LEARNING OBJECTIVES
-
Understand the varied clinical presentations of Tourette syndrome, recognising that it is not a unitary disorder, but has complex manifestations with frequent comorbidities
-
Appreciate the evolving understanding of the aetiological processes that contribute to Tourette syndrome, especially the complex gene–environment interactions
-
Acquire a working knowledge of current treatments for Tourette syndrome in a range of modalities, including promising experimental therapies
A complex neuropsychiatric disorder with onset in childhood, Tourette syndrome is characterised by multiple motor tics and one or more vocal/phonic tics, lasting longer than a year (World Health Organization, 1992; American Psychiatric Association 2013).
The impact of this disorder is significant and a plethora of studies by several groups have examined the quality of life (QoL) of young people who are affected by Tourette syndrome (see Table DS1 in the online data supplement). Despite different schedules, the results have been remarkably consistent, and also concordant with adult data, showing that people with Tourette syndrome have a reduced QoL compared with healthy individuals.
A Tourette-specific quality of life scale has now been validated (the Gilles de la Tourette Syndrome–Quality of Life scale (GTS-QOL; Reference Cavanna, Schrag and MorleyCavanna 2008) and a version for children and adolescents has been published in Italian (C&A-GTS-QOL; Reference Cavanna, Luoni and SelviniCavanna 2013). For a full discussion of QoL in people with Tourette syndrome see Reference RobertsonRobertson (2014a).
Studies have shown considerable parenting stress, caregiver burden and psychopathology in the parents of youngsters with Tourette syndrome (Reference Cooper, Robertson and LivingstonCooper 2003; Reference Lee, Chen and WangLee 2007) and this is a fruitful area for further research.
Epidemiology and prevalence
Tourette syndrome has now been described almost worldwide. Males are more commonly affected, the male/female ratio being 3 or 4 to 1. Clinical characteristics are similar irrespective of the country of origin, highlighting the biological nature of Tourette syndrome. In some instances it seems that within families, the affected males have tics whereas the females have obsessive–compulsive behaviours.
Tourette syndrome was once considered to be rare in the general population, but a comprehensive review shows that at least eight studies with similar multistaged methods documented remarkably consistent findings and suggests a global prevalence of between 0.4 and 3.8% for youngsters between the ages of 5 and 18 years, with a calculated prevalence (from raw data) of 1% worldwide, apart from Sub-Saharan Africa (Reference RobertsonRobertson 2008a,Reference Robertsonb). A more recent review of my work over 35 years, as well as that of others, suggests an updated figure of 0.85% (Reference RobertsonRobertson 2014b): in any event, the important fact is that Tourette syndrome is more common than was previously thought. The prevalence in special educational populations, such as individuals with intellectual disabilities and emotional and behavioural disorders, is much higher, and in the case of autism spectrum disorders (ASD) it is as high as 6–11% (for individual references see Reference RobertsonRobertson 2008a,Reference Robertsonb).
Aetiological theories
Aetiological suggestions for Tourette syndrome include genetic factors and environmental influences. The latter might be infections and neuroimmunological effects, pre- and/or perinatal difficulties, psychosocial stressors and/or androgen influences. The idea that the aetiology of Tourette syndrome is psychological has now been discredited.
Genetic theories
No single gene has been identified to date, and although the scientific community has been enthused by the ‘discovery’ of various genes (e.g. SLITRK1), it is likely that more than one gene is responsible. For full reviews of the genetics of Tourette syndrome see Reference O'Rourke, Scharf and YuO'Rourke et al (2009) and Reference Deng, Gao and JankovicDeng et al (2012). Recent genetic data implicate a genetic variant of HTR2C, a rare functional mutation in the HDC gene encoding L-histidine decarboxylase, and the DLGAP3 gene. Another study conducted a genome-wide linkage analysis in a large high-risk Utah pedigree examining a qualitative trait (a diagnosis of Tourette syndrome) and a quantitative phenotype based on tic severity. Two regions of interest were found: chromosome 3p for the Tourette syndrome phenotype and chromosome 1p for tic severity (Reference Knight, Coon and JohnsonKnight 2010). These results are all exciting, but emphasise the need for studies on large numbers of individuals using rare variants, sib-pair analysis, extended pedigrees or large cohorts. At least two international collaborative efforts are in place. Of importance is the fact that in the vast majority of individuals with Tourette syndrome the chromosomes are normal (Reference Robertson and TrimbleRobertson 1993).
Neuroimmunological theories
Perhaps stimulated by the fact that no gene(s) have been positively implicated in Tourette syndrome, neuroimmunological theories have been a focus of interest. These include hypotheses of (a) autoimmunity and (b) lowered immunity. These theories began when Reference Swedo, Leonard and GarveySwedo et al (1998) described a group of 50 children with obsessive–compulsive disorder (OCD) and tic disorders, designated as paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). The diagnostic criteria included: presence of OCD and/or a tic disorder, prepubertal symptom onset (usually acute and dramatic), association with group A beta-haemolytic streptococcal (GABHS) infections, episodic course of symptom severity and association with neurological abnormalities. The relapsing, remitting course was associated with significant psychopathology, including emotional lability, separation anxiety, nighttime fears, bedtime rituals, cognitive deficits, oppositional behaviours and hyperactivity. It must be emphasised that PANDAS and Tourette syndrome are not the same entity.
