C-reactive protein was the first acute-phase protein to be described with a capacity to precipitate the somatic C-polysaccharide of streptococcus pneumonia (Reference Tillett and FrancisTillett & Francis, 1930). It is a sensitive systemic marker of inflammation and tissue damage and is produced by hepatocytes predominantly under transcriptional control by the pro-inflammatory cytokine interleukin 6 (IL-6) (Reference Pepys and HirschfieldPepys & Hirschfield, 2003). The sole determinant of circulating C-reactive protein is the synthesis rate (Vigushin et al, 1983), and in general its production increases rapidly in response to a pathological stimulus but also falls rapidly upon resolution of the given stimulus.
Major depression has been shown to be associated with activation of the inflammatory response. These changes include increased numbers of peripheral leucocytes, both monocytes and neutrophils (Reference MaesMaes, 1999). Positive acute-phase proteins (including C-reactive protein) are increased (Reference Sluzewska, Rybakowski and BosmansSluzewska et al, 1996; Reference Berk, Wadee and KuschkeBerk et al, 1997; Reference MaesMaes, 1999; Reference Tuglu, Kara and CaliyurtTuglu et al, 2003), whereas negative acute-phase proteins (e.g. albumin) are decreased (Reference MaesMaes, 1999). This acute-phase response is an integral part of the inflammatory response and its purpose is to enable protein mobilisation, which serves to limit tissue damage and stimulate repair (Reference KushnerKushner, 1982).
Levels of C-reactive protein have been used for monitoring many diseases for decades. With the advent of highly sensitivite tests it has become possible to measure levels of this protein that lie within the normal range (Reference Rifai, Tracy and RidkerRifai et al, 1999). Using these high-sensitivity kits, C-reactive protein may be useful in predicting cardiovascular events in patients with coronary heart disease (Reference Haverkate, Thompson and PykeHaverkate et al, 1997; Reference Ridker, Hennekens and BuringRidker et al, 2000). Depressed individuals have been shown to be at increased risk of developing angina and myocardial infarction compared with their non-depressed counterparts (Reference DinanDinan, 1999; Reference Rozanski, Blumenthal and KaplanRozanski et al, 1999). When traditional risk factors for coronary heart disease are adjusted for, depressed individuals have a risk double that of the non-depressed population (Reference Rozanski, Blumenthal and KaplanRozanski et al, 1999). There is some evidence that risk of coronary heart disease is associated with a dose–response effect, with higher risk of the disorder in individuals with greater exposure to depression (Reference Penninx, Beekman and HonigPenninx et al, 2001). The Amsterdam Longitudinal Ageing study reported a relative risk of cardiac mortality of 1.6 associated with depressive symptoms. However, this cardiac mortality relative risk increased to 3.8 for those who were clinically depressed (Reference Penninx, Beekman and HonigPenninx et al, 2001). The link between depression and coronary heart disease may be mediated through inflammation. Atherosclerosis is preceded by inflammation with increased production of acute-phase proteins, including C-reactive protein and pro-inflammatory cytokines (Reference Libby, Ridker and MaseriLibby et al, 2002; Reference Lesperance, Frasure-Smith and TherouxLesperance et al, 2004). It seems reasonable to assume that depression increases the risk of coronary heart disease through its pro-inflammatory biology.
The purpose of our study was to examine C-reactive protein levels in depression and to determine the impact of selective serotonin reuptake inhibitor (SSRI) therapy.
METHOD
Sample
Study 1 used a between-subjects design. Thirty-two people with a history of DSM–IV major depression (American Psychiatric Association, 1994) were recruited. All were treated with an SSRI in standard dosages. At the time of C-reactive protein measurement all participants had had at least 4 weeks of therapy. Twelve (4 men, 8 women) were defined as euthymic, with a score of 7 or less on the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960), and had a mean age of 45.3 years (range 23–70). The remaining 20 currently depressed patients (7 men, 13 women) had an HRSD score greater than 17 and a mean age of 43.3 years (range 22–71). The comparison group consisted of 20 healthy individuals (10 men, 10 women) whose mean age was 37.6 years (range 21–58).
