Introduction
Apolipoprotein E (APOE) is a glycoprotein involved in the transport and redistribution of lipids in the intravascular and extravascular compartments in the body (Li et al., Reference Li1988). Three common alleles of APOE differ by single amino acid variations encoding isoforms ϵ2, ϵ3, and ϵ4. Racial/ethnic differences have been reported in studies regarding the distribution of APOE (Schipper, Reference Schipper2011; Zannis et al., Reference Zannis, Kardassis and Zanni1993), especially in Africa where the greatest genetic diversity is found (Corbo and Scacchi, Reference Corbo and Scacchi1999).
The role of APOE in the occurrence of cognitive impairment and/or dementia has been extensively evaluated. To date, APOE is the strongest genetic factor recognized in the onset of cognitive decline and late-onset Alzheimer’s disease (AD), particularly in Caucasian populations (Lipnicki et al., Reference Lipnicki2017; Seripa et al., Reference Seripa2009; Wisdom et al., Reference Wisdom, Callahan and Hawkins2011). However, it showed a weaker strength in African populations (Chen et al., Reference Chen2010; Guerchet et al., Reference Guerchet2009; Gureje et al., Reference Gureje2006; Hendrie et al., Reference Hendrie2014; Reitz et al., Reference Reitz2013).
Neuropsychiatric symptoms are strongly associated with cognitive disorders among older people (Prince et al., Reference Prince2015). They have a multifactorial origin involving environmental factors, neurochemical abnormalities, psychiatric history (including premorbid personality), social history (e.g. intellectual achievement and lifelong learning), family history, and genetic susceptibility.
Genetic determinants of neuropsychiatric symptoms have been identified in family studies based on the strong association between dementia and neuropsychiatric symptoms among older adults. It was, therefore, assumed that alleles/genes which increase the risk of cognitive disorders could also be contributing to the occurrence of neuropsychiatric symptoms (Burke et al., Reference Burke2016; Holmes et al., Reference Holmes1996).
Several studies have thus hypothesized that the ϵ4 allele of the APOE gene (APOE ϵ4) may also be associated with the presence of neuropsychiatric symptoms, especially among people with dementia (Borroni et al., Reference Borroni, Costanzi and Padovani2010; DeMichele-Sweet and Sweet, Reference DeMichele-Sweet and Sweet2010). An underlying hypothesis is that the role of APOE ϵ4 status on the relationship between cardiovascular risk biomarkers and systemic inflammation can explain its association with neuropsychiatric symptoms (Treiber et al., Reference Treiber2008). However, studies investigating the association between APOE ϵ4 and neuropsychiatric symptoms have produced inconsistent results (Liu et al., Reference Liu2002; Lyketos et al., Reference Lyketos1997; Panza et al., Reference Panza2012; Pritchard et al., Reference Pritchard2007; Ramachandran et al., Reference Ramachandran1996; Scarmeas et al., Reference Scarmeas2002; Seignourel et al., Reference Seignourel2008; Zdanys et al., Reference Zdanys2007); with studies supporting associations with various neuropsychiatric symptoms while others found no evidence. According to a study by Pink et al., a significant association was found between APOE ϵ4 and depression (joint effect HR = 2.2; 95% CI: 1.2–3.9) as well as between APOE and apathy (joint effect HR = 1.9; 95% CI: 0.9–3.9) (Pink et al., Reference Pink2015).
The association of APOE and neuropsychiatric symptoms have been mostly studied in people with dementia. However, neuropsychiatric symptoms are also reported in older people without cognitive impairment or those with Mild Cognitive Impairment (MCI)) (Baiyewu et al., Reference Baiyewu2012; Paddick et al., Reference Paddick2015; Yoro-Zohoun et al., Reference Yoro-Zohoun2019).
In sub-Saharan Africa (SSA), no studies have focused on the association of APOE and neuropsychiatric symptoms despite their high prevalence in sub-Saharan countries, especially in Central Africa: 63.7% (95% CI: 59.5–67.8) in the Central African Republic (CAR) and Republic of Congo (ROC), regardless of cognitive status (Yoro-Zohoun et al., 2018).
Considering the high frequency of APOE ϵ4 reported in SSA compared to European populations (Tishkoff et al., Reference Tishkoff2009) and the lack of studies on its association with neuropsychiatric symptoms, the purpose of this study was to evaluate the association between the APOE ϵ4 allele and neuropsychiatric symptoms among older people living in two countries of Central Africa (CAR and ROC).
