait-specific germplasm (Tripathi et al., Reference Tripathi, Kumari, Gore, Mishra, Singh, Mishra, Gayacharan, Dikshit, Singh, Semwal and Mehra2022). These trait-specific germplasm hold great potential as parental lines for further breeding programmes (Rajpal et al., Reference Rajpal, Singh, Kathpalia, Thakur, Khan, Pandey, Hamurcu and Raina2023). Leveraging diverse germplasm in lentil cultivation, two lentil lines ‘Jammu Lentil 144’ and ‘Jammu Lentil 71’, have been developed through an interspecific crosses (Singh et al., Reference Singh, Kumar, Mehra, Sood, Malhotra, Sinha, Jamwal and Gupta2022). Nevertheless, selection solely based on seed yield without considering other important traits becomes ineffective. For instance, early flowering and early maturity are key traits in the context of adverse climatic scenarios that enable the crop to overcome terminal drought and reproductive heat stress for achieving the sustainable production. Further, it has also been demonstrated that heat stress at reproductive stages adversely affects the yield and nutritional quality of lentil (Choukri et al., Reference Choukri, Hejjaoui, El-Baouchi, El Haddad, Smouni, Maalouf, Thavarajah and Kumar2020). In addition, the early varieties of lentil can fit into the rice fallows and can effectively use the residual soil moisture and enhance crop intensification. In India several states have a major share of rice fallows especially, Chhattisgarh (4.1 Mha), which accounts for almost 35% of total rice fallows, whereas Madhya Pradesh and Orissa have nearly 1.8 Mha (15%) under rice fallows. The states like Jharkhand, Maharashtra and West Bengal possess ~5–8% of area under rice fallows. Similarly, other states like Telangana, Assam and few others have <3% of total rice fallows. Very few efforts were made in the past to understand the genetic variability for earliness in combination with higher grain yield in lentil. For example, wild accession 'ILWLS 118' was identified after evaluating 70 accessions from six distinct wild species (Lens orientalis Boissier: 37; Lens odemensis Ladizinsky: 4; Lens nigricans M. Bieb: 8; Lens ervoides Brignoli: 18; Lens lamottei Czefranova: 2; and Lens tomentosus Ladizinsky: 1) for early flowering but not having high grain yield (Kumar, Reference Kumar2016). None of the efforts in the past made on a larger germplasm set to screen out early flowering and early maturity in combination with higher grain yield. Therefore, in this study, we report the first systematic evaluation of 158 genetically diverse lentil accessions to identify and determine early flowering and early maturity habits in combination with superior agronomic traits including yield. The findings from this investigation hold considerable implications for lentil breeding and genomics, which will contribute effectively to the development of sustainable lentil cultivation practices, addressing the challenges faced in lentil production and ensuring its long-term success in rain-fed areas.
Material and methods
Plant material and experimental design
A total of 158 accessions of lentil were obtained from the Indian Council of Agricultural Research (ICAR)- Indian Agricultural Research Institute (IARI), New Delhi, India. These accessions comprised 104 exotic germplasm, 12 cultivars, 34 ICARDA germplasm and 8 advanced breeding lines (Table S1). These accessions, including a large number of exotic germplasm may holds valuable genes for improving crop yield and combating various pests and environmental challenges. It offers an opportunity to discover beneficial genetic variations that can be directly utilised or integrated into Indian lentil breeding initiatives.
Characterization of germplasm was conducted during the winter seasons of 2020–2021 and 2021–2022 at two different research farms of Dr Rajendra Prasad Central Agricultural University, Pusa, Bihar. The first farm is located in Pusa, Samastipur, Bihar, at a latitude of 25°58'N and a longitude of 85°32'E. The second farm is located in Dholi, Muzaffarpur, Bihar, at a latitude of 25°59'N and a longitude of 85°36'E. The lentil accessions were sown under natural field conditions, following recommended practices for lentil cultivation. The experimental design involved an augmented block design with five popular checks: Precoz (early flowering, high 100-seed weight), Sehore 74-3 (resistance to fusarium wilt), K 75 (drought tolerant), L 4076 (late maturing and resistant to wilt and rust) and L 4717 (early maturing). The experiment was conducted in four blocks with 40 accessions sown per block uniformly along with replicating checks. The fourth block was incomplete, consisting of 38 accessions. The lentil accessions were sown in single rows of 2 m in length with 30 cm of row-to-row distance.
