INTRODUCTION
Shiga toxin-producing Escherichia coli O157 (STEC O157) causes acute gastroenteritis characterized by abdominal cramps and profuse, often bloody, diarrhoea. In 1997, an estimated 73 000 cases occurred in the United States [Reference Mead1]. Approximately 8% of reported infections progress to haemolytic uraemic syndrome (HUS), a potentially life-threatening condition comprised of acute renal failure, anaemia, and thrombocytopenia [Reference Slutsker2].
Contaminated food is thought to be an important source of STEC O157 infections in the United States [Reference Mead1]. Although ground beef has been of particular concern [Reference Slutsker2–Reference Pai5], outbreak investigations have identified a wide variety of other food vehicles, including other meat products [Reference Keene6], unpasteurized milk and fruit juices [7–Reference Keene9], and produce items [Reference Sivapalasingam10]. Non-foodborne sources have included contaminated municipal water [Reference Olsen11, Reference Swerdlow12], recreational water [Reference Ackman13–Reference Keene15], and contact with cattle [Reference Crump16, Reference Crump17] and infected persons [Reference Belongia18].
Between 1996 and 1999, the United States Department of Agriculture's Food Safety Inspection Service (USDA-FSIS) implemented the Pathogen Reduction: Hazard Analysis and Critical Control Point Systems (HACCP) Final Rule. The HACCP rule was designed to reduce contamination of meat and poultry products with bacterial pathogens, including STEC O157. At the inception of the HACCP programme, the Foodborne Diseases Active Surveillance Network (FoodNet) conducted a case-control study of sporadic STEC O157 infections [Reference Kassenborg19]. The study identified ground beef and farm visits as sources of sporadic STEC O157 infection. We report results of a second case-control study conducted during 1999–2000 to monitor and further define risk factors for STEC O157 infection.
METHODS
FoodNet monitors the incidence of pathogens commonly transmitted through food by comprehensive ascertainment of laboratory-confirmed infections and focused epidemiological studies [Reference Allos20]. Laboratory-confirmed cases of STEC O157 infection were ascertained through active laboratory-based surveillance in Connecticut, Georgia, Minnesota, Oregon and selected counties in California, Maryland, and New York. These sites comprise 11% of the US population. FoodNet staff in each site contacted all clinical laboratories in their area at least monthly to ascertain laboratory-confirmed cases. All laboratory-confirmed cases ascertained during a 12-month consecutive period in each site, beginning between February and April 1999, were considered for enrolment in the case-control study. Isolates from enrolled patients were forwarded to their respective state public health laboratory for confirmation.
Patient and control enrolment, and criteria for eligibility, exclusion, and matching, were the same as those used in the 1996–1997 FoodNet STEC O157 case-control study [Reference Kassenborg19]. Patients were asked about the symptoms, severity, and treatment of their illness. Patients and controls were asked about antibiotic use in the month before the patient's onset of illness. Additionally, patients and controls were asked about exposures in the 7 days before the patient's onset date including: meals eaten in the home or a restaurant, fruit and vegetable consumption, meat consumption and handling practices, cattle and other farm animal exposures, petting zoos or fair visits, day-care centre attendance, drinking and recreational water exposures, travel history, and food safety knowledge and practice. We obtained appropriate informed consent from all participants and conducted this study in accordance with guidelines for human research as specified by the U.S. Department of Health and Human Services.
Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA). We used a main effects log-linear Poisson regression model to estimate the change in incidence of all laboratory-confirmed STEC O157 infections from 1996 to 2000. The model estimated the effect of time on the incidence by treating calendar year as a categorical variable and adjusted for the increased population and variation in the incidence among sites. We used multivariable conditional logistic regression analysis to estimate the odds ratio (OR) and 95% confidence interval (CI) for exposures associated with STEC O157 infection in bivariate analysis of the case-control study data or exposures identified in the 1996–1997 FoodNet case-control study [Reference Kassenborg19]. Model fit was assessed by the Hosmer and Lemeshow goodness-of-fit test. For exposures that appeared in multiple questions in the questionnaire, we created composite variables in the analysis. For example, we combined the results of questions about eating pink ground beef in a restaurant and eating pink ground beef in the home into one variable that recorded any pink ground beef consumption. We coded pink hamburger consumption as a subset of hamburger consumption to estimate the incremental risk of undercooked hamburgers among those who consumed hamburgers. Using the final multivariable model, we calculated population attributable fractions (PAF) [Reference Bruzzi21] to evaluate the relative importance of each exposure.
