Introduction
Deep-sea hydrothermal-vent ecosystems utilize organic matter (OM) produced by chemoautotrophic microbes. Because prey–predator relationships provide fundamental knowledge about the ecology of organisms and relevant biogeochemistry, many studies have reported on the feeding ecology of megabenthos and chemoautotrophic microbes (e.g. Cavanaugh et al., Reference Cavanaugh, Gardiner, Jones, Jannasch and Waterbury1981; Felbeck, Reference Felbeck1985; Belkin et al., Reference Belkin, Nelson and Jannasch1986; Duperron et al., Reference Duperron, Bergin, Zielinski, Blazejak, Pernthaler, McKiness, DeChaine, Cavanaugh and Dubilier2006; Watsuji et al., Reference Watsuji, Yamamoto, Takaki, Ueda, Kawagucci and Takai2014). Meiofauna – organisms that pass through a 1-mm mesh sieve but are retained on a 63- or 32-μm mesh sieve (Giere, Reference Giere2009) – have received less attention at hydrothermal-vent habitats (Vanreusel et al., Reference Vanreusel, De Groote, Gollner and Bright2010), although meiofauna comprises a large biomass and plays essential roles in deep-sea ecosystems in general (e.g. Rex et al., Reference Rex, Etter, Morris, Crouse, McClain, Johnson, Stuart, Deming, Thies and Avery2006; Gooday et al., Reference Gooday, Nomaki and Kitazato2008). Among the deep-sea meiofauna, nematodes typically dominate the community, followed by harpacticoid copepods (e.g. Shimanaga et al., Reference Shimanaga, Nomaki, Suetsugu, Murayama and Kitazato2007). Vanreusel et al. (Reference Vanreusel, De Groote, Gollner and Bright2010) reviewed biogeographic data on nematodes associated with chemosynthetic environments in the deep sea and showed high similarity between vents and adjacent typical, non-vent sediment communities. Similar conclusions were reported from studies of vent meiofaunal communities at the East Pacific Rise (Gollner et al., Reference Gollner, Riemer, Arbizu, Bris and Bright2010b) and nematode communities at the hydrothermal-vent field and adjacent non-vent seafloor at the Izu-Ogasawara Arc, western North Pacific (Setoguchi et al., Reference Setoguchi, Nomaki, Kitahashi, Watanabe, Inoue, Ogawa and Shimanaga2014). These faunal similarities suggest low endemism in deep-sea vent meiofaunal species (cf. Gollner et al., Reference Gollner, Riemer, Arbizu, Bris and Bright2010b) and that current vent meiofauna invaded from adjacent sediments, a pattern presumably related to the absence of a planktonic life stage (cf. Vanreusel et al., Reference Vanreusel, Bossche and Thiermann1997). The low endemism of nematodes at hydrothermal vents suggests that most taxa do not rely solely on chemoautotrophic microbial production at vent chimneys.
The family Dirivultidae (Copepoda: Siphonostomatoida), an exceptionally abundant meiofaunal taxon at deep-sea hydrothermal vents, are free-living on hard substrates and often co-occur with aggregations of various sessile vent megafauna (Gollner et al., Reference Gollner, Ivanenko, Arbizu and Bright2010a). Their distribution patterns, their mouthpart morphologies, and observations of partly digested bacteria in their foregut, suggest tolerance of a wide range of hydrothermal fluid flux regimes and strong nutritional relationships with chemoautotrophic microbes inhabiting hydrothermal-vent chimneys (Dinet et al., Reference Dinet, Grassle and Tunnicliffe1988; Heptner & Ivanenko, Reference Heptner and Ivanenko2002; Gollner et al., Reference Gollner, Ivanenko, Arbizu and Bright2010a). Among Dirivultidae, Stygiopontius is the most diverse genus in hydrothermal-vent ecosystems (currently 28 accepted species; Walter & Boxshall, Reference Walter and Boxshall2021), implying a nutritional advantage in these ecosystems. Measurements of stable carbon isotopic composition (δ13C) and natural-abundance radiocarbon concentration confirmed that Stygiopontius senokuchiae described from the Izu-Ogasawara Arc hydrothermal-vent system (Uyeno et al., Reference Uyeno, Watanabe and Shimanaga2018) depends largely on chemoautotrophic bacteria on the chimney walls (Nomaki et al., Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019).
Stygiopontius copepods have a lecithotrophic mode of nutrition and are expected to have a planktic nauplius larval stage (Ivanenko et al., Reference Ivanenko, Martinez-Arbizu and Stecher2007). However, details about their ecologies, particularly the development and life cycles of dirivultid copepods, are still limited (Ferrari & Dahms, Reference Ferrari and Dahms2007; Ivanenko et al., Reference Ivanenko, Martinez-Arbizu and Stecher2007). The high genetic connectivity between dirivultid copepods at different hydrothermal-vent fields (Gollner et al., Reference Gollner, Stuckas, Kihara, Laurent, Kodami and Martinez Arbizu2016; Watanabe et al., Reference Watanabe, Senokuchi, Nomaki, Kitahashi, Uyeno and Shimanaga2021) suggests that they have high dispersal ability across several tens to thousands of kilometres. Indeed, abundant copepodites of dirivultids have been found in the water column above a vent field, enabling them to disperse to or colonize other vent fields (Gollner et al., Reference Gollner, Govenar, Arbizu, Mills, Le Bris, Weinbauer, Shank and Bright2015b). The coexistence of adult S. senokuchiae along with juveniles of different copepodite stages on the same hydrothermal-vent chimney walls at our study sites (Senokuchi et al., Reference Senokuchi, Nomaki, Watanabe, Kitahashi, Ogawa and Shimanaga2018) suggests that both adults and juveniles feed on organic matter on hydrothermal-vent chimneys.
