Introduction
Zoonotic diseases are infections that are transmitted among vertebrate animal populations and human societies (Carmena & Cardona, Reference Carmena and Cardona2014). About one-fifth of zoonoses belong to parasitic infections (Torgerson & Macpherson, Reference Torgerson and Macpherson2011). Cystic echinococcosis (CE) is a zoonotic neglected tropical disease caused by larval stages (metacestodes) of Echinococcus granulosus, the smallest cestode of medical importance and the most common reason for contagion in production animals (Cardona & Carmena, Reference Cardona and Carmena2013). Echinococcosis is a cyclozoonosis that employs two vertebrate hosts to maintain its life cycle (Cardona & Carmena, Reference Cardona and Carmena2013; Khademvatan et al., Reference Khademvatan, Yousefi, Rafiei, Rahdar and Saki2013). It mostly occurs as a domestic cycle in cattle- and sheep-raising countries, although a sylvatic cycle among wild carnivore/wild herbivore populations also exists (Cardona & Carmena, Reference Cardona and Carmena2013; Carmena & Cardona, Reference Carmena and Cardona2014). Being in the small intestine of canids (definitive hosts), adult worms lay eggs or detach gravid proglottids, which are excreted with the faeces and contaminate the environment, such as grazing pastures; infection of domestic and wild ungulates (intermediate hosts) following ingestion of eggs would then occur by chance. In the intermediate hosts, metacestodes frequently migrate to the liver and lung parenchyma and develop into hydatid cysts (Cardona & Carmena, Reference Cardona and Carmena2013). Humans are considered to be dead-end intermediate hosts for unilocular echinococcosis. Disease consequences vary from asymptomatic to development of hydatid cysts in the liver, lung and any other parts of the body (McManus et al., Reference McManus, Zhang, Li and Bartley2003). The annual global occurrence of this parasitic complication ranges between 1 and 200 cases per 100,000 individuals (Pawłowski et al., Reference Pawłowski, Eckert, Vuitton, Ammann, Kern, Craig, Dar, Rosa, Filice and Gottstein2001). Furthermore, in various medical centres of Iran, 1.18–3 cases per 100,000 persons have been identified during surgical operations (Fasihi Harandi et al., Reference Fasihi Harandi, Budke and Rostami2012). CE has been proved to be an important human and animal health concern in many rural regions of western and central Asia, China, Australia, North and East Africa, the Mediterranean territory and South America (Jenkins et al., Reference Jenkins, Romig and Thompson2005; Jenkins, Reference Jenkins2006; Magambo et al., Reference Magambo, Njoroge and Zeyhle2006; Moro & Schantz, Reference Moro and Schantz2006a; Romig et al., Reference Romig, Dinkel and Mackenstedt2006; Sadjjadi, Reference Sadjjadi2006; Wang et al., Reference Wang, Wang and Liu2008; Dakkak, Reference Dakkak2010; McManus, Reference McManus2010; Romig et al., Reference Romig, Omer, Zeyhle, Huttner, Dinkel, Siefert, Elmahdi, Magambo, Ocaido, Menezes, Ahmed, Mbae, Grobusch and Kern2011).
There is an inextricable link among human health, animal and environmental health. This association is well-represented by revitalization of infectious zoonoses such as CE in recent years, which accounts for a major worldwide burden. So, characterization of CE prevalence among animal species involved in the transmission cycle is of utmost medical and veterinary importance. Up to now, there have been many research papers on the prevalence of CE in animal hosts in Iran, but the lack of collective data on this subject has led us to design a systematic review and meta-analysis in the country.