Recently, researchers have found laboratory evidence of GABHS infections in some patients with Tourette syndrome, and/or documented in several controlled studies that some individuals with Tourette syndrome have increased anti-basal ganglia antibodies (ABGAs). One suggestion in this context is that people with Tourette syndrome have a predisposition to autoimmune responses, as indicated by the reduced frequency of regulatory T-cells that induce tolerance towards self-antigens. Another is that there may be a general lowered immunity, as evidenced by an immunoglobulin A (IgA) dysgammaglobulinaemia. Low levels of IgA may then lead to increased risk of upper respiratory tract infections (see Reference RobertsonRobertson 2012).
Pre- and perinatal difficulties and androgen influences
Reference Leckman, Bedard, Agid and ChouinardLeckman (2003) has suggested that the mothers of children with tics are 1.5 times as likely to have experienced a complication during pregnancy than the mothers of children who did not have tics. Among monozygotic twins discordant for Tourette syndrome, the index twins with Tourette syndrome have had lower birth weight than their unaffected twins. It has also been demonstrated that the severity of maternal life stress during pregnancy, and severe nausea and/or vomiting during the first trimester, are risk factors for tic disorders in offspring. Other studies have shown that prematurity, low birth-weight, low Apgar scores and more frequent maternal prenatal visits were associated with Tourette syndrome (see Reference Leckman, Bedard, Agid and ChouinardLeckman 2003). One controlled study (Reference Burd, Severud and KlugBurd 1999) demonstrated that people who developed Tourette syndrome had had more pre- and perinatal difficulties than a control group. Maternal smoking during pregnancy has been associated with: (a) a diagnosis of Tourette syndrome (Reference Motlagh, Katsovich and ThompsonMotlagh 2010; Reference Zhang, Liu and WangZhang 2012); (b) increased tic severity (Reference Mathews, Bimson and LoweMathews 2006); and (c) an increased likelihood of comorbid attention-deficit hyperactivity disorder (ADHD) in Tourette syndrome (Reference Pringsheim, Sandor and LangPrinsgsheim 2009; Reference Cui, Zheng and YangCui 2010; Reference Motlagh, Katsovich and ThompsonMotlagh 2010). Finally, Leckman and his group have suggested that androgen exposure (‘prenatal masculinisation of the brain’) may also be important in the aetiopathogenesis of Tourette syndrome and tic-related disorders.
Thus, the aetiopathology of Tourette syndrome is much more complex than previously recognised, with complicated genetic mechanisms, some infections, pre- and perinatal difficulties, maternal smoking, life stressors and androgens affecting the phenotype (Reference Leckman, Bedard, Agid and ChouinardLeckman 2003; Reference RobertsonRobertson 2012, Reference Robertson2014a).
Clinical features
Onset and characteristic features
The age at onset of Tourette syndrome ranges from 2 to 21 years, with a mean of 7 years commonly reported. The onset of vocal tics is usually some months to years later, many studies reporting a delay of around 11 years. Tics usually begin in the head and face, and eye blinking is often the first (and one of the most common) tics. Tics can be simple (e.g. blinking, eye rolling) or complex (e.g. touching, hopping). Simple vocal tics include sniffing, throat clearing, gulping, snorting and coughing. Complex vocal tics include barking, making animal noises and uttering strings of words. Tics characteristically wax and wane, are usually preceded by premonitory sensations, diminish during goal-directed behaviour and increase with emotional excitement and fatigue.
Other important and characteristic features include echolalia (copying what others say), echopraxia (copying what others do), palipraxia (repeating one's action) and palilalia (repeating the last word or part of one's sentences). Non-obscene socially inappropriate behaviour and self-injurious behaviour are both common and difficult to treat.
Echo phenomena
Echo phenomena have long been understood to be part of Tourette syndrome, described by Gilles de la Tourette himself in 1885. In one study, echo phenomena were significantly associated with the longer duration and severity of Tourette syndrome, as well as measures of obsessionality, depression and anxiety (Reference Robertson, Trimble and LeesRobertson 1988). Echo phenomena are healthy in children up to 36 months of age, but in older children clinicians should investigate for neuropsychiatric pathology. Echo phenomena are a feature of Tourette syndrome, with patients echoing both healthy movements and tics, although, as expected, echoes were predominantly part of the tic repertoire (Reference Ganos, Ogrzal and SchnitzlerGanos 2012a).