To explore risk factors for illness from hamburger consumption, we created a multivariable model that included consumption of hamburger or pink hamburger that was prepared either in the home or in a restaurant. To explore age-specific effects and potential differences in respondent recall between direct interview and proxy interview, we analysed the data in separate multivariable models for respondents aged <12 years and ⩾12 years. Food safety knowledge and practice analysis was restricted to either patients or the parent/guardian who was interviewed and was the primary foodhandler and food purchaser in the household in matched bivariate analyses.
RESULTS
Active surveillance, 1996–2000
The incidence of laboratory-confirmed STEC O157 in the FoodNet sites did not change significantly from 1996 (the start of the first FoodNet case-control study) to 2000 (the end of this study). The crude incidence (cases/1 00 000 population) declined from 2·7 in 1996 to 2·1 in 2000. However, the size and geographic composition of the FoodNet catchment area also changed during this period. Using a multivariable negative binomial model to adjust for these changes, the incidence of STEC O157 infection in 2000 increased by 9% (95% CI −16% to +41%) relative to 1996.
Case-control study enrolment, 1999–2000
Among the 531 cases with laboratory-confirmed STEC O157 infection ascertained by FoodNet surveillance during the study period, 390 met the eligibility criteria for inclusion (Fig.). Of 141 cases not eligible, 79 were outbreak-associated including 58 (73%) cases associated with an outbreak caused by well water contaminated with cattle faeces [22], 11 associated with an outbreak caused by contaminated ground beef, seven associated with an outbreak at a childcare centre, and three associated with an outbreak caused by contaminated romaine lettuce. Among the 390 eligible cases, 107 (27%) were not enrolled in the study (Fig.). A total of 283 sporadic cases and 534 controls were enrolled; 32 cases were matched to one control and 251 cases were matched to two controls. Enrolled patients were similar to non-enrolled eligible patients by age and sex. The geographic distribution of cases enrolled in the study was similar to cases ascertained through active surveillance with the exception of New York, which had a large number of outbreak cases excluded from the study. The median time from patient illness onset to interview was 17 days for cases (mean 17 days, range 3–54 days) and 21 days for controls (mean 21 days, range 7–50 days). The median age of the enrolled patients was 23 years (mean 29 years, range 0·42–93 years). Of the 104 patients aged <12 years, 49% were female and of the 179 patients aged ⩾12 years 59% were female.

Fig. Ascertainment and enrolment of laboratory-confirmed Shiga toxin-producing Escherichia coli O157 infections in the FoodNet case-control study, 1999–2000.
Clinical findings
Among the 283 patients enrolled, 90% reported bloody stools, 46% reported fever, and 46% reported vomiting. The median duration of diarrhoea was 6 days (mean 7·1 days, range 1–74 days). All patients sought medical attention; 61% presented to an emergency department and 39% were admitted to the hospital. Among those admitted, the median length of hospital stay was 4 days (mean 4·4 days, range 1–21 days). Intravenous fluids were given to 53% of enrolled patients, and 63% received at least one antibiotic for their illness. Forty-nine percent of patients reported buying an over-the-counter medication specifically for their illness. Eighty-five percent of the patients who were employed at the time of their illness missed work due to their illness for a median of 4 work days with at least 4 h missed (mean 4·6 days, range <1–8 days). Among children, 88% missed events such as attending school, participating in sports or social events, or going on vacation for a median of 1 day (mean 2·6 days, range <1–9 days). Seven children and one adult (3% of enrolled patients) developed HUS. The median age of patients with HUS was 3 years (mean 12 years, range 1–62 years) and 50% were female. All patients with HUS were hospitalized. The median length of stay was 7·5 days (mean 8 days, range 4–18 days). There were no deaths.
