A previously healthy 49-year-old male presented to the hospital with a 5-week history of progressive sensorimotor deficits, with a preceding history of Coronavirus Disease-19 (COVID-19) infection four weeks prior. His strength was grade 4+/5 in the upper extremities and 4−/5 in the lower extremities, with brisk reflexes throughout and mute plantars. He had severe sensory ataxia and a sensory level at T5. Magnetic resonance imaging (MRI) of the brain and spinal cord, both with and without contrast, were unremarkable; however, somatosensory-evoked potentials showed dorsal column dysfunction at the thoracic spinal cord. His clinical presentation was in keeping with imaging-negative myelopathy. Cerebrospinal fluid analysis was bland, and electromyography/nerve conduction studies were normal. Extensive investigations for infectious (zoster, Zoster, Ebstein-Barr, herpes simplex, cytomegalovirus, adenovirus, enterovirus, Coxsackie B virus, herpes virus 6, HIV, HTLV 1 and 2, Cryptococcus, Aspergillus), autoimmune (Systemic Lupus erythematosus, Sjogren’s, Behcet’s syndrome), malignant and paraneoplastic (anti-Hu, anti-collapsin-responsive mediator protein 5, antiamphiphysin), metabolic (B12, nitric oxide, copper, Vitamin E) genetic (adrenomyeloneuropathy, leukodystrophy, Friedreich’s ataxia), and neurodegenerative causes (Amyotrophic lateral sclerosis and primary lateral sclerosis) were unremarkable.
He was treated for clinically suspected imaging-negative myelitis with intravenous methylprednisolone (IVMP), but symptoms progressed to non-ambulation. He received a second pulse IVMP followed by oral prednisone and intravenous immunoglobulin (IVIG), which transiently improved his sensorimotor symptoms, and ultimately underwent plasmapheresis. MRI was repeated and revealed linear, increased T2 signal in the posterior limb of the internal capsule and midbrain (Figure 1) and subtle symmetric longitudinally extensive increased T2 signal within the lateral columns extending from the craniocervical junction to the conus (Figure 2).
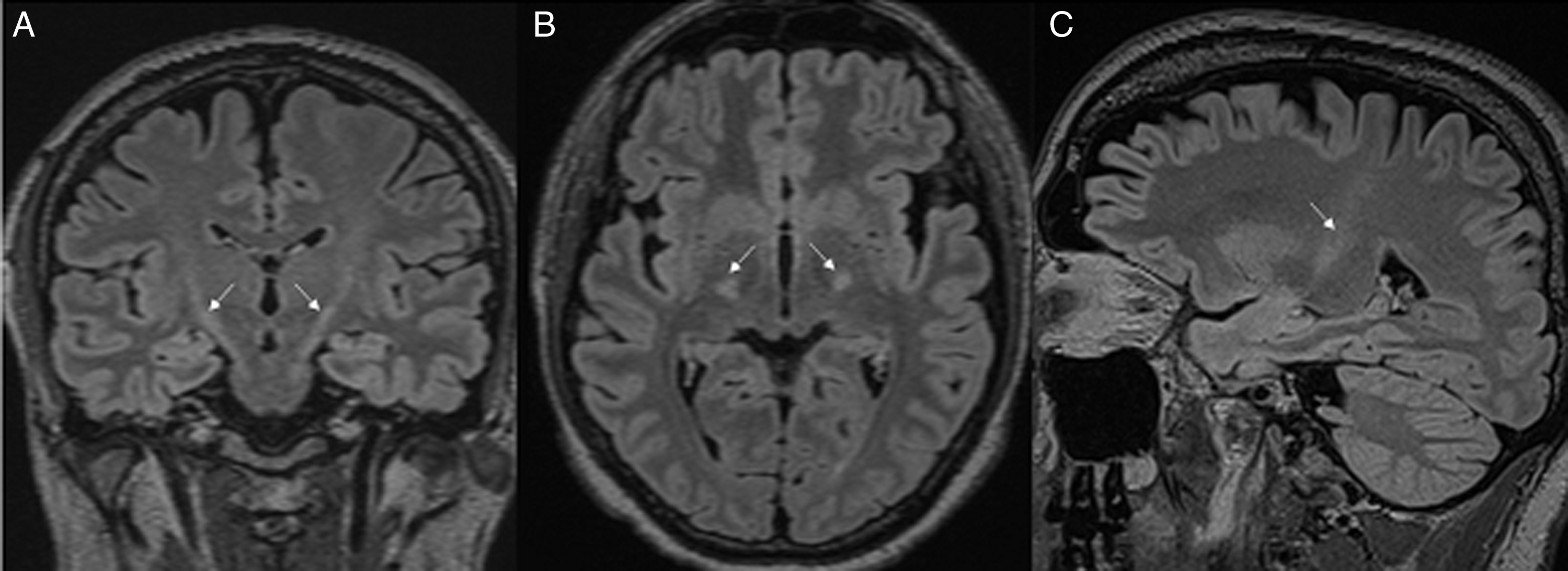
Figure 1: T2 FLAIR hyperintensities in the posterior limb of the internal capsule and midbrain (A – coronal view, B – axial view, C – sagittal view).