The δ13C values of juvenile dirivultids were sometimes lower than those of adults (Nomaki et al., Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019). The adult δ13C values were almost identical to those of co-occurring Paralvinella spp., which rely for their nutrition on chemoautotrophic microbes that use the rTCA carbon fixation pathway. This implies that juvenile dirivultids get part of their nutrition other than from chemoautotrophic bacteria, whereas adult dirivultids may rely solely on such bacteria for their nutrition. These presumed changes in dirivultid feeding habits are expected to play important roles in their adaptation to hydrothermal-vent areas. However, the timing of their colonization on hydrothermal-vent chimneys and their dependence on chemoautotrophic bacteria during their development have not been investigated in detail.
In this study, we used transmission electron microscopy (TEM) to observe gut contents of Stygiopontius senokuchiae collected from a Myojin Knoll caldera hydrothermal-vent chimney. Different developmental stages of S. senokuchiae, from the first copepodite juvenile (CI) to adult, were observed in detail. The focus was mainly on the presence or absence of bacteria near mouth parts, in the oral tubes, and in the guts. For comparison, we also observed the gut contents of the co-occurring copepods Ectinosoma sp. 1 (Copepoda: Harpacticoida: Ectinosomatidae) and an unidentified calanoid copepod from the same hydrothermal-vent chimney. To highlight the uniqueness of bacteria ingestion by Stygiopontius among the order Siphonostomatoida, we further observed the gut contents of two other copepod species belonging to Siphonostomatoida: the parasitic copepod Hatschekia labracis (Copepoda: Siphonostomatoida: Hatschekiidae), which was attached to a scarbreast tuskfin (Choerodon azurio), and Asterocheres sp. 1 (Copepoda: Siphonostomatoida: Asterocheridae), which was attached to a spirastrellid sponge, both collected in Kagoshima Bay, Japan.
Furthermore, we measured stable carbon and nitrogen isotopic compositions of the fourth and fifth juvenile copepodite (CIV, CV) and adult stages of S. senokuchiae and adult stages of Ectinosoma sp. 1 to identify any changes in nutritional ecology. We speculated that among Siphonostomatoida, which consists mainly of parasitic copepods, Stygiopontius is specialized for bacterivory. We further hypothesized that the dominance of Stygiopontius copepods at hydrothermal-vent chimneys (Uejima et al., Reference Uejima, Nomaki, Senokuchi, Setoguchi, Kitahashi, Watanabe and Shimanaga2017; Senokuchi et al., Reference Senokuchi, Nomaki, Watanabe, Kitahashi, Ogawa and Shimanaga2018) can be explained by this specialized feeding, which is not observed in co-occurring Ectinosoma sp. 1 or Calanoida.
Materials and methods
Sampling
We sampled the detritus on a hydrothermal-vent chimney at Myojin Knoll caldera, located in the Izu-Ogasawara Arc of the western North Pacific (Honsho et al., Reference Honsho, Ura, Kim and Asada2016; Bernhard et al., Reference Bernhard, Nomaki, Shiratori, Elmendorf, Yabuki, Kimoto, Tsuchiya and Shimanaga2023), during cruise KT-18-3 of the RV ‘Shinsei-maru’ using the remotely operated vehicle (ROV) ‘Hyper-Dolphin 4500’ (Figure 1A). Hydrothermal-vent meiofaunal communities in the Izu-Ogasawara Arc area were previously described by Uejima et al. (Reference Uejima, Nomaki, Senokuchi, Setoguchi, Kitahashi, Watanabe and Shimanaga2017) and Senokuchi et al. (Reference Senokuchi, Nomaki, Watanabe, Kitahashi, Ogawa and Shimanaga2018).

Fig. 1. Images of sampling sites and copepod species examined in this study. (A) Hydrothermal-vent chimney at Myojin Knoll caldera. (B) Copepod specimens isolated from glutaraldehyde-fixed detritus samples from the hydrothermal-vent chimney. (C) Stygiopontius senokuchiae (adult female) stained with rose-Bengal. (D) Stygiopontius senokuchiae (adult male). (E) Ectinosoma sp. 1 (adult female) stained with rose-Bengal. (F) Ectinosoma sp. 1 (adult male) stained with rose-Bengal. (G) Spirastrellid sponge off Sakurajima, Kagoshima Bay, Japan. (H) Asterocheris sp. 1. (adult female) collected from the spirastrellid sponge. (I) Hatschekia labracis (adult female) attached to a gill filament of the scarbreast tuskfin Choerodon azurio in Kagoshima Bay. Scale bars: B, 500 μm; C, D, E, F, 100 μm; H, I, 500 μm.
The detritus on a Paralvinella polychaete colony on a hydrothermal-vent chimney was sampled with a suction sampler attached to the ROV with exchangeable sample containers equipped with a 30-μm mesh. On board, suction samples were split in half using a plankton splitter (RIGO Co. Ltd, Japan). One half of the sample was immediately fixed and preserved in 99.5% EtOH for study of molecular phylogeny. After removing excess seawater, the other half was preserved with 2.5% glutaraldehyde in filtered seawater at 4°C for TEM observations. In the laboratory on land, the samples fixed with glutaraldehyde were sieved with seawater, and the different developmental stages of S. senokuchiae and Ectinosoma sp. 1, and a single specimen of Calanoida, were isolated under a binocular microscope with the assistance of a differential interference microscope (Table 1, Figure 1B–F). The isolated specimens were put into 2.5% glutaraldehyde and kept at 4°C prior to further processing for TEM.