Methods
Participants
Participants aged 65 and above were recruited from the EPIDEMCA program. This study included the participants assessed for both neuropsychiatric symptoms and APOE genotype.
Design
The EPIDEMCA program is a cross-sectional multicenter study conducted in CAR and ROC from November 2011 to December 2012, as described elsewhere (Guerchet et al., Reference Guerchet2014). Four sites were included as one rural and one urban area in each country: the capitals of CAR (Bangui) and ROC (Brazzaville) as urban areas, and Nola in CAR and Gamboma in ROC as rural areas. A door-to-door approach was used in rural areas and a random sampling proportional to the size of each main subdivision of the city was performed in urban sites. The sample size for the dementia prevalence survey was estimated at around 500 participants per site, based on a 5% expected prevalence of dementia and 2% precision.
Participants were evaluated through a two-stage design. In the first stage, sociodemographic, vascular, and lifestyle factors were collected through a standardized structured questionnaire before performing a physical examination. Blood samples were taken at this stage from all consenting participants.
Participants were cognitively screened in the first phase and the cognitive diagnosis was established in the second stage by a neurologist. During this second stage, neuropsychiatric symptoms were evaluated using the brief version of the Neuropsychiatric Inventory (NPI-Q) (Kaufer et al., Reference Kaufer2000).
All assessments were performed in local languages (“Sango” in CAR,” Lari”, “Kituba”, and “Lingala” in ROC). To ensure conceptual equivalence from French to local languages, a process of translation and back translation (following WHO guidelines) was achieved by a group of independent linguistic professionals followed by a consensus between the clinicians and researchers in the study.
Measurements
APOE genotypes
At the participants’ homes, blood samples (20 ml) were collected in two polypropylene EDTA tubes by dedicated nurses. Within hours of blood sampling, the samples were centrifuged, aliquoted, and frozen at −20 °C or −80 °C in a local laboratory to prevent degradation due to high temperatures. Samples were then stored at the Pasteur Institute of Bangui (urban CAR) and the National Laboratory of Public Health in Brazzaville (urban ROC) before dry ice shipping to the University of Limoges, where the biobank is located. Genomic DNA was extracted from white blood cells using standard procedures in the Pasteur Institute (Lille, France). APOE genotyping was performed by the polymerase chain reaction (PCR) as previously described by Lambert et al. (Reference Lambert1998).
Neuropsychiatric assessment
The brief version of the Neuropsychiatric Inventory (NPI-Q) was developed to assess 12 neuropsychiatric symptoms among older people: delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep and nighttime behavioral disorders, and appetite and eating disorders (Kaufer et al., Reference Kaufer2000). It is based on an informant interview and assesses neuropsychiatric symptoms over the last 30 days. It measures the severity and distress rating for each of the 12 symptoms. Its original version, the Neuropsychiatric Inventory (NPI), is mainly used to assess Behavioral and Psychiatric Symptoms of Dementia (Van der Linde et al., Reference Van der Linde2013). It has already been used and validated in African populations (Baiyewu et al., Reference Baiyewu2003; Paddick et al., Reference Paddick2015). The French version of the NPI-Q was used in this study and was translated into local languages relevant to each country (“Sango” in CAR, “Lari” “Kituba”, and “Lingala” in ROC) (Yoro-Zohoun et al., 2018). As for the other assessments, the NPI-Q was back translated from local languages to French in order to provide a high level of translation accuracy. Furthermore, to ensure an accurate description of the symptoms, the NPI-Q was performed by trained clinicians with experience in neurological and psychiatric assessments.
Cognitive diagnosis
Cognitive screening of older participants was performed during the first stage of the study using the Community Screening Interview for Dementia (CSI-D) (Hall et al., Reference Hall1993) and mental state was evaluated through the Geriatric Mental State (GMS) (Copeland et al., Reference Copeland, Dewey and Griffiths-Jones1986). Participants with a low cognitive score at the CSI-D (≤24.5) were subsequently invited to a neurological assessment.
During the second phase, participants underwent other psychometric tests such as the Free and Cued Selective Reminding Test (Grober et al., Reference Grober1988), Zazzo’s cancelation task (Zazzo, Reference Zazzo1974), and Isaac’s Set Test of verbal fluency (Isaacs and Kennie, Reference Isaacs and Kennie1973).