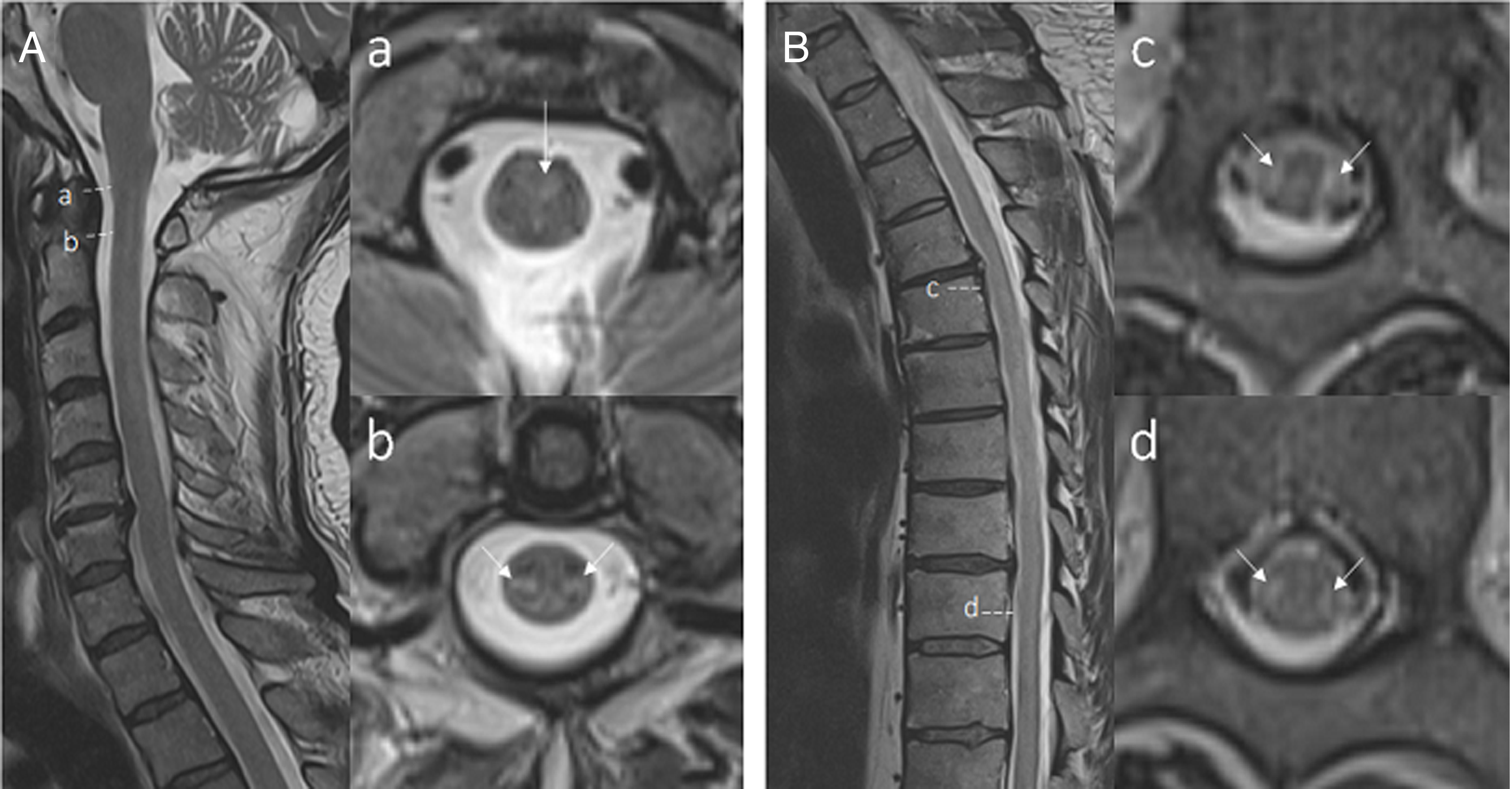
Figure 2: T2 hyperintensities from craniocervical junction to the conus (A – sagittal C-spine view. a/b – axial C-spine view, B – sagittal T and L-spine view, c – axial T-Spine view, d – axial L-spine view).
His symptoms stabilized after 5 sessions of plasmapheresis and another infusion of maintenance IVIG. He was transferred to a rehabilitation center, and at a 4-month follow-up, he was able to ambulate with a 4-wheel-walker. Repeat MRI showed improvement of the T2 signal changes involving the dorsal and lateral spinal columns.
Although COVID-19 is primarily a respiratory illness, neurological manifestations of the infection are increasingly described. Reference Whittaker, Anson and Harky1 Several viruses including COVID-19 have been associated with post-infectious myelitis, but these presentations typically show central longitudinal T2 changes without corresponding enhancement. Reference Chow, Magnussen, Ip and Su2–Reference Baghbanian and Namazi4 This case highlights a unique delayed MRI pattern of increased symmetrical T2 signal starting in the posterior limb of the internal capsule, extending to the conus, preceded by COVID-19 infection four weeks prior.
This unique neuroimaging pattern has been described in five other similar cases with good outcomes, suggesting that this may occur rarely with COVID-19 infection. Reference Huang, Shah and McNally5 Lateral and dorsal column involvement is relatively atypical in viral myelitis and has generally been described in paraneoplastic myelitis. In one studied cohort of paraneoplastic myelitis, patients almost invariably progressed despite immunotherapy with poor prognosis. Reference Flanagan, McKeon and Lennon6 These unique neuroimaging findings further reinforce the increasingly expanding spectrum of neurological manifestations of COVID-19, specifically a lateral tract longitudinal extensive myelitis, where patients may have a better prognosis compared to the similar pattern seen in lateral-column paraneoplastic myelitis. Neuroimaging findings may occur later despite significant early neurological deficits, highlighting the importance of clinical suspicion repeat imaging.
Statement of authorship
AA, RH, and TC were responsible for drafting the manuscript and the editing. AA was responsible for the submission of the final draft.
Competing interests
The authors do not report any conflict of interest, financial, or otherwise.