Study 2 employed a within-subject design. Twenty women were recruited with a mean age of 37.9 years (range 23–56). All were diagnosed with DSM–IV major depression with melancholic features and had an HRSD score greater than 17. They had been medication-free for at least 6 weeks at the time of recruitment. Following baseline investigation they were treated for 3 weeks with either fluoxetine (20 mg), paroxetine (20 mg) or sertraline (50 mg). The choice of antidepressant was made by the treating clinician.
All participants had a full physical examination to rule out significant physical illness, including acute or chronic infections and inflammatory or immune disorders; individuals were excluded if they had any endocrine, immune or metabolic disorder such as an autoimmune disorder, inflammatory bowel disease or acquired immunodeficiency syndrome. Similarly, participants who in the past 2 weeks had had an allergic, infectious or inflammatory response were excluded from the study. Laboratory testing was performed on the whole sample, including full blood count and renal and liver profile. Patients with comorbid axis I disorders, including substance misuse, were excluded.
Procedure
Participants provided a 4 ml sample of blood, collected in a serum clot activator container between 09.00 h and 11.00 h. Severity of depression was assessed using the 17-item version of the HRSD (Reference HamiltonHamilton, 1960) on the morning of blood sampling. The levels of C-reactive protein were measured using an immunoturbidimetric assay by Olympus (http://www.olympus-global.com). This system was able to detect concentrations of this protein as low as 1.57 mg/l.
Data analysis
A one-way analysis of variance (ANOVA) was performed to determine group differences between both patient groups and the control group in study 1. In the within-subject study, paired t-tests and Pearson's product moment correlation coefficients were used as appropriate. All results are expressed as mean (s.e.m.) and the data were analysed by GraphPad Prism, version 4.0 for Windows (Graph Pad Software, San Diego, California, USA; http://www.graphpad.com).
RESULTS
Study 1
Overall, levels of C-reactive protein did not differ between the group treated with SSRIs (whether currently depressed or euthymic) and the healthy comparison group (Fig. 1). The mean level of C-reactive protein in the currently depressed group was 4.68 mg/l (s.e.m.=0.93), in the currently euthymic depression group it was 2.50 mg/l (s.e.m.=0.94) and in the healthy control group it was 3.44 mg/l (s.e.m.=0.50). A one-way ANOVA yielded a between-group comparison which was not significant (F=1.68, d.f.=50, P=0.19). No relationship could be established between severity of depression (HRSD score) and C-reactive protein level (r=-0.002, NS).
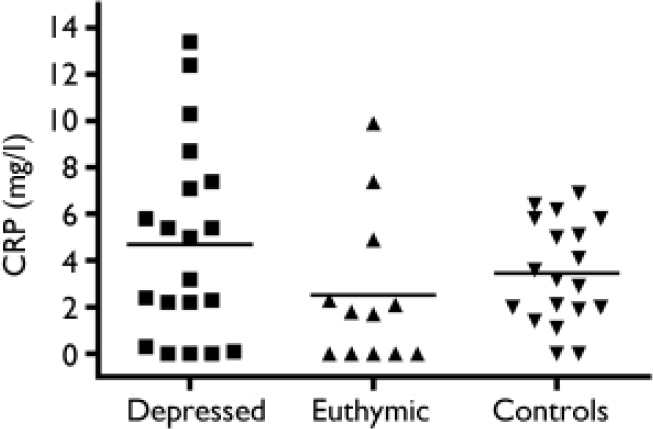
Fig. 1 Levels of C-reactive protein (CRP) in patients with recurrent depression and in healthy controls.