Cognitive diagnosis was performed following the DSM-IV-TR criteria for dementia (American Psychiatric Association, 2000), Petersen’s criteria for MCI (Petersen, Reference Petersen2004), and clinical criteria proposed by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) for AD (Lyketsos et al., Reference Lyketsos2000). The Clinical Dementia Rating (CDR) scale was used to rate dementia severity (Morris, Reference Morris1993). Medical history, test performances, and clinical features were used to differentiate dementia subtypes.
Covariates
Sociodemographic variables collected included age, sex, education level, and marital status. Education level was dichotomized as “no formal education” and “some formal education (i.e. attended primary school at least)” while marital status was also dichotomized into “married/ living with a partner” and “not married” (comprising of single, divorced, and widowed participants).
Vascular and lifestyle factors investigated were smoking status (current smoker or not), frequency of alcohol consumption (“none”, “sometimes” or “regularly”), physical activity (yes/no), hypertension (yes/no), and diabetes (yes/no). Regular consumption of alcohol was defined by a consumption of alcoholic beverages at least 5 times a week while older people consuming on a less regular basis (but not abstinent) were entered into the “sometimes” category. Participants were considered to be physically active when they reported doing at least 150 min of walking/cycling in the past week.
Participants were considered to have hypertension when they reported taking an antihypertensive drug and/or when systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg during the physical assessment (World Health Organization, 2013). Similarly, participants were considered diabetic when they reported antidiabetic treatment and/or their glycemia ≥126 mg/dl if the fasting period > 2 hours or ≥200 mg/dl in non-fasting participants (World Health Organization, 2006).
Psychological and psychosocial factors were also assessed. The presence of dependent personality disorder was evaluated by the Personality Diagnostic Questionnaire-4+ (Hyler, Reference Hyler1994), the number of stressful life events recorded according to Persson and Skoog’s questionnaire (Persson and Skoog, Reference Persson and Skoog1996) and the presence of psychoactive drug abuse recorded through the GMS assessment (Copeland et al., Reference Copeland, Dewey and Griffiths-Jones1986). Participants were considered as having a psychoactive drug abuse if they reported taking at least one drug listed in the GMS (opium, heroin, morphine-like analgesics, cocaine, hallucinogens, cannabis, other psychostimulants, e.g. amphetamines, barbiturates, other hypnotics, and sedatives).
Statistical analysis
Data were computerized with Epidata (version 3.1) and statistical analysis was conducted with Stata Software version 14 for Windows (StataCorp LP, College Station, Texas).
Qualitative variables were described using numbers and percentages and compared using χ2 or Fisher’s exact tests depending on theoretical numbers. Quantitative variables were summarized into means (and their standard deviation) or medians (and their interquartile range) and compared using analysis of variance or Kruskal–Wallis tests depending on their distribution.
The analysis was performed with APOE ϵ4 allele in three categories: no ϵ4/heterozygous ϵ4 (one ϵ4)/homozygous ϵ4 (two ϵ4). We considered that participants had at least 1 neuropsychiatric symptom when they answered yes to 1 of the 12 symptoms in the NPI-Q during all the analysis.
Neuropsychiatric symptoms were grouped into categories. Participants were considered to have at least one effective symptom when they reported depression, anxiety, euphoria, or apathy in the NPI. In the same way, participants were considered to have at least one psychosis symptom when they reported hallucinations or delusions, and to have hyperactivity symptoms when they reported at least one symptom such as agitation, irritability, disinhibition, and aberrant motor behavior. Participants who reported at least one symptom of sleep, nighttime behavior disorders, or appetite and eating disorders were considered to have other behavioral symptoms.
We explored the association between the APOE ϵ4 allele and neuropsychiatric symptoms using logistic regression models. Five models were designed, the first one assessing the unadjusted association between APOE ϵ4 and neuropsychiatric symptoms, and the four following models with stepwise adjustments to potential confounders. These models were fitted using neuropsychiatric symptoms (individual or groups) as the dependent variable and age, sex, education level, marital status, cognitive status (categorized as no MCI nor dementia, MCI, and dementia), smoking status, alcohol consumption, hypertension, diabetes, physical activity, dependent personality disorder, number of stressful life events, and psychoactive drug abuse as independent variables. Confounders and interactions between independent variables were examined in all the analyses. Odds Ratios (OR) and 95% confidence intervals (CI) were calculated. The threshold of significance for the p-value was 5%.