Table 1. Sampling details for copepod specimens observed by transmission electron microscopy (TEM) and notes on bacteria appearance in TEM images

A parasitic copepod Hatschekia labracis associated with the scarbreast tuskfin (Choerodon azurio), and Asterocheres sp. 1, which might be a commensal species attached to a spirastrellid sponge, were collected by divers using scuba gear in Kagoshima Bay, Japan (Figure 1G–I). Copepods were removed from the host animals and sorted under a dissection microscope (Table 1). Subsequently, the copepods were fixed with 2.5% glutaraldehyde and kept overnight at room temperature. They were then kept chilled at 4°C until processing.
Hydrothermal-vent copepods were also sampled for measurement of stable carbon isotopic composition (δ13C). During cruise NT13-09 of the RV ‘Natsushima’, suction samples were collected from the surface of a Paralvinella colony at Myojin-sho caldera for the isolation of S. senokuchiae (Table 2). During cruise NT14-06 of the RV ‘Natsushima’, suction samples were collected from the surface of a bacterial mat at Bayonnaise caldera for the isolation of Ectinosoma sp. 1 (Table 2). The suction samples were preserved in 99.5% EtOH after removing excess seawater. On land, copepods were isolated from the samples under a binocular microscope and kept in 99.5% EtOH prior to sample processing for δ13C analyses. Previous studies have reported that ethanol preservation sometimes causes a shift in δ13C values, typically within a few ‰ (e.g. Enge et al., Reference Enge, Wanek and Heinz2018). However, these isotopic shifts are relatively small compared with the δ13C variation in hydrothermal-vent ecosystems (typically around 30‰) (Bell et al., Reference Bell, Aquilina, Woulds, Glover, Little, Reid, Hepburn, Newton and Mills2016).
Table 2. Details of hydrothermal-vent copepods used for carbon isotopic composition analysis
TEM observations
Glutaraldehyde-fixed samples were post-fixed and embedded in resin following previously published protocols for TEM sample preparation (Nomaki et al., Reference Nomaki, Chikaraishi, Tsuchiya, Toyofuku, Suga, Sasaki, Uematsu, Tame and Ohkouchi2015, Reference Nomaki, LeKieffre, Meibom, Escrig, Yagyu, Richardson, Matsuzaki, Murayama, Geslin and Bernhard2018). In brief, the specimens were post-fixed with 2% osmium tetroxide in filtered artificial seawater for 2 h at 4°C, dehydrated in a graded ethanol series, and embedded in epoxy resin (Quetol 651; Nisshin EM, Tokyo, Japan). Embedded specimens were sectioned into several semi-thin sections (500 nm thick) using an ultramicrotome (Ultracut S; Leica, Germany). Sections were observed with an optical microscope (BX51; Olympus, Japan) to assess the condition and appearance of oral tubes or gut contents. Selected specimens were further sectioned into ultra-thin sections (60 nm thick) (Table 1). These sections were stained with 2% aqueous uranyl acetate and lead staining solution (0.3% lead nitrate and 0.3% lead acetate; Sigma-Aldrich, USA) and were observed by TEM (Tecnai G2 20; FEI, Hillsboro, OR, USA) at 200 kV.
Analysis of stable carbon isotopic composition
EtOH-fixed copepod samples were rinsed twice in the ultrapure water and then put directly into pre-cleaned tin capsules and dried at 60°C to remove water and to determine the dry weight. Typically 5–15 individuals were pooled into one sample to provide enough sample mass for analysis, with the exception of one adult female S. senokuchiae that had a large body size (Table 2). They were then decalcified with 0.1 M HCl and completely dried again, and then the tin capsules were sealed using pre-cleaned forceps.
The stable carbon isotopic compositions were determined using an isotope ratio mass spectrometer (Delta plus XP; Thermo Finnigan, Cambridge, MA, USA) connected to an elemental analyzer (FlashEA1112; Thermo Finnigan) through a continuous-flow interface (ConFloIII; Thermo Finnigan) following the procedure and using standard materials reported previously (Ogawa et al., Reference Ogawa, Nagata, Kitazato, Ohkouchi, Ohkouchi, Tayasu and Koba2010; Tayasu et al., Reference Tayasu, Hirasawa, Ogawa, Ohkouchi and Yamada2011; Isaji et al., Reference Isaji, Ogawa, Boreham, Kashiyama and Ohkouchi2020). We used six inter-laboratory verified organic standards (L-tyrosine, L-alanine, L-proline, L-glutamic acid, L-valine and nickel octaethylporphyrin; δ13C values of −34.17‰ to +0.18‰) as working standards to calibrate isotope ratios. The L-tyrosine listed above was used as a standard for the quantification of TOC contents. Errors for repeated analyses (from 5–20) of these standards were ±0.12‰ (SD, 1σ).