Coprolalia and copropraxia
Coprolalia has been widely misunderstood as a pathognomonic feature. It denotes inappropriate, involuntary, out-of-context swearing, often disguised by the patient and without offensive intent. Instead of the whole swear word, many individuals say only part of the word (e.g. ‘fu fi’) and disguise it, for example, by coughing. It occurs in about 20–30% of patients of Tourette syndrome clinics and begins within 5 years of tic onset. It seldom occurs in community samples. The Tourette Syndrome Association in the USA suggests that as few as 10–15% of all people with Tourette syndrome have this feature.
Many physicians are still under the misapprehension that coprolalia must be present for the diagnosis to be made. Coprolalia is often used in the media as a symptom of Tourette syndrome, probably because its sensational effect increases viewing numbers.
It has been suggested that copropraxia (the inappropriate making of obscene gestures) occurs in about 6–18% of clinic patients (Reference Robertson, Trimble and LeesRobertson 1988; Reference Freeman, Zinner and Muller VahlFreeman 2009). Those authors have also documented that coprophenomena are associated with tic severity, repetitive behaviours, the amount of comorbid psychiatric disorders and the number of anti-tic medications taken.
Peak of severity and premonitory sensations
The peak of tic severity is around 10–12 years of age. Symptoms usually begin with transient bouts of tics, but by 10 years, most children notice nearly irresistible urges that precede the tics. These premonitory sensations appear to be in the child's conscious awareness and are likely to reflect a defect in sensorimotor gating because they intrude and become a source of distraction. Premonitory sensations are common, occur with the majority of tics and may be either localised (around the area of the tic) or generalised (covering a wide area of the body). They have been likened to the ‘urge’ or ‘tight’ sensation before a sneeze and, as with the sneeze in healthy people, they are usually relieved by the tics (Reference Kwak, Dat Vuong and JankovicKwak 2003). It has been understood that most people can suppress tics, and that this is in part due to the premonitory sensations. In a recent study, however, it was demonstrated that there was no correlation between the perceived strength of premonitory sensations and the ability to suppress tics: in other words, the urges and tic inhibition are not directly related (Reference Ganos, Kahl and SchunkeGanos 2012b).
Over the course of hours, tics occur in bouts, with a regular inter-tic interval (Reference Leckman, Bloch and ScahillLeckman 2006). It has been suggested that there may be a fractal, deterministic and possibly chaotic process in the tic time series (short-term bouts, and longer-term waxing and waning) (Reference Peterson and LeckmanPeterson 1998).
Comorbidity with Tourette syndrome
The comorbid psychiatric disorders most commonly seen in Tourette syndrome include ADHD, obsessive–compulsive behaviours, OCD and ASD. The relationships between these and Tourette syndrome are complex and are summarised in Table 1.
TABLE 1 Comorbid disorders and coexistent psychopathology in young people with Tourette syndromea
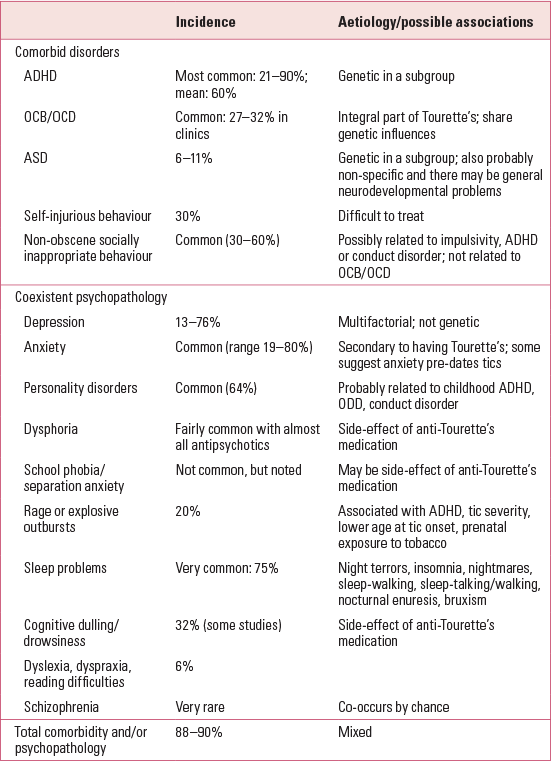
An investigation embracing 3500 clinic patients with Tourette syndrome worldwide demonstrated that at all ages, 88% of individuals had reported comorbidity/psychopathology. The most common was ADHD, followed by obsessive–compulsive behaviours and OCD. Anger control problems, sleep difficulties, coprolalia and self-injurious behaviours reached high levels only in patients with comorbid disorders. Males were more likely than females to have comorbid disorders (Reference Freeman, Fast and BurdFreeman 2000). This has also been shown to be true in community studies, with around 90% of people with Tourette syndrome having attracted other diagnoses (for studies, see Reference RobertsonRobertson 2012). Thus, both in clinic populations and in the community, only about 10% of people with just Tourette syndrome (‘Tourette syndrome alone’) have tics and 90% have comorbid psychiatric diagnoses.