Ethics
All the participants and/or their families were informed and gave their consent before their inclusion in the study. The study was approved by the Ministry of Public Health in CAR, the “CERSSA (Comité d’Ethique de la Recherche en Sciences de Santé)” in ROC and the “Comité de Protection des Personnes du Sud-Ouest et d’Outre-Mer 4 (CPP-SOOM4)” in France.
Results
Study sample
As illustrated in Figure 1, among the 2002 participants of EPIDEMCA study, 775 had CSI-D ≤ 24.5 and were invited to the second phase. Of those, 532 participants were assessed with the NPI-Q and were eligible for this study. However, 432 had blood samples available of whom 110 had missing APOE genotype. Thus, the overall study sample was 322 participants.

Figure 1. Presents the flowchart of the EPIDEMCA study including the selection of the study sample.
Participants’ characteristics
As presented in Table 1, 81.1% of the participants were female. Median age was 75.0 [IQR: 69.0–80.0]. Most of them had no formal education (90.6%) and the majority was not married or not living with a partner (74.9%). Slightly more than half of the participants had neither MCI nor dementia after the neurological examination (182 participants, i.e. 57.1%), 21.6% (n = 69) had MCI and 21.3% (n = 68) had dementia. AD was the most common subtype with 52 participants (16.1%) followed by vascular dementia with 7 participants (2.2%). Three participants remained without a cognitive diagnosis.
Table 1. Participants’ characteristics according to the site, EPIDEMCA, 2011–2012

Abbreviations: IQ, interquartile range; SD, standard deviation; CAR, Central African Republic; ROC, Republic of Congo; n, number.
Regarding the overall APOE distribution (Table 1), at least 1 APOE ϵ3 allele was identified in the majority of participants (277 participants, 86.0%) followed by at least 1 APOE ϵ4 allele (135 participants, 41.9%) and at least one APOE ϵ2 allele (79 participants, 24.5%). Among the 135 (41.9%) APOE ϵ4 carriers, 18 (5.6%) were ϵ4 homozygous and 117 (36.3%) were ϵ4 heterozygous (99 ϵ3/ϵ4 and 18 ϵ2/ϵ4).
Demographic characteristics (age, sex, marital status, education level, and cognitive status) of included (n = 322) and excluded (n = 210) participants were statistically comparable (Table 2).
Table 2. Sociodemographic characteristics of 532 eligible participants of the second stage of EPIDEMCA survey with (included) or without APOE genotyping (excluded), EPIDEMCA, 2011–2012
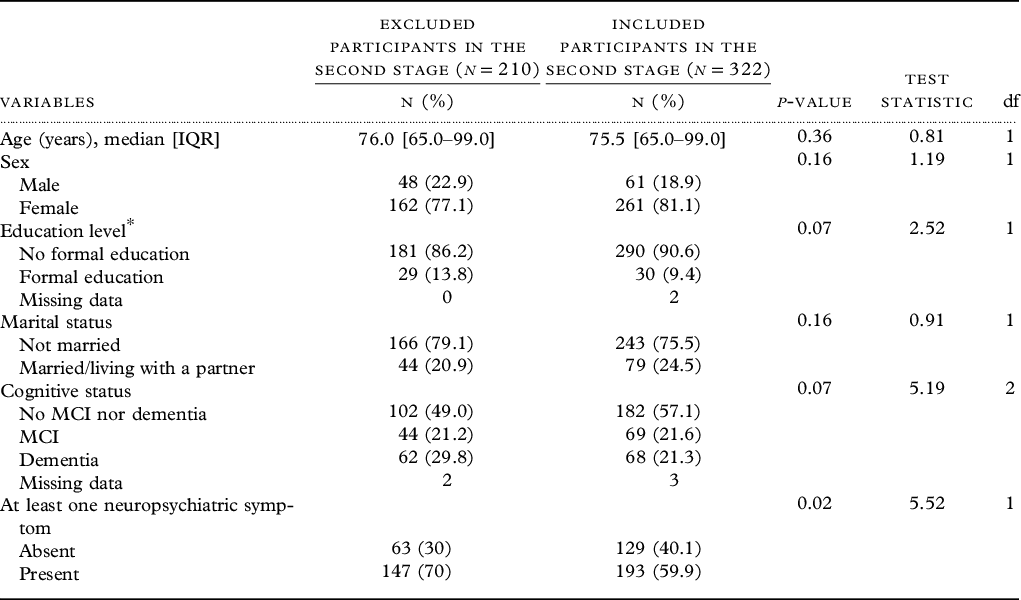
Abbreviation: IQR, interquartile range.