Phenotypic evaluation for early flowering and other agronomic traits
We evaluated 158 germplasm for one season at Pusa (2022–23) and for two seasons at Dholi (2021–22 and 2022–23). Phenotypic data was recorded on five randomly selected plants of each lentil accession for morphological traits (plant height, PH, cm; primary branches per plant, PB) and grain yield and yield related traits (number of pods per plant, PPP; hundred seed weight, HSW, g; grain yield per plant, YPP, g) with the exception of phenological traits (days to 50% flowering, DF; days to maturity, DM), which were recorded on plot basis. The descriptors published by ICARDA and IBPGR (International Board for Plant Genetic Resources) in 1985 were used for lentil germplasm characterisation to measure these traits.
Statistical analysis
The phenotypic data collected from two locations was used to estimate BLUP (best linear unbiased prediction) values using the Meta-R software (Alvarado et al., Reference Alvarado, Rodriguez, Pacheco, Burgueño, Crossa, Vargas, Pérez-Rodríguez and Lopez-Cruz2020). BLUP was used for the estimation of random effects and calculated values were utilised in further analysis. The analysis of variance (ANOVA) was performed using the ‘augmentedRCBD’ package in R (Aravind et al., Reference Aravind, Mukesh Sankar, Wankhede and Kaur2020). Correlation coefficient analysis was conducted and visualised using the ‘ggplot2’ and ‘ggcorrplotin’ R packages, respectively (Wickham, Reference Wickham2016). Additionally, the BoxPlotR web-based tool (Spitzer et al., Reference Spitzer, Wildenhain, Rappsilber and Tyers2014) was utilised to construct boxplots for all phenological and agronomic traits, showing the distribution of the phenotypic data. Principal component analysis (PCA) was conducted using the ‘factoextra’ package in R (Kassambara and Mundt, Reference Kassambara and Mundt2020). To perform hierarchical clustering using the UPGMA method, we used the ‘dendextend’ and ‘factoextra’ packages in R programming language.
Results
Characterization of lentil germplasm
The analysis of variance (ANOVA) results revealed highly significant (P < 0.01) differences among treatments for all the agronomic traits except the PB (Table 1). A comparative analysis of field experiments conducted at Pusa and Dholi revealed a considerable range of variation, especially in the case of traits like PH, DM and PPP. In Pusa, the PH ranged from 19.00 to 43.25 cm with an average value of 30.86 cm. While at Dholi, the PH was higher compared to Pusa, ranging between 24.98 and 59.80 cm with an average of 42.33 cm. Similarly, we observed significantly lower number of PPP at Pusa (13–106) compared to Dholi (20–140). Although DM varied between the two locations Dholi (109–122 days) and Pusa (111–128 days), we did not observe statistically significant difference between the locations. Nevertheless, genotypes tested at Dholi matured 6 days earlier compared to Pusa.
Table 1. Analysis of variance (ANOVA) for phenological and morphological characters of lentil accessions evaluated in augmented block design
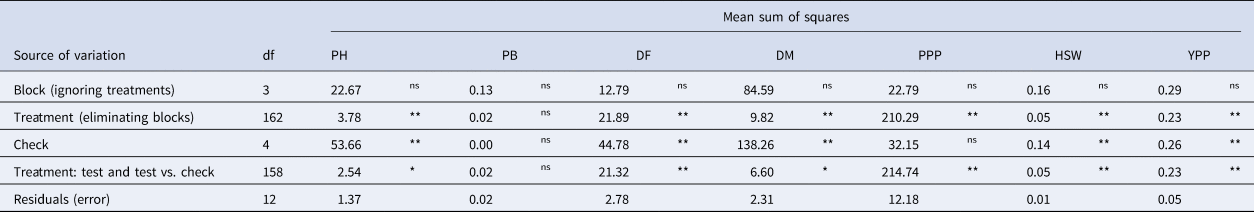
Note: Plant height (PH), number of primary branches (PB), days to 50% flowering (DF), days to maturity (DM), number of pods per plant (PPP), hundred seed weight (HSW), grain yield per plant (YPP), degrees of freedom (df), nonsignificant (ns), *significant at 5% probability level, **significant at 1% probability level.
We observed a wide range of variability for all the traits in terms of descriptive statistics like means, minimum and maximum value, standard error, standard deviation as well as measures of skewness and kurtosis (Table S2). Skewness measures the asymmetry of a distribution, while kurtosis indicates its peakedness. In this study, the kurtosis was not significant (P > 0.05) for PB, DF and PPP traits, suggesting that these traits fit a normal distribution. However, for PH, DM, HSW and YPP traits, the kurtosis was significant (P ≤ 0.05), indicating non-normal distributions. Specifically, DF (0.58), PPP (0.71) and YPP (1.00) traits showed positive skewness (P ≤ 0.01), implying that more accessions were below the mean than expected in a normal distribution. Conversely, PH (−0.17) and DM (−0.27) traits exhibited negative skewness, indicating that more accessions were above the mean than expected in a normal distribution. Additionally, the traits PH, DM, HSW and YPP showed significant (P < 0.05) and positive kurtosis, heavily suggesting leptokurtic distributions (Table S2). Boxplots represent the distribution of quantitative traits, revealed a wide range of variability among the germplasm (Fig. 1).