Attention-deficit hyperactivity disorder
Relatively recently, research groups have separated individuals with Tourette syndrome into subgroups on the basis of clinical symptoms, specifically separating those with and without ADHD. Thus, they have examined children with Tourette syndrome alone, and compared them with groups such as Tourette syndrome plus ADHD, ADHD alone, and unaffected controls. Youngsters with Tourette syndrome alone did not differ from unaffected controls on many ratings, including aggression, delinquency and conduct difficulties. By contrast, children with Tourette syndrome plus ADHD were significantly above unaffected controls, and similar to those with ADHD alone, on the indices of disruptive behaviours. Studies further showed that youngsters with Tourette syndrome plus ADHD demonstrated more internalising and behaviour problems and poorer social adaptation than children with Tourette syndrome alone or controls. Of importance is that youngsters with Tourette syndrome alone were not significantly different from unaffected controls on most measures of externalising behaviours and social adaptation, but had more internalising symptoms.
In summary, individuals with Tourette syndrome alone appear to be similar to healthy controls and, except for the internalising problems, significantly different from those with Tourette syndrome plus ADHD, and this clearly has major management and prognostic implications (Reference RobertsonRobertson 2006a).
Obsessive–compulsive disorder and obsessive–compulsive behaviour
In other controlled studies, young people with Tourette syndrome have been shown to have more obsessional symptoms than control participants. Importantly, the obsessive–compulsive behaviours encountered in Tourette syndrome are statistically and clinically different from those behaviours in OCD (for a review, see Reference RobertsonRobertson 2000, Reference Robertson, Bedard, Agid and Chouinard2003). The obsessive–compulsive behaviours in people with Tourette syndrome include thoughts (obsessions) of violence, sex and aggression, and actions (compulsions) concerning touching of the self and/or others, symmetry and ordering. This differs from many people with OCD, who are often more preoccupied with dirt, germs and contamination. It is also clear that there is a genetic link between Tourette syndrome and obsessive–compulsive behaviours (Reference Eapen, Pauls and RobertsonEapen 1993).
Autism spectrum disorder
It has long been documented that Tourette syndrome and ASD have clinical similarities and share many symptoms. Evidence has been emerging from phenomenological, epidemiological and pathogenetic perspectives (Reference StateState 2010) that Tourette syndrome and ASD overlap. It has been suggested that shared molecular pathways affect the development of both disorders: examples include disruption of the NRXN1, NLGN4X and CNTNAP2 genes (Reference Clarke, Lee and EapenClarke 2012; Reference EapenEapen 2012).
It seems likely that the disorders discussed in this section are comorbid with Tourette syndrome because they have neurodevelopmental similarities, clinical similarities and in some cases are probably genetically related.
Coexistent psychopathology
The most common coexistent psychopathologies found in Tourette syndrome include depressive illness, depressive symptoms, anxiety, phobias, intellectual disability and, in adolescents and adults, personality disorder. These appear to be coexisting rather than comorbid, as they are unlikely to share a genetic underpinning. The psychoses, such as bipolar affective disorder, are probably related to other disorders such as OCD and ADHD, whereas schizophrenia is rare and the two disorders co-occur only by chance (Reference Robertson, Bedard, Agid and ChouinardRobertson 2003, Reference Robertson2012). The relationships between Tourette syndrome, comorbid disorders and coexistent psychopathology are summarised in Table 1.
There is some exciting evidence emerging that specific aspects of the aetiology, phenotype and indeed endophenotype (e.g. specific brain changes related to a particular clinical type) may be found in Tourette syndrome and this is a worthwhile direction for future research (Reference Robertson, Eapen, Martino and LeckmanRobertson 2013).
Aggression and behavioural difficulties
It has been suggested that aggressive behaviours are probably influenced by both genetic (e.g. the MAOA gene) and epigenetic (e.g. risk to the fetus, pre- and perinatal difficulties) factors (Reference LiuLiu 2011a). For example, violence or aggression to women during pregnancy can result in obstetric problems and altered personality in offspring if the child has an individual vulnerability. Environmental effects such as early-life stressors may also play a part (Reference Verhoeven, Booij and KruijtVerhoeven 2012). There are, of course, also cultural theories of aggression, for example that it is related to the male dominance in society or that children learn it from their early role models such as parents.
As already mentioned, one of the most common comorbidities in youngsters with Tourette syndrome is ADHD (Reference RobertsonRobertson 2012). Compared with unaffected peers, adults with Tourette syndrome are more likely to have personality disorders (e.g. Reference Robertson, Banerjee and Fox HileyRobertson 1997). In youngsters with both Tourette syndrome and ADHD (compared with Tourette syndrome alone) there are significantly more disruptive behaviours (Reference RobertsonRobertson 2006a), and adults with Tourette syndrome have additional problems with substance misuse, aggression and forensic encounters (Reference Haddad, Umoh and BhatiaHaddad 2009). Some of the explanations may be integral to Tourette syndrome, whereas others may well be generic, as outlined above.