Study 2
Following 3 weeks of treatment with an SSRI, 2 patients had an HRSD score greater than 17, and 2 were clinically euthymic with an HRSD score below 7 (Fig. 2). Levels of C-reactive protein dropped significantly following treatment (Fig. 3): the pre-treatment level was 12.0 mg/l (s.e.m.= 0.95) and the level following treatment was 8.0 mg/l (s.e.m.=0.68) (t=4.88, d.f.=18, P<0.0001). Two patients had an increase in HRSD score post-treatment but in both cases their level of C-reactive protein dropped. There was no relationship between protein level and HRSD score or between change in protein level and change in HRSD score.
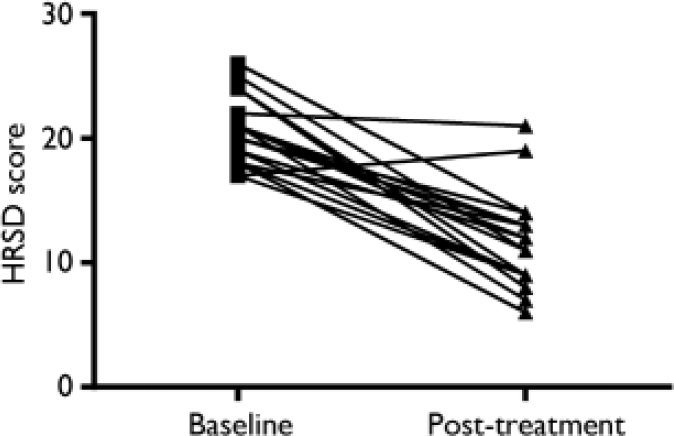
Fig. 2 Hamilton Rating Scale for Depression (HRSD) scores at baseline and following treatment.
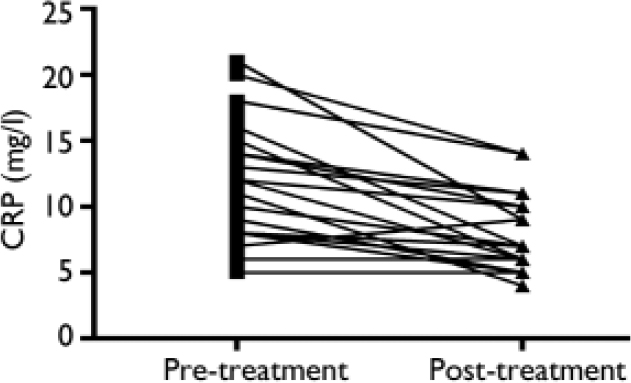
Fig. 3 Levels of C-reactive protein (CRP) before and after treatment with a selective serotonin reuptake inhibitor.
DISCUSSION
The major finding of this study is that following antidepressant treatment there is a significant drop in C-reactive protein concentrations independent of the resolution of depressive symptoms. In our first study we measured C-reactive protein in patients with major depression who were currently depressed and in patients who were euthymic. Patients in both depression groups continued taking their SSRI medication for the purpose of this study. Levels of C-reactive protein did not differ significantly among both groups and healthy controls. Previous studies have found increased levels of C-reactive protein in people with major depression compared with healthy individuals, prior to initiation of antidepressant therapy (Reference Sluzewska, Rybakowski and BosmansSluzewska et al, 1996; Reference Berk, Wadee and KuschkeBerk et al, 1997; Reference Tuglu, Kara and CaliyurtTuglu et al, 2003). Our study is the first to compare euthymic and non-euthymic patients both currently receiving treatment.
Implications
The findings suggest that the inflammatory response seen in depression may not have a role in the pathophysiology of depression since it is possible to decrease the inflammatory response without any improvement in the clinical state. However, the inflammatory response may have significant implications for peripheral disorders such as coronary heart disease.