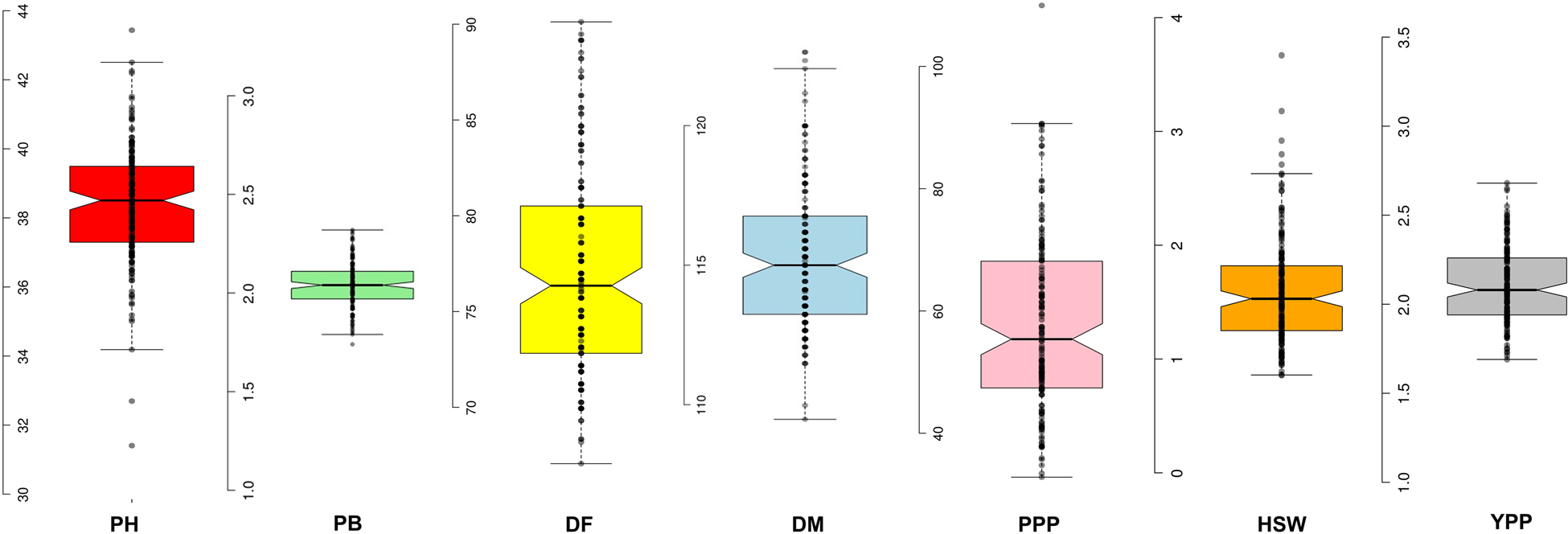
Figure 1. Box-plot showing phenotypic distribution of all seven agronomic traits in the 158 accessions of lentil using calculated BLUPs value. Note: Plant height (PH, cm), number of primary branches (PB), days to 50% flowering (DF), days to maturity (DM), number of pods per plant (PPP), hundred seed weight (HSW, g), grain yield per plant (YPP, g).
Correlation coefficient analysis is commonly employed to assess the strength and direction of relationships among traits. We computed correlation coefficient amongst six traits (two phenological, one morphological and three yield-related) measured on the set of 158 lentil accessions. The results indicated a prevailing tendency for positive correlations among the recorded traits. DM had highly significant and positive correlation with DF (0.75; P ≤ 0.01). On the other hand, YPP exhibited a positive and significant correlation with PPP (0.77; P ≤ 0.01). Notably, there was a negative correlation observed between DF and YPP. Additionally, PH showed a positive and significant correlation with all traits except YPP (Figure S1).