It appears that Tourette syndrome and conduct disorder are not intimately (i.e. aetiologically, genetically) connected. In a person with Tourette syndrome and conduct disorder, the latter is associated with ADHD in the proband and a family history of violence, aggression and forensic encounters (Reference Robertson, Cavanna and EapenRobertson 2015).
Types of Tourette syndrome
There have been many studies indicating that Tourette syndrome is not a unitary condition as suggested, indeed stipulated, by both American Psychiatric Association (DSM-5) and World Health Organization (ICD-10) diagnostic criteria. An early study of 90 people with Tourette syndrome found a high incidence of obsessionality, depression and hostility (Reference Robertson, Trimble and LeesRobertson 1988). Importantly, depression was not related to medication. Aggression, hostility and obsessionality were significantly associated with some core features of Tourette syndrome, including copro- and echophenomena and a family history of the syndrome.
The first study to formally investigate the phenotype was that of Reference Alsobrook and PaulsAlsobrook & Pauls (2002), who used hierarchical cluster analysis (HCA) and principal component factor analysis (PCFA) and reported four significant factors, which accounted for 61% of the variance. These were:
-
aggressive phenomena (e.g. kicking, temper fits and argumentativeness)
-
motor and vocal/phonic tics only
-
compulsive phenomena
-
tapping and absence of grunting.
Since then there have been many other analytical studies conducted on clinical cohorts (see Reference RobertsonRobertson 2014d), and all have reported more than one type or phenotype. A large multiply affected pedigree living in the community was submitted to HCA and PCFA and again four types (phenotypes) were found (Reference Robertson and CavannaRobertson 2007). Moreover, in all studies that examined for it, one type was ‘pure Tourette syndrome’, i.e. motor and vocal tics only. This is in agreement with the clinical data of Reference Freeman, Fast and BurdFreeman et al (2000) and community data of Reference Khalifa and von KnorringKhalifa & von Knorring (2003, Reference Khalifa and von Knorring2005), who found that only about 10% of all individuals with Tourette syndrome have only motor and vocal tics. Although not directly comparable, all studies using HCA, PCA or latent class analysis (LCA) have shown two or more factors, in terms of both tics and psychopathology. Further studies have used similar methods, and although their results give different slants, they agree that there is more than one phenotype (Reference RobertsonRobertson 2014d). Reference RobertsonRobertson (2014b) highlights the neurological heterogeneity as well as other clinical differences (other than comorbidity and psychopathology) encountered in Tourette syndrome.
All these studies add to the growing body of evidence that Tourette syndrome is not a unitary condition. Thus, one is able to conclude that the Tourette syndrome phenotype is heterogeneous and not unitary as previously suggested and shown in DSM and ICD. In conclusion, whether using complex statistical methods (e.g. HCA, PCA, LCA) or material derived from clinical or community settings, one type (phenotype) or clinical presentation of Tourette syndrome consists of ‘pure simple motor and vocal tics only’, whereas other phenotypes include complex tics and the comorbid disorders, various coexistent psychopathologies and complex behaviours.
Assessment
The assessment of patients with Tourette syndrome requires a thorough personal and family history, as well as full mental state and neurological examinations. Several standardised schedules may be useful for accurately diagnosing Tourette syndrome, assessing the response to medication and in research (Box 1). The physician-rated Diagnostic Confidence Index and the self-rated MOVES in particular are useful for the practising clinician, as they will ensure that the main symptoms for diagnosis (Diagnostic Confidence Index) and severity (MOVES) are assessed. The Diagnostic Confidence Index highlights the phenomenological characteristics of tics, including the presence of coprolalia, echolalia and palilalia, complex tics, premonitory urges/sensations, relief after tics, suppressibility, rebound, suggestibility, variability of tics, and the waxing and waning course (Reference Robertson, Banerjee and KurlanRobertson 1999).
BOX 1 Some instruments for diagnosing Tourette syndrome, assessing medication response, and research
-
National Hospital Interview Schedule
-
Yale Global Tic Severity Scale
-
Premonitory Urge for Tics Scale (self-rated)
-
Motor tic, Obsessions and compulsions, Vocal tic Evaluation Survey (MOVES)
-
Hopkins Motor and Vocal Tic Severity Scale
-
Rush Video-Based Tic Rating Scale
-
Diagnostic Confidence Index
For reviews of these scales see Reference Robertson and CavannaRobertson & Cavanna (2008c) and Reference RobertsonRobertson (2011)
Familiarity with Tourette syndrome, as well as training by an expert, are important for implementing most of these scales. However, a good clinician, given time, will be able to elicit symptoms, make a diagnosis and give correct treatment and management.