Results
TEM observations of mouthparts, oral tubes and gut contents
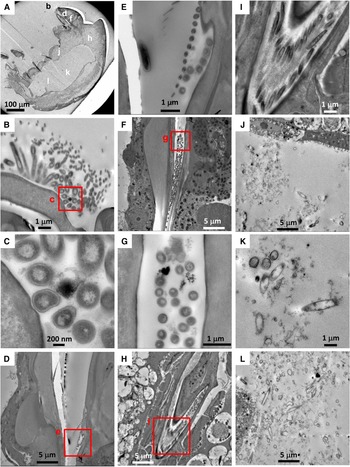
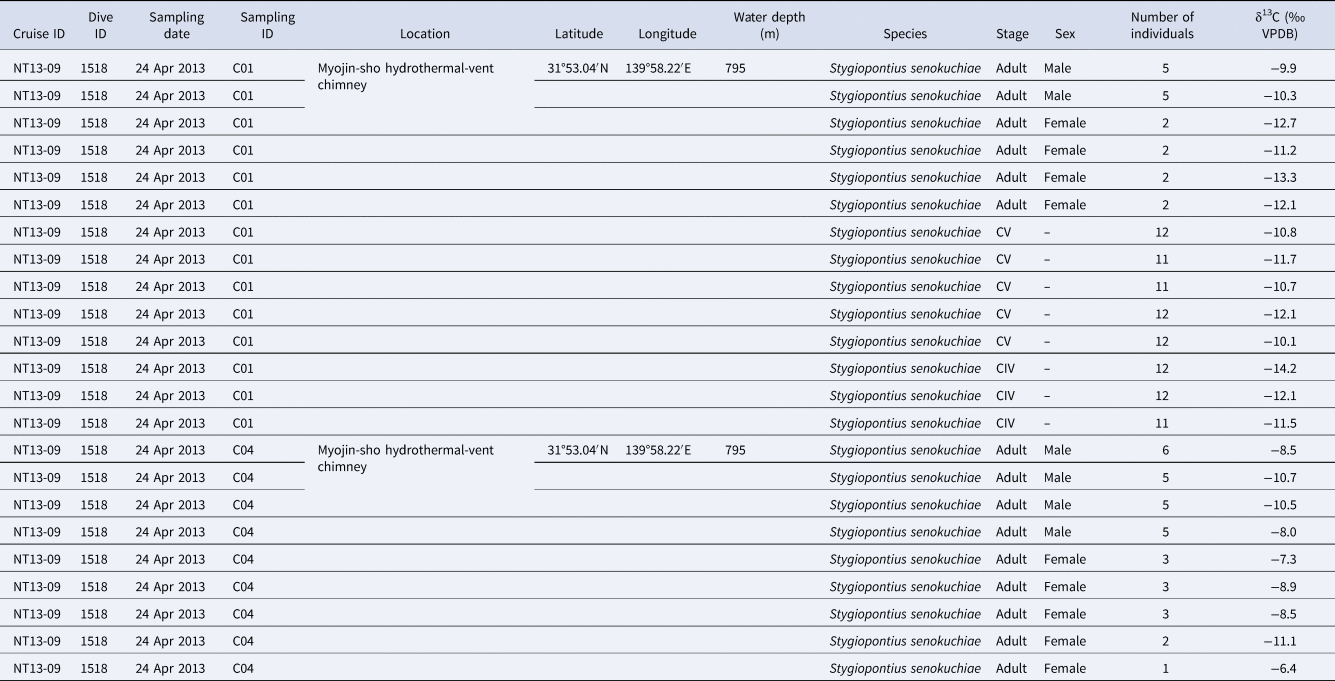
Among 14 individuals of Stygiopontius senokuchiae that were observed with TEM, 12 specimens showed degraded to almost intact bacteria in their oral tube or gut, or even around mouthparts in some specimens (Table 1, Figure 2). There were no obvious trends in bacterial appearance across different developmental stages of S. senokuchiae. A seta in the oral tube seems to prevent a backflow of bacteria toward the mouth (Figure 2D, E). Bacteria with spherical or elongated shapes sometimes completely filled the oral tube (Figure 2F–I). In the gut, bacteria were observed in many different states of degradation (Figure 2J–L). The abundance of bacteria in the gut varied between specimens (Table 1).
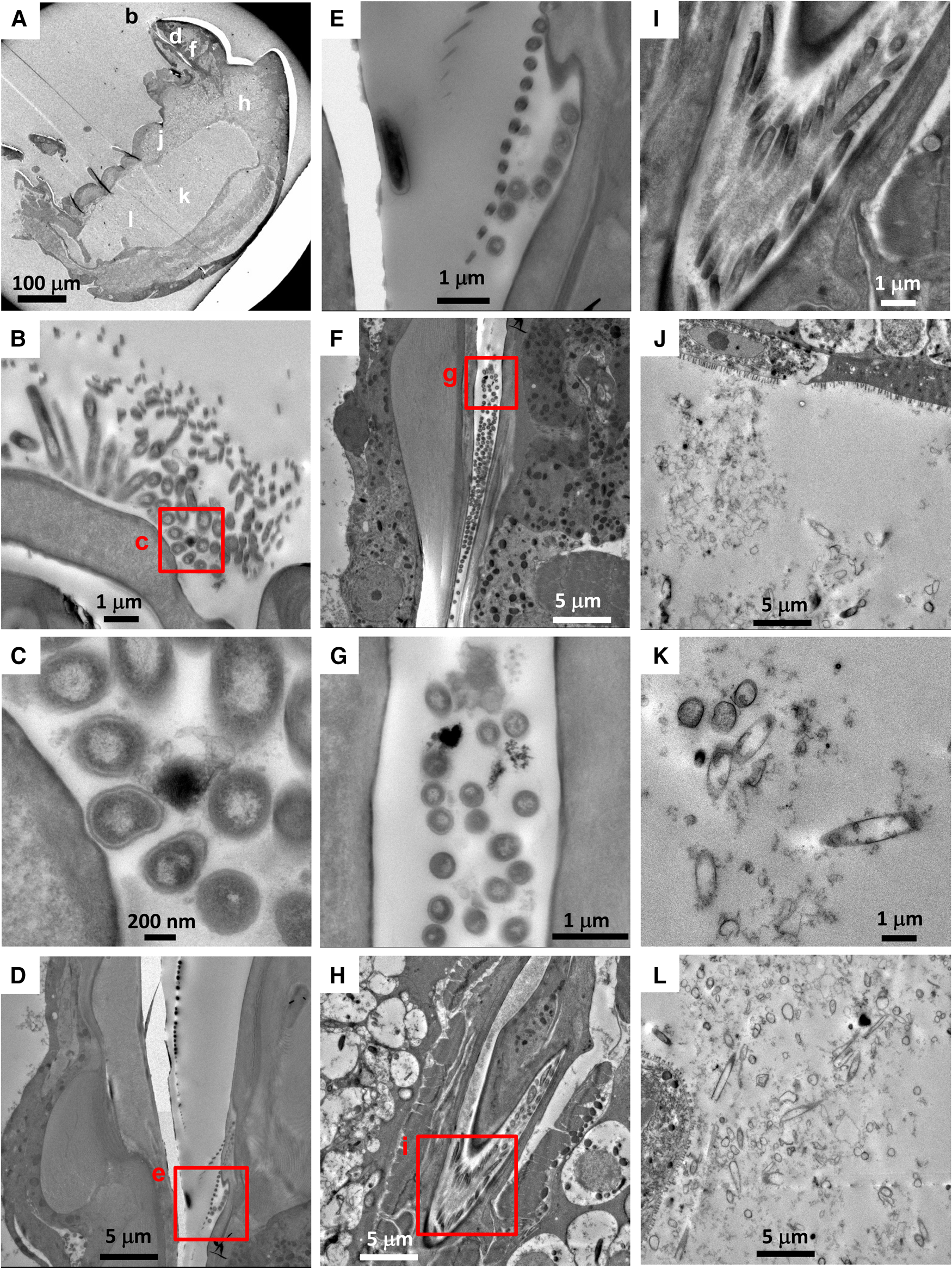
Fig. 2. Transmission electron microscopy (TEM) images of a stage CV male Stygiopontius senokuchiae (specimen C5_3 in Table 1) collected from Myojin Knoll caldera. (A) Low magnification view showing entire specimen and the regions shown at