Three distinct cluster in 158 lentil germplasm
For identification of homogenous clusters, we performed cluster analysis using Euclidean distance based on the morphological, phenological, yield and yield-related traits. UPGMA clustering grouped all 158 accessions into three distinct clusters and one genotype (EC 78438) remained out-grouped (Fig. 2). Among three distinct clusters, Cluster-II is the largest with 76 lentil genotypes (48.10%) followed by Cluster-I (72 genotypes, 45.57) and Cluster-III (9 genotypes). Of 72 accessions in Cluster-I, 44 were exotic germplasm, 14 accessions were from ICARDA and 14 breeding lines. Similarly, among 76 germplasm clustered in Cluster-II, 56 exotic germplasm, 15 ICARDA lines and five cultivated varieties were accommodated. It contained accessions like EC 267634 and EC 223212-A displaying a higher HSW (>2.6 g) and accessions EC 78425 and EC 223207 with lower seed weight (<1.7 g). Moreover, Cluster-II featured accessions with shorter PH, such as EC 223223, P8112 and EC 267536 (<36 cm). Cluster-III contained nine accessions, characterised by three exotic germplasm, four ICARDA lines and two cultivated varieties, displaying notable attributes of higher YPP and PPP (Fig. 2, Table S3). The genotype EC 78438 which out-grouped flowering in 69 days and recorded high yields, with HSW 3.6 g, can be one of the promising germplasm for utilisation in future lentil breeding programs. Interestingly, Cluster-II comprised early-flowering accessions like EC 223197-A, P8112 and EC 267696 (≤69 days), while Cluster-I encompassed late-flowering accessions such as P15121, P15115 and P13108 (>89 days) as well as accessions (P13130, EC 78395 and EC 223220) with a PH more than >41 cm. In the case of Cluster-I, DF and DM ranged from 69 to 90 days and 112 to 123 days, respectively. Similarly, in the case of Cluster-II, DF and DM ranged from 67 to 90 days and 109 to 123 days, respectively.
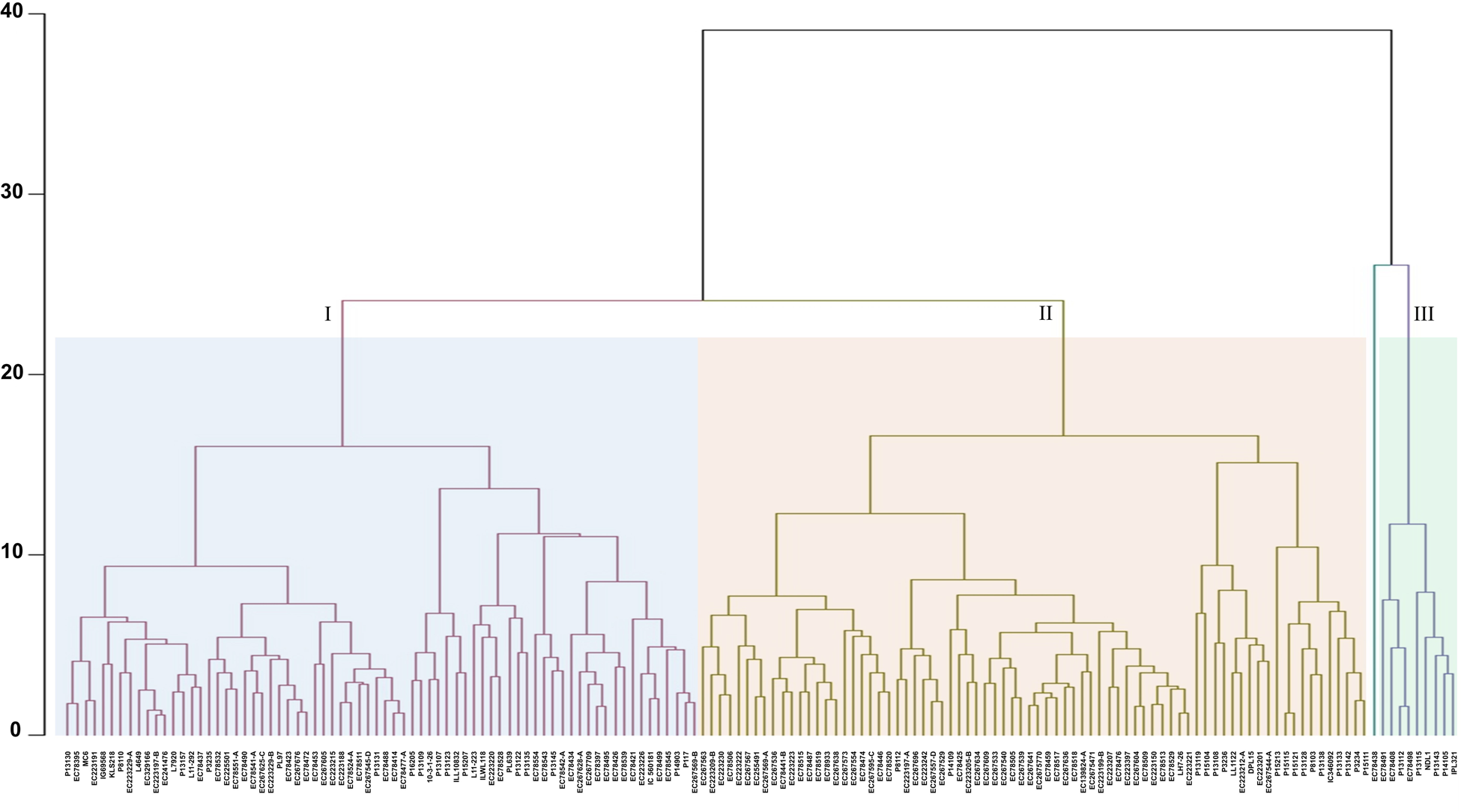
Figure 2. Hierarchical clustering of 158 lentil accessions conducted using the unweighted pair group method with arithmetic mean (UPGMA) based on BLUP values of phenotypic traits. The resulting dendrogram illustrates distinct clusters, each represented by a unique colour.