Risk factors
We first included a variable identified in bivariate analysis (drinking untreated surface water from a pond, lake, river, or stream), and exposures identified in the 1996–1997 case-control study (eating at a restaurant with table service, eating hamburger, purchasing meat from a custom slaughter arrangement, eating produce, and contact with cattle on a farm) in an exploratory multivariable model. Contact with cattle (living on, working on, or visiting a cattle farm) was associated with infection (OR 4·3, 95% CI 1·9–9·5), as was drinking untreated surface water from a pond, lake, river, or stream (OR 3·8, 95% CI 1·5–10). STEC O157 infection was not associated with eating at a restaurant with table service (OR 0·9, 95% CI 0·6–1·3), eating hamburgers (OR 1·0, 95% CI 0·6–1·6), or purchasing meat from a custom slaughter arrangement (OR 0·5, 95% CI 0·2–1·4). However, among those who ate hamburgers, patients were more likely to report eating pink hamburger (OR 1·9, 95% CI 1·1–3·4). Eating fruits and vegetables showed an inverse association with illness, with the lowest estimate among persons who consumed ⩾25 servings of fruits or vegetables during the 7-day exposure period in this multivariable model.
We created a final multivariable model that included eating hamburger and pink hamburger, eating fruits and vegetables, contact with cattle on a farm, and drinking untreated surface water (Table 1). Contact with cattle on a farm and drinking untreated surface water remained risk factors for infection, as did consumption of pink hamburger among hamburger eaters (Table 1). Consumption of fruits and vegetables continued to be protective, with an apparent dose–response effect.
Table 1. Risk factors for sporadic Shiga toxin-producing Escherichia coli O157 infection in the Foodborne Diseases Active Surveillance Network, 1999–2000

OR, Odds ratio; CI, confidence interval; PAF, population attributable fraction.
Because eating pink hamburger remained a risk factor for infection, we next examined differences in association for hamburger consumption by place of preparation in a multivariable model adjusted for produce consumption, contact with cattle and drinking untreated surface water. There was no association with eating pink hamburgers prepared in restaurants (OR 1·5, 95% CI 0·6–4·2). However, eating pink hamburgers prepared at home was associated with illness (OR 1·8, 95% CI 1·0–3·3).
The prevalence of several exposures varied by patients’ age. Therefore, we also created separate multivariable models that included all variables in the final multivariable model for persons aged <12 years and for those aged ⩾12 years. Among children aged <12 years, eating hamburgers in general was associated with infection (OR 3·0, 95% CI 1·2–7·7), although eating pink hamburger was not (OR 1·2, 95% CI 0·4–3·2). This association was driven primarily by hamburgers prepared at home (OR 2·7, 95% CI 1·3–5·7) rather than hamburgers prepared in restaurants (OR 0·5, 95% CI 0·3–1·1). Among persons aged ⩾12 years, the results were similar to the overall analysis (see Table 1). Among hamburger eaters, eating pink hamburger prepared at home (OR 1·9, 95% CI 1·0–3·6) and in restaurants (OR 2·7, 95% CI 1·0–7·7) were both associated with illness. The dose–response effect for eating fruits and vegetables was similar for persons aged ⩾12 years compared to the analysis of all cases and controls. The association for contact with cattle did not vary by age category or type of exposure (living, working, or visiting a cattle farm). The odds ratio for drinking untreated surface water was higher but less precise among persons aged <12 years (OR 7·1, 95% CI 1·7–29) compared to persons aged ⩾12 years (OR 2·2, 95% CI 0·6–7·3).