greater magnification in B–L. (B) Close-up view of mouthparts showing putative bacteria surrounded by setae. (C) Close-up view of rectangle (c) in image B showing putative bacteria. (D) Oral tube showing spherical bacteria inside. (E) Close-up view of rectangle (e) in image D showing bacteria trapped by setae. (F) Abundant spherical bacteria in the oral tube. (G) Close-up view of rectangle (g) in image F showing spherical bacteria. (H) Elongated bacteria filling the oesophagus. (I) Close-up view of rectangle (i) in image G showing abundant elongated bacteria. (J, K, L) Gut contents showing degraded spherical and elongated bacteria.
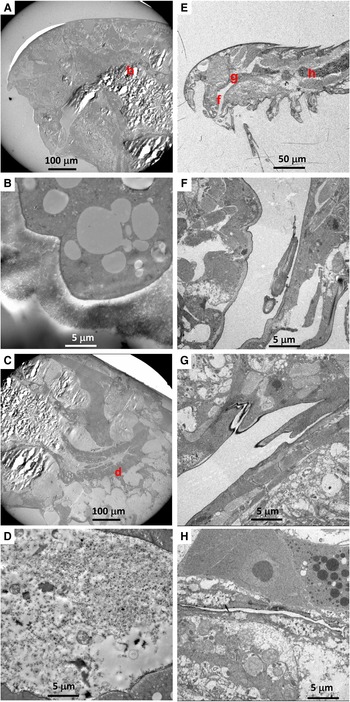
There were no visible bacteria in the co-occurring calanoid (N = 1) or Ectinosoma sp. 1 (N = 3), regardless of the location observed (i.e. mouthparts, oral tubes and guts) (Figure 3). The calanoid had longer villi than S. senokuchiae with detritus of unidentifiable origin in their gut (Figure 3B), and their guts were filled with amorphous degraded contents (Figure 3D). The oesophagus and gut of Ectinosoma sp. 1 were almost empty (Figure 3F–H).