Principal component analysis (PCA)
The analysis involved six variables and the results showed that PC1 and PC2 accounted for 37.4% and 28.1% of the total variation, respectively. The biplot representation of the PCA results revealed that the accessions were scattered in all four quarters, indicating significant variability among them based on the measured variables (Fig. 3a). We have observed that Dim1 (Dimension 1/PC1) has the highest eigenvalue and variance percent, indicating that it explains the largest amount of variance in the dataset (Table 2). The highest positive eigenvalues for DM (0.78) and DF (0.71) in PC1 signify their significant influence on the overall variability among lentil genotypes. As we move down the dimensions, the eigenvalues and variance percentages decrease, suggesting a diminishing contribution to the overall variance. In the correlation circle of a PCA (Fig. 3b), the angles formed by the vectors representing YPP and DF are nearly 90°, which indicates a lack of positive correlation between these variables. The relatively short vector length of HSW suggests a lower contribution to PC1. On the other hand, longer vector lengths, such as that of YPP, make a significant contribution of nearly 49% to both PC1 and PC2. The close proximity of the YPP and PPP vectors signifies a strong correlation between these variables. Similarly, the vectors for DM and DF also exhibit close proximity, highlighting their strong positive correlation (Fig. 3b). In the PCA results, we have observed the contribution of each trait to the PC. For PC1, DM had the highest contribution of 27.15%, meaning it had the most significant impact on the first PC. On the other hand, HSW had the lowest contribution to PC1 with only 3.27%. Additionally, in PC2, YPP made the highest contribution, accounting for 36.82% of the variance in this PC. On the other hand, PH had the lowest contribution to PC2, with only 2.37% (Table S4).
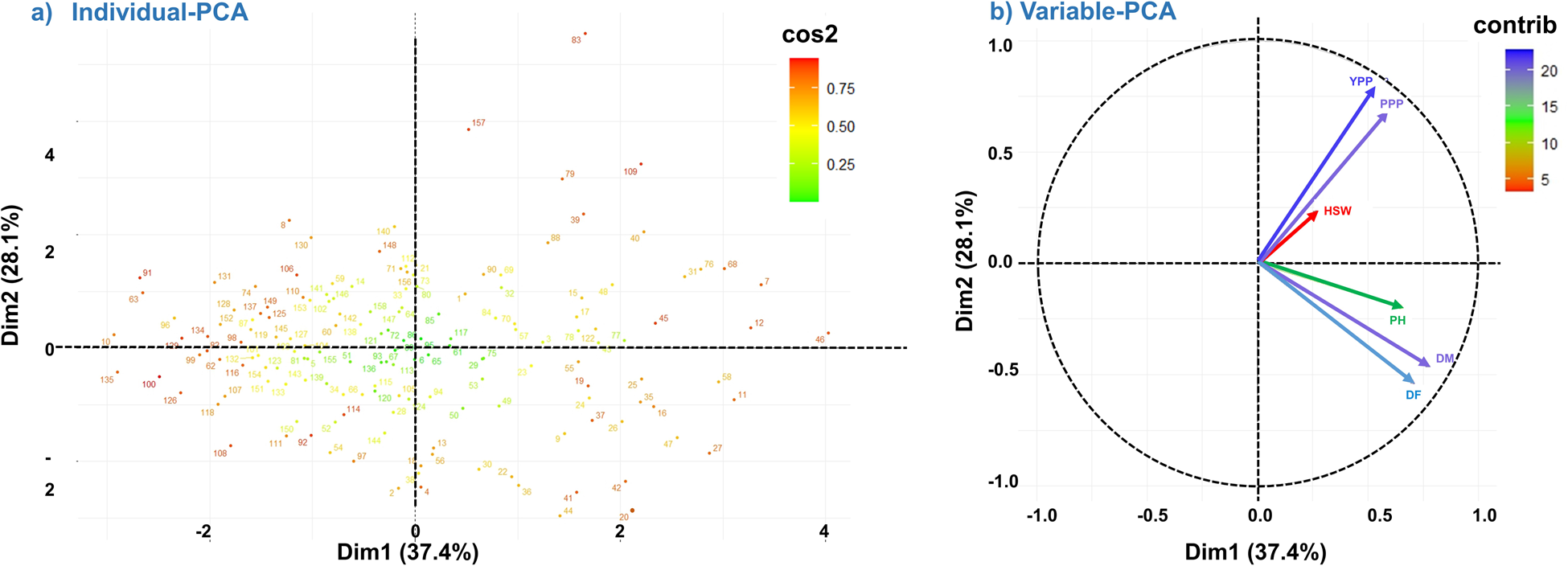
Figure 3. Principal component analysis (PCA) plot of individuals and variables. Principal components (PCs) 1 (representing 37.4% of explained variance) and PC2 (28.1% of explained variance) are plotted. (a) PCA biplot of individual samples in relation to PC1 and PC2. A high cos2 indicates a quality of good representation of the individual on the PC. (b) Correlation circle of a PCA. In a PCA correlation circle plot, vector direction and length play crucial roles in conveying information about the relationships between variables and principal components. The direction of a vector signifies the strength and nature of the correlation between an original variable and a specific principal component; proximity to the component indicates a stronger correlation. Meanwhile, vector length is equally significant, as longer vectors indicate higher correlations. This means that variables with longer vectors contribute more significantly to the variability explained by that principal component. Interpreting the angle between vectors provides insights into the relationships between variables: smaller angles denote positive correlations, while larger angles imply weaker or non-existent correlations.