Many clinicians would suggest blood sampling for copper and ceruloplasmin (to exclude Wilson's disease) and acanthocytes (to exclude neuroacanthosis) as good practice, but genetic testing for Huntington's disease would only be undertaken exceptionally. Neuroimaging in research studies shows differences between patients with Tourette syndrome and controls, and endophenotypes (brain changes specific to Tourette syndrome) have been suggested (Reference Robertson, Eapen, Martino and LeckmanRobertson 2013): in routine practice, however, computed tomography, electroencephalograms (EEGs) and electrocardiograms (ECGs) are usually non-contributory. These investigations are usually only warranted to exclude any other diagnosis, such as myoclonic (jerking) or petit mal (blinking) epilepsy or chorea with rheumatic fever.
Management and treatment
The treatment for all individuals with Tourette syndrome includes psychoeducation, reassurance and explanation. In many mild cases and young people this may in fact suffice. Medication is the mainstay for the majority of symptoms of Tourette syndrome and many of the comorbid conditions and coexistent psychopathologies. New strategies include the successful and side-effect-free habit reversal training and comprehensive behavioural intervention for tics. Injection of botulinum toxin into the periorbital tissues and vocal cords has yet to be fully evaluated, and deep brain stimulation for severe and refractory tics in adults also needs further study (see Reference RobertsonRobertson 2011, Reference Robertson2012, Reference Robertson2014a).
Table 2 includes the main management strategies and medications for Tourette syndrome currently available and used by many clinicians. Empirical evidence of efficacy, ranked A to D (‘good’ to ‘minimal’), has been collated from double-blind trials (best evidence), large series (some evidence) and case reports (minimal or anecdotal evidence) and also personal experience, which, although anecdotal, covers many patients and is representative of clinic populations.
TABLE 2 Main strategies of the management of the motor and vocal/phonic tics of Tourette syndrome in young people, showing the quality of the current evidencea
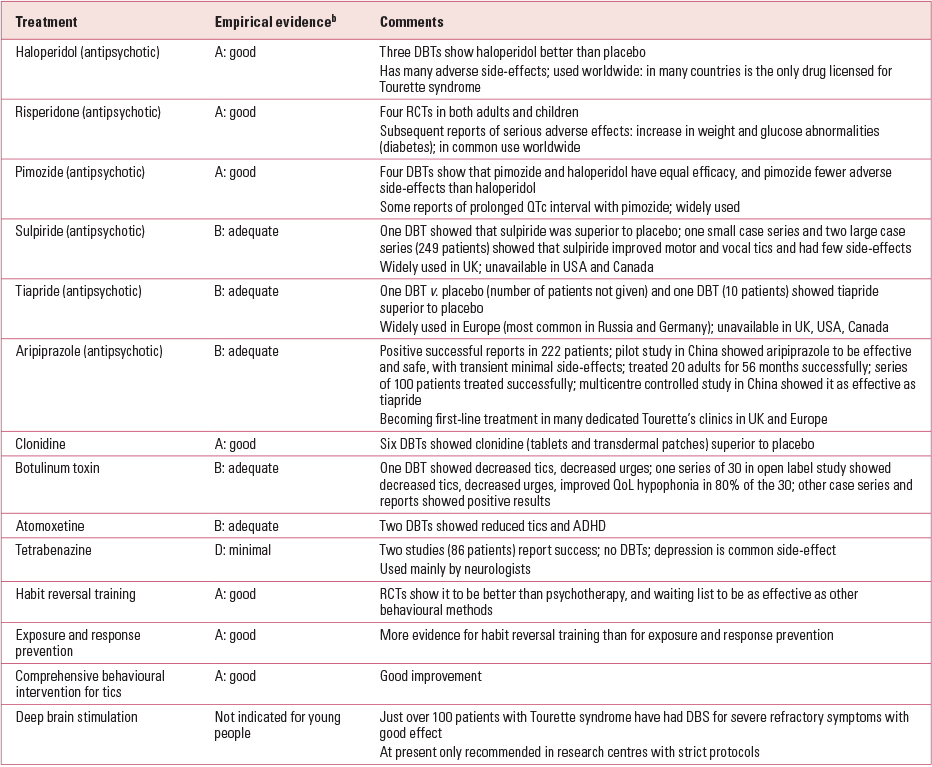
Behavioural interventions
Behavioural methods for adult and child patients may be useful alone or in combination with medications for many aspects of Tourette syndrome. Habit reversal training has been shown to be significantly better than or equal to supportive psychotherapy and better than the waiting list for adults with Tourette syndrome. Comprehensive behavioural intervention for tics was found to be helpful for young people (Reference Piacentini, Woods and ScahillPiacentini 2010). Exposure and response prevention has also proved very successful in the treatment of tics, and a novel non-pharmacological treatment using self-hypnosis was successful in 26 (almost 80%) of 33 children (see Reference RobertsonRobertson 2012). In recent European guidelines, behavioural therapies are recommended as a first-line treatment of Tourette syndrome, albeit on the basis of evidence less strong than for habit reversal training (Reference Verdellen, van de Grient and HartmannVerdellen 2011).