Elevation of C-reactive protein
To further clarify the issue we conducted a study using a within-subject design following the same group of patients with major depression before and after antidepressant treatment. Pre-treatment C-reactive protein concentrations were higher than those seen following treatment. We did not have a healthy control group for this phase, so it is not possible to say with certainty that the baseline levels were elevated in comparison with normal; however, it seems likely that this was the case. In an earlier study Sluzewska et al (Reference Sluzewska, Rybakowski and Bosmans1996) measured C-reactive protein in 49 in-patients with major depression, all of whom had been drug-free for at least 10 days. A statistically significant difference in protein concentration was found in the patients. Berk et al (Reference Berk, Wadee and Kuschke1997) also measured C-reactive protein prior to initiation of treatment in patients with major depression, and found it to be elevated in the patients relative to healthy participants. In that study increased levels of C-reactive protein were found to correlate with IL-6 concentrations, consistent with the hypothesis that major depression is associated with activation of the immune response. However, in our study 2, when C—reactive protein was measured following 3 weeks of antidepressant treatment, levels of this protein had significantly reduced. This finding was independent of response to antidepressant therapy. The data are in keeping with our first study, which showed that the patients with a current episode of major depression who continued on antidepressant medication had C-reactive protein levels comparable with those in healthy participants and in patients with recurrent depressive disorder who were euthymic at the time of the study.
Rothermundt et al (Reference Rothermundt, Arolt and Peters2001) also found that C-reactive protein levels following 2 weeks and 4 weeks of treatment did not differ significantly from those in healthy participants, providing further evidence for the anti-inflammatory potential of antidepressants. Hornig et al (Reference Hornig, Goodman and Kamoun1998) measured C-reactive protein concentrations in unipolar depression and bipolar affective disorder; in their study a significant reduction in the number of participants with levels greater than 6 mg/l was found following lithium monotherapy.
Heart disease and C-reactive protein
Minor elevations of C-reactive protein are predictive of cardiovascular events in people with coronary heart disease (Reference Haverkate, Thompson and PykeHaverkate et al, 1997). In a large prospective cohort study from Reykjavik involving 2459 patients and 3969 matched controls, a single measurement of C-reactive protein was studied in relation to the 20-year incidence of coronary heart disease. Patients with a concentration greater than 2 mg/l had a relative risk of coronary heart disease of 1.92 compared with patients with levels of the protein in the bottom third (Reference Danesh, Wheeler and HirschfieldDanesh et al, 2004). The precise mechanism underlying C-reactive protein levels and adverse outcomes is not clear. It has been suggested that statins, which are commonly used to lower cholesterol levels, may reduce systemic inflammation and therefore reduce C-reactive protein by decreasing levels of atherogenic lipoproteins. However, Nissen et al (Reference Nissen, Tuzcu and Schoenhagen2005) have demonstrated that the reduced rate of progression of atherosclerosis is significantly related to greater reduction in levels of atherogenic lipoproteins and independently related to reductions in C-reactive protein.
Conclusions
Selective serotonin reuptake inhibitor therapy for major depression results in a reduction in C-reactive protein levels. This occurs irrespective of clinical outcome. Our findings suggest that these drugs may have benefit as anti-inflammatory agents even in people without depression who have inflammatory conditions.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Antidepressants induce a drop in C-reactive protein levels in people with major depression whether or not they respond to treatment.
-
▪ Depression is associated with an inflammatory response.
-
▪ Selective serotonin reuptake inhibitors (SSRIs) have an anti-inflammatory effect.
LIMITATIONS
-
▪ A high-sensitivity C-reactive protein assay would have yielded more accurate results.
-
▪ Participants were treated with different SSRIs, which makes conclusions about individual compounds tentative.
-
▪ The study does not indicate whether the anti-inflammatory effect of SSRIs is direct or indirect, for example by acting through cortisol.
Acknowledgements
T.GD. is in receipt of funds from the Science Foundation of Ireland through the Alimentary Pharmabiotic Centre, the Health Research Board of Ireland and the Wellcome Trust.






eLetters
No eLetters have been published for this article.