Table 2. Principal component analysis based Eigen vectors and Eigenvalues explaining the variance contribution from the principal component (PC) axes

Trait-specific germplasm identification
The evaluation of genetic resources in lentil germplasm is crucial for identifying germplasm with specific desirable traits. To address the limited information on trait-specific superior accessions, a comprehensive evaluation process was conducted. In this study, trait-specific diverse lentil germplasm was identified (Table 3). The germplasm identified as superior for the YPP trait are EC 78438, EC 78498, EC 78408, P 13143 and EC 78491. These particular lines demonstrated a YPP exceeding 2.71 g, surpassing our high-yield check variety, Precoz, which yielded 2.63 g, as well as K75, which yielded 1.92 g. The accessions that have been identified as superior for the DF trait comprise EC 223197-A, P 8112, EC 267696, EC 78532 and EC 223242. Among these lines, EC 223197-A demonstrated a DF period of 67 days, which is one day earlier than our check variety L 4717, which required 68 days to reach 50% flowering. The superior germplasm for the DM trait includes EC 267696, P 3235, EC 267641, EC 78933 and EC 78551-A. In comparison, the early maturing check variety (L 4717) took approximately 102 days to reach maturity. The identified lines, on the other hand, exhibited a range of 109 to 112 days to reach maturity. Notably, EC 78491 and EC 78438 have emerged as exceptional germplasm, demonstrating both high YPP and early flowering. However, it is important to further evaluate these identified lines across different environments and multiple seasons to validate their performance and stability. These identified superior accessions for each trait can serve as potential donors in crop improvement programmes, allowing for the diversification of parental material and the enhancement of economically important traits.
Table 3. Promising trait-specific germplasm identified on the basis of combined phenotypic data along with the checks evaluated in the study
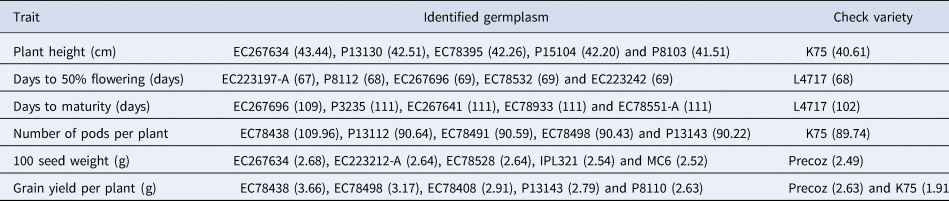
Note: Parentheses contain the values of particular traits of specific germplasm lines.