Education level*: missing values = two among the included participants.
APOE ϵ4 and neuropsychiatric symptoms
Overall, 192 older adults reported at least 1 neuropsychiatric symptom, representing a prevalence of neuropsychiatric symptoms of 59.9% (95% CI: 54.4–65.2). Among them, 61.4% were had no ϵ4 allele, 34.4% were heterozygous ϵ4 carriers, and 4.2% were homozygous ϵ4 carriers.
Neuropsychiatric symptoms were distributed as follows: depression 39.4% (95% CI: 34.5–45.3), anxiety 26.7% (95% CI: 22.1–31.9), irritability 22.7% (95% CI: 18.1–27.4), sleep and nighttime behavioral disorders 16.8% (95% CI: 12.7–20.9), apathy 11.5% (95% CI: 8.0–15.0), appetite and eating disorders 10.2% (95% CI: 6.9–13.5), delusions 9.9% (95% CI: 6.7–13.4), hallucinations 9.6% (95% CI: 6.4–12.9), agitation 7.1% (95% CI: 4.4–10.1), disinhibition 5.3% (95% CI: 2.8–7.8), aberrant motor behavior 4.7% (95% CI: 2.3–7.0), and euphoria 2.8% (95% CI: 0.9–4.6).
According to neuropsychiatric symptom groups, affective symptoms were more frequently reported with a prevalence of 47.0% (95% CI: 41.5–52.5), followed by hyperactivity symptoms (27.7%, 95% CI: 22.5–32.3), and other behavioral symptoms (23.3%, 95% CI: 18.6–27.9). Psychosis symptoms were the less reported symptoms: 15.0% (95% CI: 11.1–18.9).
Apart from delusions, the distribution of each neuropsychiatric symptoms did not significantly vary with APOE ϵ4 status, as shown in Table 3. Distribution of groups of neuropsychiatric symptoms according to APOE ϵ4 status is presented in Supplementary Table 1. Similarly, the number of neuropsychiatric symptoms did not significantly vary with the presence of the APOE ϵ4 allele (p = 0.56) (see Supplementary Table 2).
Table 3. Distribution of each neuropsychiatric symptoms according to APOE ϵ4 status, EPIDEMCA, 2011–2012

Neuropsychiatric symptoms and APOE ϵ4 allele were not significantly associated in the unadjusted model (heterozygous OR: 0.7, 95% CI: 0.5–1.2, homozygous OR: 0.5, 95% CI: 0.2–1.4). However, a nonsignificant trend toward a risk reduction for homozygous APOE ϵ4 allele carriers was detected after adjustment, except in model 3, where it was significant (OR: 0.3, 95% CI: 0.1–0.9) (Table 4).