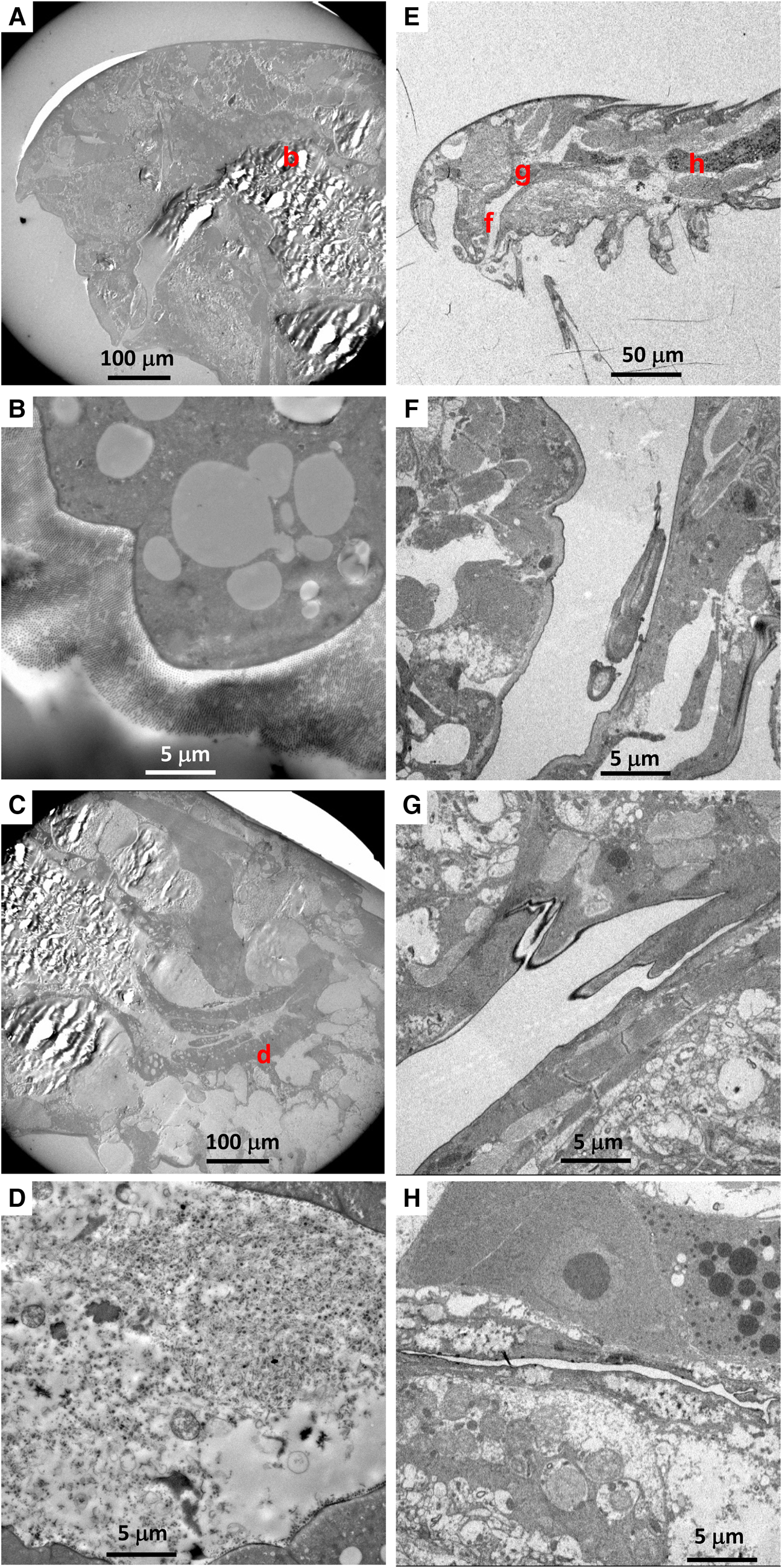
Fig. 3. (A–D) Transmission electron microscopy (TEM) images of a calanoid copepod collected from Myojin Knoll caldera. (A) Low magnification view of anterior, showing position of image B. (B) Intestinal wall filled by dense villi. (C) Low-magnification view of posterior, showing position of image D. (D) Amorphous degraded contents in the hindgut. (E–H) TEM images of an adult female Ectinosoma sp. 1 collected from Myojin Knoll caldera (specimen Kai_1_2 in Table 1). (E) Low-magnification view showing anterior and regions shown at higher magnification in F–H. (F, G) Empty oesophagus. (H) No obvious gut contents.
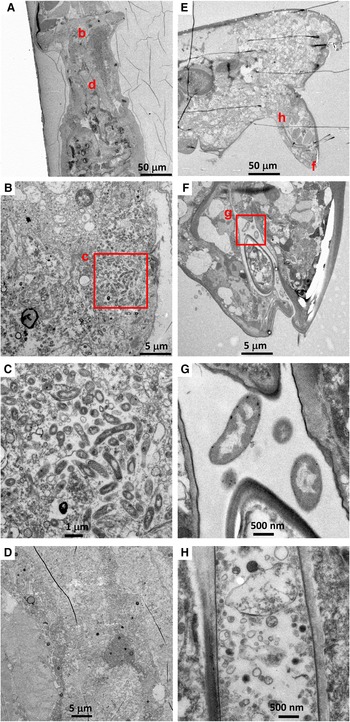
Among six Hatschekia labracis specimens observed, two had abundant bacteria in their guts (Figure 4B, C), and one (specimen Kai_2_3) had a single bacterial cell in its oral tube (image not shown). In general, their gut contents consisted of amorphous, degraded organic matter (Figure 4D).

Fig. 4. (A–D) Transmission electron microscopy (TEM) images of an adult female Hatschekia labracis attached to a scarbreast tuskfin Choerodon azurio collected from Kagoshima Bay, Japan (specimen Kai_2_1 in Table 1). (A) Low-magnification view of anterior showing positions of images B and D. (B) Aggregation of bacteria found in the oesophagus. (C) Close-up view of rectangle (c) in image B showing elongated bacteria. (D) Amorphous, degraded contents in the guts. (E–H) TEM images of an adult male Asterocheris sp. 1 attached to a spirastrellid sponge collected from Kagoshima Bay (specimen Kai_4_1 in Table 1). (E) Low-magnification view of anterior, showing positions of images F and H. (F) Close-up view of mouthparts and oral tube. (G) Close-up view of rectangle (g) in image F showing bacteria in the oral tube. (H) Degraded contents in the oesophagus.
There were no bacterial cells or remnants in the gut contents of six Asterocheres sp. 1 individuals, however two specimens had a few bacterial cells in their oral tube (Figure 4F, G). The content of their gut or oesophagus was mainly amorphous degraded organic matter of unknown origin (Figure 4H).