Discussion
We have characterised 158 lentil accessions for morphological, phenological, yield and yield-related traits in the present study. Our research stands out distinctly when compared to earlier studies for several compelling reasons. First and foremost, we have expanded our germplasm panel to encompass a wider and more varied range, providing a richer dataset and more comprehensive insights. Furthermore, our study places particular emphasis on two key traits: early maturity and high grain yield. These traits are not merely incidental but are strategically selected due to their paramount importance in addressing the challenges of climate resilience in rain-fed agriculture systems. Early maturity ensures that crops reach maturity stages before potential adverse climatic conditions, thereby minimising risks and optimising yields. On the other hand, achieving a high grain yield is essential for food security and sustainability, especially in regions vulnerable to erratic rainfall patterns and other climate-related uncertainties. In past, although early flowering trait has been studied, none of the studies used the accessions that we reported in this study. Substantial amounts of variability observed among the evaluated genotypes increased the likelihood of obtaining better lentil genotypes through selection and provided the opportunity for identification of new recombinants for genetic improvement. Numerous researchers have previously reported highly significant differences in morpho-agronomic traits between lentil genotypes (Sharma et al., Reference Sharma, Singh, Gill, Kumar and Parihar2020; Hussain et al., Reference Hussain, Iqbal, Akbar, Arshad, Munir, Ali, Masood, Ahmad, Shaheen, Tahir and Khan2022; Takele et al., Reference Takele, Mekbib and Mekonnen2022). In our study, we did not observe a significant difference in the number of primary branches. However, we did not consider this trait for further analysis. The DF ranged from 66 to 89 days, similar to another study (El Haddad et al., Reference El Haddad, Rajendran, Smouni, Es-Safi, Benbrahim, Mentag, Nayyar, Maalouf and Kumar2020) with a range of 51–78 days. Comparatively, the ranges we observed in our study are lower than the broader range of 51–123 days reported by Tripathi et al. (Reference Tripathi, Kumari, Gore, Mishra, Singh, Mishra, Gayacharan, Dikshit, Singh, Semwal and Mehra2022). We also found significant variation in DM, ranging from 109 to 124 days, somewhat similar to the reports of earlier researchers (El Haddad et al., Reference El Haddad, Rajendran, Smouni, Es-Safi, Benbrahim, Mentag, Nayyar, Maalouf and Kumar2020; Tripathi et al., Reference Tripathi, Kumari, Gore, Mishra, Singh, Mishra, Gayacharan, Dikshit, Singh, Semwal and Mehra2022). These findings underline the diverse nature of lentil genotypes with respect to their flowering and maturity traits. In terms of location, very less differences in phenological traits were observed because of the reason that genotypes were exposed to more or less similar climatic conditions. However, noticeable differences in morphological and yield related traits such as PH and PPP were observed. These distinctions might be attributed to variations in soil composition and fertility levels.
The selection of appropriate genotypes is a critical aspect of crop improvement, necessitating proper screening to identify traits that exhibit strong and positive correlations. For instance, in our studies, we found that PH displayed positive and significant correlations with both DF and DM. Similar results were obtained in a previous study under two different conditions, namely, heat stress and normal conditions (El Haddad et al., Reference El Haddad, Rajendran, Smouni, Es-Safi, Benbrahim, Mentag, Nayyar, Maalouf and Kumar2020). It is also reported that PH serves as a pivotal factor influencing both shoot yield and overall biomass production (Islam et al., Reference Islam, Karim, Oliver, Urmi, Hossain and Haque2018). Our findings suggested that higher PH is associated with late flowering, which can be important consideration for selecting genotypes with desirable traits. Similar positive correlations between PH and DF were previously reported (Dalbeer et al., Reference Dalbeer, Verma and Kavita2013; Mekonnen et al., Reference Mekonnen, Mekbib, Kumar, Ahmed and Sharma2014; Tripathi et al., Reference Tripathi, Kumari, Gore, Mishra, Singh, Mishra, Gayacharan, Dikshit, Singh, Semwal and Mehra2022). We have observed that the average PH (38.48 cm) is higher than that reported in previous studies (Kumar and Solanki, Reference Kumar and Solanki2014; Tripathi et al., Reference Tripathi, Kumari, Gore, Mishra, Singh, Mishra, Gayacharan, Dikshit, Singh, Semwal and Mehra2022). These traits have been key focal points during the process of domestication and subsequent plant breeding (Kaur et al., Reference Kaur, Atri, Akhatar, Mittal, Kaur and Banga2021). The timing of flowering is very important as plants must accumulate sufficient biomass before the initiation of flowering to support progressively emerging reproductive shoots. We also observed that flowering time was negatively associated with HSW and YPP. A similar correlation was reported earlier in lentil studies (Saxena et al., Reference Saxena, Tikle, Paliya and Singh2015; Takele et al., Reference Takele, Mekbib and Mekonnen2022). This type of association is not exclusive to lentils but also evident in other legumes like chickpea (Devasirvatham et al., Reference Devasirvatham, Gaur, Raju, Trethowan and Tan2015; Traore et al., Reference Traore, El-Baouchi, En-Nahli, Hejjaoui, Metougui, Hamwieh, Sohail, Istanbuli, Boughribil and Amri2022), pigeonpea (Sarsamkar et al., Reference Sarsamkar, Kadam, Kadam, Kalyankar and Borgaonkar2007) and cluster bean (Sharma et al., Reference Sharma, Mahla, Kumar and Gaikwad2021). Although early flowering might lead to a slight yield reduction, its importance remains prominent. This is especially relevant in situations encountered with terminal heat and moisture stress conditions for lentil production. In such scenarios, early flowering can be crucial. This will help in minimising the risk of dehydration or heat stress during the sensitive flowering and post-anthesis grain-filling stage (Aktar-Uz-Zaman et al., Reference Aktar-Uz-Zaman, Haque, Sarker, Alam, Rohman, Ali, Alkhateeb, Gaber and Hossain2022). Additionally, the PPP not only directly contributes to overall yield but also indirectly influences other important yield components. As such, traits like HSW, peduncle length and number of peduncles should be given due consideration during the selection and development of high-yielding lentil varieties (Mishra et al., Reference Mishra, Dikshit, Kumari, Priti, Tripathi, Devi, Aski, Mehra, Sarker and Kumar2020).