Table 4. Association between neuropsychiatric symptoms and APOE ϵ4 genotype, EPIDEMCA, 2011–2012
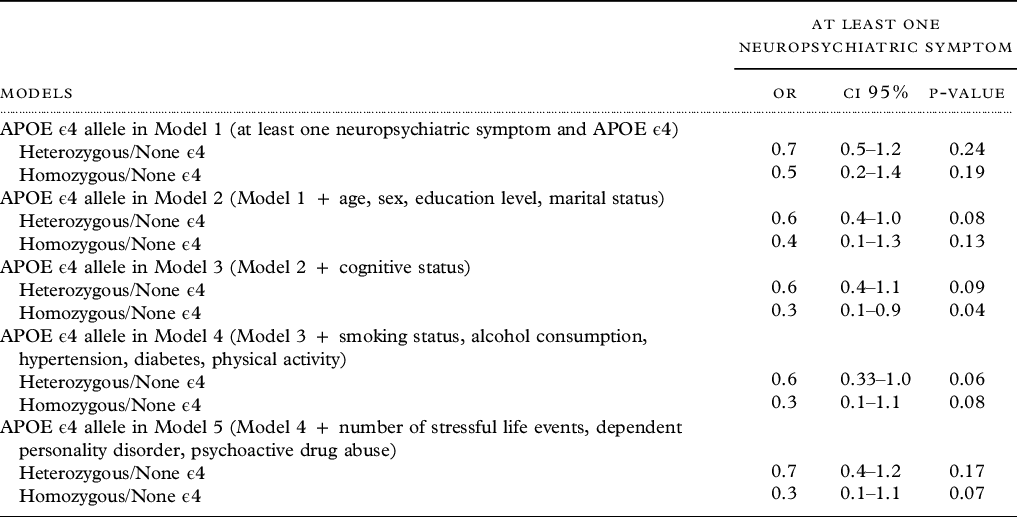
Overall, none of the symptom groups were significantly associated with APOE ϵ4 in the unadjusted and adjusted models. A trend toward an increased risk for homozygous APOE ϵ4 allele carriers among participants with psychosis symptoms was detected after adjustment, especially in model 2 where it reached significance (OR: 3.3, 95% CI: 1.0–10.7). Detailed results of the associations between APOE ϵ4 and affective, hyperactivity, psychosis, and other behavioral symptoms are reported in Supplementary Tables 3, 4, 5, and 6.
Discussion
Main results
In this study, we aimed at evaluating the association between neuropsychiatric symptoms and the APOE ϵ4 allele in older populations in CAR and ROC. APOE ϵ4 was the second most frequent allele after APOE ϵ3 and was identified in more than 40% of the sample. Among participants with at least one neuropsychiatric symptom, more than half had no ϵ4 allele and very few were homozygous ϵ4 carriers. The distribution of each neuropsychiatric symptom did not significantly vary with APOE ϵ4 status in our sample. Overall, only one model showed a significant association between at least one neuropsychiatric symptom and APOE ϵ4 allele, the other ones showing a trend toward a protective effect of ϵ4 in homozygous carriers.
Comparison with other studies
The frequency of APOE polymorphism in humans varies considerably from one population to another (Schipper, Reference Schipper2011; Zannis et al., Reference Zannis, Kardassis and Zanni1993). However, it appears that the APOE ϵ3 allele is often the most frequent one followed by ϵ4 and then ϵ2, as also reported in this study (Corbo and Scacchi, Reference Corbo and Scacchi1999; Eisenberg et al., Reference Eisenberg, Kuzawa and Hayes2010; Schipper, Reference Schipper2011; Zannis et al., Reference Zannis, Kardassis and Zanni1993; Zekraoui et al., Reference Zekraoui1997).
A trend toward a reduction of neuropsychiatric symptoms for APOE ϵ4 allele carriers was emerging in our study. This result differs from those reported in a large cross-sectional study of AD patients in the U.S.A. (Zdanys et al., Reference Zdanys2007) where no significant association was found between any neuropsychiatric symptom and APOE ϵ4 allele. However, participants included were only of the AD subtype and the sample size was larger than ours (226 with AD compared to 68 with dementia in our study). Due to those methodological differences, comparisons between both studies are limited.
Very limited evidence is available at the moment on this specific association between neuropsychiatric symptoms considered together and APOE ϵ4 allele, which restricts comparisons to other studies. Many studies focused only on specific neuropsychiatric symptoms among older people (Panza et al., Reference Panza2012). A protective effect of the ϵ4 allele against specific neuropsychiatric symptoms (delusion, hallucination, anxiety, depression, apathy) or groups of symptoms (affective, psychotic) was reported in various studies although many others have not confirmed it (Panza et al., Reference Panza2012).
The APOE ϵ4 allele might increase the risk of depression or apathy for people with AD. Additionally, the APOE ϵ4 allele was not associated with anxiety alone in patients with AD but could be associated with both anxiety and depression. Similar findings were found regarding psychotic symptoms: delusions and/or hallucinations were sometimes associated with APOE ϵ4 (Panza et al., Reference Panza2012). A trend toward an association between APOE ϵ4 and specific neuropsychiatric symptoms was also observed in fitted models only (Zdanys et al., Reference Zdanys2007). Indeed, Zdanys et al. reported that the presence of ϵ4 was significantly associated with psychotic symptoms adjusting for age, sex, education, and Mini-Mental State Examination score. The association between APOE ϵ4 and neuropsychiatric symptoms could be more complex than one might think, especially due to a high genetic variability observed in Africa regarding APOE distribution (Corbo and Scacchi, Reference Corbo and Scacchi1999). Indeed, findings on the association between AD and APOE ϵ4 are more heterogeneous in SSA (Chen et al., Reference Chen2010; Gureje et al., Reference Gureje2006; Hendrie et al., Reference Hendrie2014; Reitz et al., Reference Reitz2013) with no significant or weaker effect, including in the EPIDEMCA study (Guerchet et al., personal data).