Pharmacological interventions
Medication is often required for the treatment of moderate to severe tics and psychopathologies in patients with Tourette syndrome. Double-blind trials have demonstrated that many medications (Table 2) are superior to placebo. Importantly, the dose given for Tourette syndrome is small compared with the dose for schizophrenia or mania. Thus, a daily dose of 0.5–3 mg of haloperidol may be sufficient in Tourette syndrome, whereas 30 mg may be required in severe mania or schizophrenia in adults. Tetrabenazine can also be effective and is prescribed mainly by neurologists: a side-effect can be depression. Clonidine (or guanfacine in the USA) can be given for tics, impulse control and ADHD (and it may also help with insomnia). A baseline ECG is advisable, as is regular monitoring of pulse and blood pressure. One can commence at a dose of 25 μg, increasing gradually to 150 μg daily. It is advisable to take blood for a baseline prolactin, as many antipsychotic medications result in hyperprolactinaemia, which can have endocrinal repercussions (Reference RobertsonRobertson 2000, Reference Robertson2012).
Antidepressants, especially the selective serotonin reuptake inhibitors (SSRIs), are useful for depression (e.g. fluoxetine at a standard dose of 20 mg/day) and obsessive–compulsive behaviours or OCD (e.g. fluoxetine at 40–60 mg/day). Clomipramine (a tricyclic antidepressant) may also be useful in obsessive–compulsive behaviours or OCD, but it usually has more side-effects than the SSRIs and is dangerous in overdose. In the obsessive–compulsive behaviours and OCD associated with Tourette syndrome, a small dose of an antipsychotic is useful as an augmentation agent. It is important to know that the response to individual antipsychotics is idiosyncratic; a patient may respond to one but not another and, unfortunately, not to a second trial of an original medication, if discontinued.
Recently, the newer atypical (second-generation) antipsychotics have been shown to be useful in treating Tourette syndrome. The main side-effects are weight increase and, in some individuals, precipitation of diabetes or metabolic syndrome. It is therefore advisable to check fasting glucose levels in patients receiving the atypicals, especially if they have put on weight. Atypical antipsychotics used successfully in treating Tourette syndrome include risperidone, olanzapine, quetiapine, ziprasidone and aripiprazole. In both the literature and my clinical experience, patients treated with antipsychotics can have raised prolactin levels, which in some cases requires discontinuation of the drugs (Reference RobertsonRobertson 2012). Rickards et al (2012) have reported findings from a survey of European prescribing practices in Tourette syndrome which showed that, in the management of various symptom clusters, risperidone was most frequently prescribed for tics, sertraline for obsessive–compulsive behaviours and methylphenidate for ADHD. The use of aripiprazole has gained momentum in Europe, the UK and the USA, and one controlled trial in China showed it to be as effective as tiapride (Reference Liu, Chen and ZhongLiu 2011b). Aripiprazole is therefore rated B in Table 2.
As stated earlier, clonidine has been used in the treatment of Tourette syndrome and of ADHD, and thus it may well be a useful treatment for individuals with both disorders. Good evidence for the safety and efficacy of the combination of stimulants and clonidine comes from a large randomised double-blind trial including over 130 children who had ADHD and a tic disorder and were treated with clonidine alone, methylphenidate alone, clonidine and methylphenidate, or placebo (Tourette's Syndrome Study Group 2002). Compared with placebo, the greatest benefit was with the combination of clonidine and methylphenidate. Of importance was that the proportion of participants reporting a worsening of tics was no higher in those treated with methylphenidate than in those receiving clonidine or placebo.
Thus, it does appear from evidence-based studies that stimulants, if used judiciously in patients with Tourette syndrome or tics and comorbid ADHD, do not necessarily increase tics. In addition, the combination of stimulants and clonidine appears to be safe. Atomoxetine is a relatively new agent for the treatment of ADHD and it may prove useful in the treatment of Tourette syndrome plus ADHD – further research is needed.
A less used but successful treatment is botulinum toxin injected into affected areas (e.g. the vocal cords for loud distressing vocal tics and coprolalia); treatment is usually carried out by a neurologist with special expertise, in expert clinics.
Deep brain stimulation is used worldwide for Parkinson's disease, tremor and dystonia, and even for depression, OCD and Tourette syndrome (e.g. Reference Hariz and RobertsonHariz 2010). It has been used in over 100 people with Tourette syndrome, but only those with severe refractory illness. It is unlikely that it would be used in youngsters under the age of 20.
Continuity into adult life
It was initially thought that Tourette syndrome was lifelong. Several studies have now reported that tic severity reduces during adolescence and it seems that by the age of 18, tics decrease in many patients. Only greater tic severity in childhood has been associated with increased tic severity at follow-up. Although the prognosis for Tourette syndrome is better than originally thought concerning tic symptomatology (for a review, see Reference RobertsonRobertson 2008a,Reference Robertsonb), the comorbidity often changes with age (Reference Rizzo, Gulisano and CaliRizzo 2012) and the psychopathology (e.g. depression) worsens with age (e.g. Reference RobertsonRobertson 2006b).