Stable carbon isotopic composition
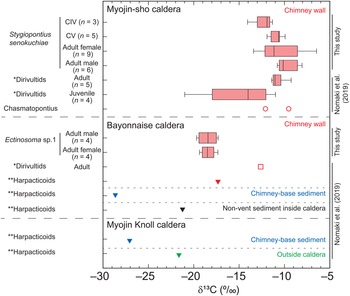
The δ13C values of S. senokuchiae ranged between −14.2 and −9.9‰ (Table 2), which is a range similar to previously reported values for dirivultid copepods at Myojin-sho caldera (Nomaki et al., Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019). There were no significant differences across developmental stages, or between sexes of adults of this species (Kruskal–Wallis test, P > 0.05, Figure 5). Multiple comparisons did not detect any significant difference between any pair of copepodite stages of the species, either (Tukey tests, Ps > 0.05). Ectinosoma sp. 1 had consistently lower δ13C values (–19.9 to −17.5‰) than S. senokuchiae. There were no significant differences in δ13C values between adult females and males (Wilcoxon rank sum exact test, P > 0.05, Figure 5).

Fig. 5. Stable carbon isotope ratios (δ13C) of Stygiopontius senokuchiae collected from Myojin-sho, and of Ectinosoma sp. 1 collected from Bayonnaise Knoll. Box-and-whisker plot denotes minimum, maximum, lower and upper quartiles, and median of δ13C values of mixtures of 2–15 individuals for each category in cases with more than 3 replicates (Table 2). The δ13C values of copepods previously reported at these sites (Nomaki et al., Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019) are plotted together for comparison. *Dirivultids, reported as ‘Dirivultidae: mainly consist of Stygiopontius specimens’; **Harpacticoids, reported as ‘Other copepods: other copepods than Dirivultidae, mainly (~90%) consist of harpacticoids’ in Supplementary Table S3 in Nomaki et al. (Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019). CIV, copepodite IV stage; CV, copepodite V stage.
Discussion
Bacterivory of S. senokuchiae
Among three copepod species examined at a hydrothermal-vent field, Stygiopontius senokuchiae almost consistently showed bacterial cells in its oral tube or gut contents (Table 1). In some cases, the oral tube was filled with spherical and elongated bacterial cells (Figure 2F, I). Guts were also filled with relatively intact to mostly degraded bacterial cells (Figure 2J–L). The existence of degraded bacteria supports the idea that S. senokuchiae digests ingested bacteria and gains nutrition from them. The presence of at least two morphotypes of bacterial cells suggests that S. senokuchiae does not selectively ingest specific bacterial species from the flourishing bacteria on hydrothermal-vent chimneys. Limen et al. (Reference Limen, Stevens, Bourass and Juniper2008) reported the selective ingestion of bacteria by Stygiopontius quadrispinosus, which has a small mouth opening. Stygiopontius senokuchiae, which has a similar mouth size, seems to ingest the bacterial mat in bulk without any obvious selection, probably by grazing (Heptner & Ivanenko, Reference Heptner and Ivanenko2002).
There were no clear trends in gut contents or δ13C values of S. senokuchiae with developmental stage, at least from CI to adult, in terms of gut contents (Table 1), or from CIV to adult, in terms of δ13C values (Figure 5). After settling on a hydrothermal-vent chimney in the CI stage (Ivanenko et al., Reference Ivanenko, Martinez-Arbizu and Stecher2007), S. senokuchiae may continuously utilize chemoautotrophic bacteria as a nutritional source. It should be noted that one sample of juvenile Dirivultidae, which consisted mainly of Stygiopontius, had δ13C values as low as −21.2‰ (Nomaki et al., Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019; Figure 5). This might be explained by the contribution from photosynthetic organic matter, or mixtures of chemoautotrophic bacteria that use CBB and rTCA carbon-fixation pathways with different degrees of isotope fractionation (House et al., Reference House, Schopf and Stetter2003). Although we do not have the exact data for the composition by developmental stage measured by Nomaki et al. (Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019), it is possible that their samples contained smaller copepodite stages such as CI–CIII.
At venting chimneys, high in situ primary chemoautotrophic production is limited to areas of active hydrothermal flow, and those environmental stresses have a stronger negative effect on smaller meiofaunal species than on macrofauna (Gollner et al., Reference Gollner, Govenar, Fisher and Bright2015a). Among meiofaunal copepods, dirivultids appear to be exceptionally successful in those extreme environments, probably using their larger body sizes (adult females are usually >1 mm long) and higher swimming speeds to avoid sudden environmental changes (Gollner et al., Reference Gollner, Govenar, Fisher and Bright2015a). On the other hand, their youngest copepodites are much smaller (<0.3 mm in this study) and probably slower than the adults. Thus, it is plausible that the smallest Stygiopontius copepodites avoid areas with the most active hydrothermal eruptions and utilize detritus derived from photosynthetic organic matter deposited ubiquitously on chimneys, although they also ingest bacteria (Table 1). It should also be noted that gut contents analysis and biomass δ13C values reflect feeding habits at different time scales; gut contents show information about food items at a scale from hours to a few days, whereas δ13C values represent the average signature of food items over days to months, depending on turnover rates and the timing of moults.