The lack of association between APOE ϵ4 and neuropsychiatric symptoms in this study could be also explained by the no-significant association between dementia and APOE ϵ4 in the EPIDEMCA study (Guerchet et al., personal data), suggesting that this might be the same for the association between neuropsychiatric symptoms and APOE ϵ4.
Furthermore, the data suggest the potential existence of one or more additional genetic susceptibility factors in the APOE sequence, modifying the risk associated with the APOE ϵ4 allele in the occurrence of AD (Lambert et al., Reference Lambert1998). We can hypothesize that the same is likely to be relevant for the APOE association with neuropsychiatric symptoms. This could partly explain that a significant protective effect of APOE ϵ4 only appeared under some conditions in our study. We can also hypothesize that our results could be due to chance, particularly due to low statistical power. This might explain the mostly nonsignificant results in the study. The protective effect of homozygous APOE ϵ4 allele carriers on neuropsychiatric symptoms in our study could also reflect the distribution of APOE ϵ4 allele among participants with at least one neuropsychiatric symptom as homozygous ϵ4 carriers were less represented among older people with at least one neuropsychiatric symptom. Many studies have specifically focused on the association between APOE genotypes (rather than allele distribution) and specific neuropsychiatric symptoms as reported in a review (Panza et al., Reference Panza2012). However, unlike our results, homozygous ϵ4 participants have often been identified as having a greater risk of developing a neuropsychiatric symptom than homozygous ϵ3 or heterozygous ϵ4 carriers (Michels et al., Reference Michels2012; Panza et al., Reference Panza2012). Indeed, APOE ϵ4 homozygous carriers might have a greater amyloid burden than heterozygous carriers, which would increase metabolic differences and impact on the occurrence of noncognitive symptoms among people with dementia as proposed by Levy et al. (Reference Levy1999).
The role of APOE in the occurrence of neuropsychiatric symptoms remains unclear due to the inconsistency of study results. Inconsistent evidence and variations in results between studies might reflect differences in methodology (design, population, sample sizes, diagnostic criteria, statistical methods) (Panza et al., Reference Panza2012). In essence, studies performed to identify the association between APOE and neuropsychiatric symptoms were mainly cross-sectional and only a few were longitudinal (Panza et al., Reference Panza2012; Pritchard et al., Reference Pritchard2007; Scarmeas et al., Reference Scarmeas2002). Also, the majority of studies focused on AD populations rather than cognitive disorders or dementia (Panza et al., Reference Panza2012; Persson and Skoog Reference Persson and Skoog1996; Zdanys et al., Reference Zdanys2007). Furthermore, data were mostly available from high-income countries (Panza et al., Reference Panza2012). Our study expanded to a population including participants with dementia, participants with MCI, and participants without dementia nor MCI although our number of participants with dementia was small compared with other studies (Panza et al., Reference Panza2012). The definition of neuropsychiatric symptoms varies from study to study. While we considered the presence of 1 of the 12 symptoms from the NPI-Q, most other studies used the score (greater than or equal to 1) of each symptom to define neuropsychiatric symptoms (Zdanys et al., Reference Zdanys2007). Moreover, other instruments have been used to evaluate individual neuropsychiatric symptoms (Scarmeas et al., Reference Scarmeas2002). Depression assessed as an illness and not as a symptom, and the challenges of diagnosing anxiety or differentiate it from depression among people with dementia could also be major limitations to the investigation of the association between depression, anxiety, and APOE (Seignourel et al., Reference Seignourel2008).