In cluster analysis, the grouping of germplasm into different clusters enabled an exploration of their genetic diversity. The differences we observed in traits might be because these large number of exotic accessions were collected from ICARDA, Syria and are being maintained at NBPGR and IARI, New Delhi, India. The wide range of euclidean distances we computed in our study underscores the significance of diverse lentil accessions as valuable sources of diverged breeding material. Notably, Cluster-III is primarily composed of lentil accessions from India, while other clusters predominantly feature exotic accessions from Syria. The clustering we observed in our study arises from the genetic variability within the lentil accessions. Cluster-I comprises accessions characterised by early-flowering and a shorter PH. On the other hand, Cluster-II is composed of accessions that exhibit late-flowering characteristics with higher PH. Notably, a single exotic accession, EC 78438, stands out for its exceptional combination of high yield and early flowering, and it holds great potential for future breeding programmes.
The decline in pulse cultivation is linked to the expansion of high-yielding varieties of wheat and rice in northern and eastern India, respectively. This trend could persist unless extra-early varieties suitable for adoption under these systems are developed (Shrestha et al., Reference Shrestha, Turner, Siddique and Turner2006). Similarly, rice-fallow regions in South Asia face challenges due to dry soil at harvest, hindering subsequent sowing. Here, early maturing lentil germplasm could contribute as a useful genetic resource to breeding efforts, converting mono-cropped areas into double-cropped ones, and enhancing lentil production in rice-based systems. Therefore, in a previous study, a genotype ILWLS 118 was identified for early flowering from a wild accession, which belongs to Lens orientalis Boissier (Kumar, Reference Kumar2016). Additionally, IC 241529 and IC 241532 accessions were identified that flowered in just 51 days (Tripathi et al., Reference Tripathi, Kumari, Gore, Mishra, Singh, Mishra, Gayacharan, Dikshit, Singh, Semwal and Mehra2022). In our study, the exotic germplasm ‘EC 223197-A’ was identified as an early-flowering entry with a substantial HSW (>1.9 g) and flowered a day ahead of the national check L 4017. Such early entry holds great promise for expanding lentil cultivation in rice-fallow regions of eastern India, where crops are sown under moisture-conserved conditions (Kumar et al., Reference Kumar, Mishra, Upadhyay and Hans2019). Additionally, these genotypes will also be the best option in drought-prone areas such as those in India, including Gujarat, Maharashtra and Madhya Pradesh. The introduction of early maturing lentil germplasm offers a potential solution, enabling the development of varieties suited to these systems. Additionally, lines with high YPP were identified such as EC 78438 and EC 78498 (>3.1 g). Among the lentil accessions that were found to mature early, EC 78933 was also recognised earlier for its high iron content (Mehra et al., Reference Mehra, Sarker, Dixit, Aski, Khandia and Munjal2018). These highly valuable trait-specific germplasm selections hold potential for utilisation in breeding endeavours.
The lentil accessions exhibited considerable diversity and allelic richness across all traits, except for PB, as indicated by the summary statistics. The novel sources for various traits in lentil have been identified in this study, such as EC 223197-A for early flowering and maturity, EC 78438 for high yield, EC 267696, EC 78491 and EC 78438 for earliness and high yield. Identified and validated trait-specific germplasm may represent a promising reservoir of new alleles. To capitalise on this genetic diversity and expedite the overall improvement of lentil, the identified germplasm with specific traits will be strategically utilised in hybridization programmes. This approach aims to foster the development of high-performing cultivars that exhibit improved agronomic traits, yield potential and overall resilience to varying environmental conditions. Such efforts in lentil breeding hold the potential to positively impact food security and agricultural sustainability, contributing to the advancement of global crop production and nutrition.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262124000042.
Acknowledgements
MT acknowledges financial support from Dr Rajendra Prasad Central Agricultural University, Pusa, Bihar and University of Southern Queensland, Toowoomba, Queensland, Australia. YDN acknowledges the Department of Biotechnology, Government of India, for the DBT-JRF fellowship for his Ph.D. program.