Food safety behaviour
Among the 191 cases and 384 controls who reported preparing ground beef in the home, a similar proportion of cases (96%) and controls (93%) reported always or almost always washing their hands after handling ground beef (OR 0·6, 95% CI 0·3–1·5). Of those who reported washing hands after handling raw ground beef, 92% used soap and water. A similar proportion of cases (3%) and controls (2%) used a meat thermometer to check the internal temperature of hamburger patties while cooking (OR 1·3, 95% CI 0·4–4·3). Among the 196 cases and 378 controls who reported purchasing ground beef for the household, 42% of cases and 48% of controls placed meat in a separate plastic bag before placing it in the shopping cart with other groceries (OR 1·1, 95% CI 0·7–1·9). Overall, 72% of respondents noticed a label detailing safe cooking instructions on meat packages, 59% read the label, and 34% reported that the label included the temperature to cook ground beef to ensure that it was safe. There was no association between noticing or reading the label and illness.
DISCUSSION
More that 20 years after it was first recognized as a source of STEC O157 infections [Reference Ostroff23, Reference Riley24], consumption of undercooked ground beef continues to be identified as a source of sporadic cases. Since the 1996–1997 FoodNet case-control study, the proportion of cases caused by undercooked ground beef may have fallen (Table 2). The proportion of cases in the present study attributed to eating pink hamburger in restaurants was only 2% of cases, compared with 7% in the 1996–1997 FoodNet study [Reference Kassenborg19]. There was less difference in the proportion of cases attributed to eating pink hamburgers in the home (6% in 1999–2000 vs. 8% in 1996–1997). A high proportion of respondents reported proper food safety knowledge and behaviours, such as frequent hand washing with soap after handling raw ground beef and thorough cooking of hamburgers. However, consumption of undercooked ground beef remained an important vehicle for STEC O157 transmission to the end of 2000.
Table 2. Population attributable fraction percentages for sporadic Shiga toxin-producing Escherichia coli O157 infection risk factors in the Foodborne Diseases Active Surveillance Network from the 1996–1997 and the 1999–2000 case-control studies
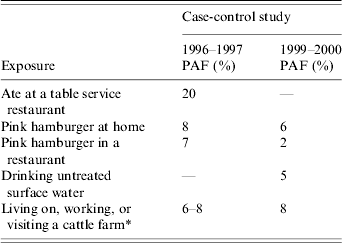
PAF, Population attributable fraction.
* Variable was calculated by age: ‘Lived on or visited a farm’ for patients aged <6 years (6%) and ‘Visited a farm with cows’ for patients aged ⩾6 years (8%).
Direct contact with cattle was also an important risk factor in this study. Persons who lived on, worked on, or visited a cattle farm were at increased risk for infection. This finding is consistent with findings of several other studies and outbreak investigations [Reference Bender and Shulman25, 26]. The risk did not change by age group or duration of exposure. Guidelines for the prevention of transmission of STEC O157 and other pathogens following animal contact in petting zoo settings have been developed [27]. The overall impact of these recommendations has yet to be evaluated.
In contrast to the 1996–1997 FoodNet case-control study, we found STEC O157 infections were associated with drinking untreated surface water. Furthermore, during this study a large outbreak of STEC O157 infections associated with contaminated drinking water occurred, resulting in 79 laboratory-confirmed infections that were excluded from the present study [22]. This outbreak illustrates how drinking water can become contaminated with STEC O157 from cattle and result in human illness.