It might be possible that the APOE ϵ4 allele acts in a different and specific way on each neuropsychiatric symptom assessed alone rather than together, which could also explain the lack of significance of our results. Moreover, as demonstrated for depression, environmental and genetic differences between populations could influence the strength of association between this symptom and APOE ϵ4 (Jeste et al., Reference Jeste, Depp and Vahia2010). Unfortunately, due to the lack of statistical power, we were not able to investigate the association between the APOE ϵ4 allele and each neuropsychiatric symptom and are therefore not able to confirm this hypothesis. Additionally, we were unable to explore the influence of the level of severity or subtype of dementia (Monastero et al., Reference Monastero2006) or assess the role of sex for this association, as suggested in some studies (Michels et al., Reference Michels2012; Müller-Thomsen et al., Reference Müller-Thomsen2002). In the literature, the appearance of some specific neuropsychiatric symptoms could vary by sex. For example, depression might be associated with APOE ϵ4 in women but not in men (Müller-Thomsen et al., Reference Müller-Thomsen2002). However, the mechanisms remain unclear. Based on such reported differences, we can imagine that the APOE ϵ4 allele acts in a different and specific way on each neuropsychiatric symptom according to sex. Therefore, our results might be more generalizable to female populations rather than males.
Strengths/limitations
This study is the first to assess the association between APOE ϵ4 and neuropsychiatric symptoms among older people in SSA. Unlike many existing studies on this specific association, it was a population-based study. Its strengths also include a high-quality methodology regarding the assessment of both main measures: APOE genotypes and neuropsychiatric symptoms. Indeed, the NPI is reported among the most widely used instruments (Van der Linde et al., Reference Van der Linde2013) to assess behavioral and psychological symptoms of dementia. Although not specifically validated in the context of this study, the NPI-Q was previously used in several studies conducted in SSA (Baiyewu et al., Reference Baiyewu2003; Paddick et al., Reference Paddick2015). In addition to a rigorous translation process, we aimed at limiting possible issues related to the meaning of symptoms by working with experienced clinicians in the field (neurological and psychiatric assessments) and interviewers fluent in the relevant languages. All the symptoms were described in simple words and using examples to illustrate when necessary.
However, we must also acknowledge some limitations. Our findings were mostly nonsignificant, which probably reflects a lack of statistical power to either confirm or refute the link between the APOE ϵ4 and neuropsychiatric symptoms. This is likely due to the small sample size in our study overall as the sample was derived from participants with low CSI-D cognitive scores and with available APOE genotypes (40% of the EPIDEMCA sample) (Guerchet et al., personal data). The generalizability of our finding is therefore also affected, and results might not be extended to any older population from those countries or SSA countries.
The specificity of the study population included (i.e. with low cognitive score) could also affect the findings and limit their interpretation. Indeed, the prevalence of neuropsychiatric symptoms in this population might have been overestimated and the strength of the association between APOE ϵ4 and neuropsychiatric symptoms also affected. Finally, no statistical correction method was used on our multiple analyses, thus limiting the interpretation of the results and potentially leading to the overestimation of the effect sizes.
In conclusion, the association between neuropsychiatric symptoms and APOE ϵ4 allele remains unclear and seems complex. The APOE ϵ4 allele might be protective against neuropsychiatric symptoms among older adults in Central Africa in certain conditions. It is therefore required to perform in-depth research to better examine this relationship in populations from low- and middle-income countries and SSA.
Acknowledgments
This study was funded by the French National Agency (ANR) through the ANR-09-MNPS-009-01 grant. The authors would like to thank the staffs of Universities of Bangui (Central African Republic) and Marien Ngouabi in Brazzaville (Republic of Congo), Institut Pasteur in Bangui and Laboratoire National de Santé Publique in Brazzaville, Health ministries of the Central African Republic and the Republic of Congo, University of Limoges, Doctoral School of Limoges University, and Limousin Regional Council. They also thank all the investigators and the participants of this survey.
Conflict of interest
None.
Description of authors’ roles
I. Yoro-Zohoun conducted data analysis and wrote the first draft. M. Guerchet, P-M Preux were involved in data analysis and interpretation. D. Houinato, P. Nubukpo, J-P Clément, have participated in critical revision of the manuscript for important intellectual content. B. Ndamba-Bandzouzi, P. Mbelesso, and M. Guerchet supervised the data collection. J-F. Dartigues, B. Ndamba-Bandzouzi, and P. Mbelesso were responsible for diagnosing cognitive disorders. J-C Lambert supervised the genotyping. All authors reviewed the manuscript, provided further contributions and suggestions, and approved the final manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1041610220003993.