In an elegant study, Reference Pappert, Goetz and LoiusPappert et al (2003) followed up a group of 56 individuals with Tourette syndrome who had been videotaped according to a strict protocol between 1978 and 1991, when they were 8- to 14-year-olds. Thirty-one of the original cohort, now aged over 20, were contacted and included in a follow-up video study. A rater assessed the 62 tapes and rated five tic domains: the two videotapes for each participant were compared for each tic domain, as well as a composite tic disability score. Results showed that 90% of the adults still had tics. Many patients who had suggested that they were tic free were not, as no less than 50% had objective evidence of tics. The mean tic disability score reduced significantly with age. All tic domains improved with age and there were significant improvements for motor tics. The improvements in tic disability were not related to medication, as only 13% of the adults were receiving medication for tics, compared with 81% of the children. The authors concluded that although tics improve with time, most adults have persistent tics.
Reference Rizzo, Gulisano and CaliRizzo et al (2012) investigated 100 people with Tourette syndrome who were assessed at onset and at follow-up 10 years later to evaluate the severity of the tics and the presence of comorbid disorders and coexistent psychopathologies. Impairment was also evaluated. At 10-year follow-up, 58% of the 38 individuals with ‘pure’ Tourette syndrome (i.e. no comorbidity) persisted with the same ‘pure’ clinical phenotype, whereas 42% had changed to a ‘Tourette plus OCD’ phenotype. In the Tourette plus OCD subgroup, 55% required medication and fared better than those with initial comorbidities, who also had a significantly reduced quality of life.
Conclusions
Tourette syndrome is now recognised to be common, affecting about 1% of the population almost worldwide. The aetiology is more complex than was once thought, and is widely accepted to be genetic in most individuals, although no single gene has been identified. Other behaviours, however, such as obsessive–compulsive behaviours and OCD, are widely recognised phenotypes of the putative gene(s). Newer evidence is that some cases of ADHD and ASD also share some genetic underpinning. Other aetiopathological suggestions include environmental influences, such as infections, neuroimmunological effects, pre- and/or perinatal difficulties, psychosocial stressors and/or androgen influences. The old idea that Tourette syndrome is psychological and to be treated with psychoanalysis has now been discredited.
Exciting evidence is emerging that specific aspects of the aetiology, phenotype and endophenotype (e.g. brain changes related to a particular clinical type) may be identified in Tourette syndrome and this is a worthwhile direction for future research.
A wide variety of comorbid psychiatric disorders and/or psychopathology are common, and it seems that the presentation may change over time. Unexpectedly, it appears that uncomplicated Tourette syndrome (without comorbid disorders) may not have the best prognosis. Some comorbidities are common and integral (e.g. OCD, obsessive–compulsive behaviours, ADHD, ASD), whereas some coexistent psychopathologies (e.g. depression) are common but multifactorial in origin, and others (such as personality disorder in adults and bipolar affective disorder) may be due to comorbid conditions (e.g. ADHD, OCD) rather than Tourette syndrome itself.
Treatment should be symptom targeted and, ideally, should also be holistic. This is important as it not only alleviates the suffering but may also improve the outlook in terms of tics, psychopathology and psychosocial functioning. Habit reversal training, exposure response prevention and comprehensive behavioural intervention are gaining momentum in the treatment of tics in Tourette syndrome. Deep brain stimulation is currently the ‘quantum leap’ in many of our professional lives, but remains a research tool.
MCQs
Select the single best option for each question stem
-
1 Which of the following has not been shown to adversely affect quality of life in Tourette syndrome?
-
a tic severity
-
b ADHD
-
c OCD
-
d enlarged ventricles on neuroimaging
-
e employment status.
-
-
2 The prevalence of Tourette syndrome worldwide has been estimated as about:
-
a 0.05%
-
b 0.2%
-
c 0.5%
-
d 1%
-
e 3%.
-
-
3 The mean age at onset of Tourette syndrome is:
-
a 3 years
-
b 5 years
-
c 7 years
-
d 9 years
-
e 11 years.
-
-
4 Which of the following is not thought to be a putative factor in the aetiology of Tourette syndrome?
-
a prenatal androgen exposure
-
b auto immunity
-
c maternal smoking during pregnancy
-
d pathological psychodynamic defence mechanisms
-
e genetics.
-
-
5 Which of the following behavioural treatments has been shown to be effective in the treatments of tics associated with Tourette syndrome?
-
a exposure and response prevention
-
b flooding
-
c debriefing
-
d mindfulness-based meditation
-
e behavioural activation.
-
MCQ answers
| 1 | d | 2 | d | 3 | c | 4 | d | 5 | a |





eLetters
No eLetters have been published for this article.