Although contaminated produce has been implicated in outbreaks of STEC O157 infection [Reference Sivapalasingam10, Reference Rangel28], we observed a dose-dependent decrease in risk of sporadic illness associated with produce consumption. Similar protective effects of produce and a diverse diet have been noted in case-control studies of sporadic STEC O157 infections in the United States [Reference Kassenborg19] and Scotland [Reference Locking29], and in FoodNet case-control studies of sporadic Campylobacter [Reference Friedman30] and Cryptosporidium infection [Reference Roy31]. Notably, the effect remains in multivariable analysis, suggesting that it is not due to simple substitution of produce for potentially contaminated meat. Research using cattle indicates that alterations in diet can influence the presence of STEC O157 in the gut and the shedding of the organism [Reference Herriott32]. Specifically, produce items containing coumarins appear to affect the viability of STEC O157 in vitro [Reference Duncan, Flint and Stewart33, Reference Duncan34], and it is possible that similar effects occur in humans. On balance, the multiple benefits of a diet that includes ample servings of fruits and vegetables appear to outweigh the potential risks of consuming produce that is potentially contaminated with enteric pathogens such as STEC O157 [Reference Sivapalasingam10].
There are important limitations to this study. Self-reported behaviours are not necessarily an accurate reflection of actual practice, particularly when some behaviours are known or suspected to be associated with the infection [Reference Anderson35]. Moreover, the study cannot assess cross contamination in the kitchen if the respondent was not aware that it had occurred. Assessing the adequacy of cooking is difficult as pink colour is an imperfect surrogate for thorough cooking [36, Reference Hunt, Soerheim and Slinde37]. However, given the rarity of thermometer use in this study, pinkness may be the best available indicator of undercooking. Sporadic case-control studies may lack the statistical power to detect relatively infrequent (e.g. venison) or episodic (e.g. recreational water contamination) sources of STEC O157. The collective PAF for the identified risk factors in our study was <25%. These values may underestimate the true risk attributable to each exposure and should therefore be interpreted conservatively as relative values indicating the risk rank of the exposures. Nevertheless, it is notable that we observed a decrease in the PAF associated with undercooked hamburgers prepared at restaurants between the first and second case-control studies, both in absolute terms and relative to the PAF of undercooked hamburgers prepared at home. This observation is consistent with surveillance data showing a decrease in restaurant-associated outbreaks [Reference Rangel28]. These changes have occurred despite stable levels of contamination in HACCP meat samples and no overall change in disease incidence during the 4 years in between the FoodNet case-control studies.
In the time period covered by this study (1999–2000), sporadic STEC O157 infections were associated with consumption of undercooked ground beef and direct contact with cattle. Reducing the prevalence of STEC O157 in cattle on the farm would probably reduce both risks. Improved farm management practices, such as chlorination of drinking water and dietary changes prior to slaughter, may reduce the level of contamination among cattle [Reference Callaway38]. Promising future interventions in cattle include the use of sodium chlorate in drinking water and vaccination against STEC O157. The results of our study emphasize the need for continued food safety education of consumers and foodhandlers that result in safe handling and adequate cooking of ground beef. Undercooked ground beef is not the only source of E. coli O157 infections, but it remains one of the most important. Despite implementation of the USDA HACCP rule in 1996, there was no decline in the incidence of laboratory-confirmed STEC O157 infections in the FoodNet sites from 1996 to 2000. Similarly, there was no reported decline in the percent of ground beef from HACCP samples that were positive with STEC O157 to the end of 2000 [Reference Naugle39]. More recently documented declines in the incidence of STEC O157 in the FoodNet sites [40, 41] may be attributable to revisions in HACCP or changes in meat industry practices beginning in 2003. Continued efforts by industry, regulators, and consumers can reduce transmission from this source. Innovative approaches such as irradiation of ground beef could further reduce risk [Reference Osterholm and Potter42]. Strategies to reduce risk from sources other than ground beef are proving more difficult to develop and implement.
ACKNOWLEDGEMENTS
We thank the members of the CDC Emerging Infections Program Working Group for their contributions to this study and Drs Frederick Angulo, Robert Tauxe, Patricia Griffin, and Alecia Naugle for their thoughtful review of this manuscript. Financial support was provided by the Centers for Disease Control and Prevention National Center for Infectious Diseases, the U.S. Department of Agriculture Food Safety and Inspection Service, and the Food and Drug Administration Center for Food Safety and Applied Nutrition.
DECLARATION OF INTEREST
None.