Co-occurring Calanoida and Ectinosoma sp. 1 did not show any signs of bacterial ingestion (Table 1, Figure 3). Because calanoids are planktonic or epibenthic, the specimen found in our sample may have drifted from an adjacent ecosystem (Heptner & Ivanenko, Reference Heptner and Ivanenko2002) and thus relied on nutritional sources other than bacteria. The empty gut contents of Ectinosoma sp. 1 might reflect regurgitations of gut contents during sample recovery or the process of glutaraldehyde fixation, making interpretation difficult. However, their δ13C values (−19.9 to −17.5‰) do not indicate that their main nutritional source is chemoautotrophic bacteria fixing carbon through the rTCA pathway as observed for Stygiopontius (Figure 5; Nomaki et al., Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019). Furthermore, Nakasugi et al. (Reference Nakasugi, Shimanaga, Nomaki, Watanabe, Kitahashi, Motomura and Iseda2022) found that the distributional patterns of harpacticoids, including Ectinosomatidae, were not related to the availability of chemoautotrophic bacteria on hydrothermal-vent chimneys. The δ13C values of Ectinosoma in this study are attributed to the large contribution of photosynthetic organic matter, as observed in harpacticoids at surrounding non-vent areas, or to mixtures of chemoautotrophs (Nomaki et al., Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019). Because the majority of harpacticoid species at vent sites also inhabit surrounding non-vent sites and are thought to have colonized vent sites from there (Gollner et al., Reference Gollner, Riemer, Arbizu, Bris and Bright2010b, Reference Gollner, Govenar, Martinez Arbizu, Mullineaux, Mills, Le Bris, Weinbauer, Shank and Bright2020), patterns of δ13C values with developmental stage may shed light on their mechanisms for adapting to hydrothermal-vent chimneys.
TEM observations and carbon isotope ratio measurements of S. senokuchiae were carried out using samples from different hydrothermal vents – Myojin-sho and Myojin Knoll calderas – which are about 20 km apart. The δ13C values of Dirivultidae, mainly consisting of S. senokuchiae, were similar at Myojin-sho and the neighbouring seamount Bayonnaise Knoll (Figure 5; Nomaki et al., Reference Nomaki, Uejima, Ogawa, Yamane, Watanabe, Senokuchi, Bernhard, Kitahashi, Miyairi, Yokoyama, Ohkouchi and Shimanaga2019). Furthermore, haplotype network analysis of this species revealed that populations at those knolls have almost the same genetics (Watanabe et al., Reference Watanabe, Senokuchi, Nomaki, Kitahashi, Uyeno and Shimanaga2021). These observations suggest that all S. senokuchiae in this hydrothermal-vent area have comparable ecologies and similar stable carbon isotopic compositions.
Bacterivory in the order Siphonostomatoida
We presume that both Hatschekia labracis and Asterocheres sp. 1 are parasites of Choerodon azurio and the spirastrellid sponge, respectively, because the amorphous contents in the oral tube and gut of both copepods suggest the digestion of blood plasma or other components of the host organisms. We observed putative bacterial cells in the oral tube or gut (Figure 4) of two or three specimens among the six examined for each species (Table 1). Although the origins of the observed bacteria are unknown, it is possible that these copepods accidentally suck bacterial cells from the host or from the surrounding seawater, and those cells could be part of their nutrition. However, bacteria were not observed in the gut of either Mihbaicola sakamakii (Hatschekiidae) or Phrixocephalus cincinnatus (Pennellidae), which are other members of the order Siphonostomatoida (Perkins, Reference Perkins1994; Hirose & Uyeno, Reference Hirose and Uyeno2014), implying that nutritional dependence on bacteria is not common within this order.
Heptner & Ivanenko (Reference Heptner and Ivanenko2002) mentioned that the 47 species belonging to the order Siphonostomatoida (i.e. 45 species in nine genera of the family Dirivultidae and one species each in the families Ecbathyriontidae and Asterocheridae) feed on bacteria. Furthermore, they speculated that the order Siphonostomatoida was originally parasitic, and the Dirivultidae evolved to graze bacteria on hydrothermal-vent chimneys. Our gut content observations and carbon isotopic composition analyses confirmed that S. senokuchiae is highly specialized to feed on chemoautotrophic bacteria at CI through adult stages, which is apparently different from co-occurring copepods and other siphonostomatoids. However, the morphological and nutritional adaptations of Dirivultidae, including Stygiopontius, are still largely unknown. Further investigations of the general trends in nutritional dependency of Siphonostomatoida should provide a clearer idea of how the Dirivultidae evolved and adapted to hydrothermal-vent fields as bacterivores.
Conclusions
Stygiopontius senokuchiae, a hydrothermal-vent-specific dirivultid copepod, ingests bacterial cells on hydrothermal-vent chimneys from copepodite stage I to the adult stage. We found no clear differences in feeding habits between developmental stages or between males and females from gut content observations and stable carbon isotope measurements. Neither a coexisting calanoid species nor Ectinosoma sp. 1 showed any bacterial ingestion, implying that these copepods are temporarily distributed on the hydrothermal-vent chimneys or utilize food sources other than chemoautotrophic bacteria. Although two species of shallow-water siphonostomatoids sometimes possessed bacterial cells in their oral tube or gut, bacterivory seems to be specific for the family Dirivultidae among the order Siphonostomatoida. Further investigations are necessary to address the adaptation of Dirivultidae to the organic-rich, isolated, hydrothermal-vent habitat.
Acknowledgements
We are grateful to the crews and scientists of RV ‘Natsushima’ and the ROV ‘Hyper-Dolphin’ of the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) during cruises NT13-09 and NT14-06, and RV ‘Shinsei-maru’ and the ‘Hyper-Dolphin 4500’ of JAMSTEC during cruise KS-18-3. We thank Ms Kaya Oda for her help with sample preparation. We also thank an anonymous reviewer and the editors, who provided helpful comments on an earlier version of this manuscript.
Author contributions
HN and MS designed the study, KK and YM isolated and identified hydrothermal-vent copepods, DU collected and identified shallow-water copepods, AT and HN performed TEM observations, NOO and NO measured isotopic compositions, and all authors contributed to data interpretation and discussions. HN, MS and DU drafted the manuscript, and all authors edited and approved the manuscript.
Financial support
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) program (grant numbers JP26440246 and JP19H03305 to MS).
Conflict of interest
The authors declare none.