Methods
Study area
Covering a wide area in the Middle East (1,648,195 km2), Iran is the 18th largest country worldwide, with a population of approximately 80 million in 2015, located between 25°3′ and 39°47′N, and 44°5′ and 63°18′E, and bordering Iraq and Turkey in the west, Afghanistan and Pakistan in the east, the Persian Gulf and Oman Sea in the south, as well as Azerbaijan, Armenia and Turkmenistan in the north. Except for a small region on the margin of the Caspian Sea coast with a considerable annual rainfall and covered by dense vegetation, the general climate of Iran is hot and dry, forming the Iranian plateau. It is one of the world's most mountainous countries, its landscape dominated by rugged mountain ranges that separate various basins or plateaux from each other. The populous western part is the most mountainous, with ranges such as the Caucasus, Zagros and Alborz Mountains. Lower temperatures, severe winters and heavy snowfalls exist in the Zagros basin, while in the central and eastern basins there is an arid climate because of high-altitude mountain ranges in the western and northern parts. These mountain ranges are so high that rain clouds cannot reach the central and eastern basins. Annual precipitation is 680 mm in the eastern part of the plain and more than 1700 mm (66.9 inches) in the western part (https://en.wikipedia.org/wiki/Iran).
Search strategy
In order to appraise the prevalence of cystic echinococcosis in Iran, we conducted a systematic review depending on English and Persian online-released literature. The search procedure was performed in five English databases (PubMed, Scopus, Web of Science, Science Direct and Google Scholar) and four Persian databases (Magiran, Iran Medex, Iran Doc and Scientific Information Database) from January 1990 to December 2015 and the medical subject headings (MeSH) terms used for this review were as follows: ‘Echinococcus granulosus’, ‘Echinococcosis’, ‘Hydatid’, ‘Hydatic’, ‘Epidemiology’, ‘Prevalence’ and ‘Iran’, alone or in combination.
Study selection and data extraction
In accordance with the inclusion criteria, cross-sectional studies on the prevalence of CE in definitive and/or intermediate hosts were included. Two autonomous reviewers carefully read and revisited all included papers. Also, incoherence among studies was obviated by discussion and consensus. A data extraction form was designed on the basis of the kind of animals, infected organs (in the case of intermediate hosts), number of studies and positive cases, as well as geographical region, and was used for the accurate information needed. Meanwhile, two risk factors were considered for cases of human echinococcosis, i.e. place of residence and gender. We performed a current meta-analysis according to PRISMA guidelines (Preferred Reporting Items for Systematic reviews and Meta-Analysis) (Moher et al., Reference Moher, Liberati, Tetzlaff, Altman and Group2010).
Meta-analysis
The meta-analysis procedure was performed as described previously (Foroutan-Rad et al., Reference Foroutan-Rad, Khademvatan, Majidiani, Aryamand, Rahim and Malehi2016a, Reference Foroutan-Rad, Majidiani, Dalvand, Daryani, Kooti, Saki, Hedayati-Rad and Ahmadpourb; Foroutan et al., Reference Foroutan, Khademvatan, Majidiani, Khalkhali, Hedayati-Rad, Khashaveh and Mohammadzadeh2017; Khademvatan et al., Reference Khademvatan, Foroutan, Hazrati-Tappeh, Dalvand, Khalkhali, Masoumifard and Hedayati-Rad2017). Briefly, we estimated the prevalence and 95% confidence interval (CI) of animal echinococcosis for every study that was included. The findings of the meta-analysis were represented by a forest plot diagram, which shows estimates of prevalence and their respective CIs of individual studies with the summary measure. Cochran's Q and I 2 statistics were used for evaluation of heterogeneity and inconsistency, respectively. In addition, small study effects and publication bias were calculated using funnel plot diagrams based on Egger's regression test. According to outcomes of the heterogeneity test, either Der Simonian and Laird's random-effects method or Mantel–Haenszel's fixed-effects method were employed to pool the estimations. Furthermore, studies were categorized in terms of geographical areas (north, east, west, south and centre) of Iran and total prevalence was appraised in five different regions.
Results
Definitive hosts
Based on the review of literature and within the frame of inclusion criteria, 37 out of 5363 records on the prevalence of CE in definitive hosts qualified for this systematic review and meta-analysis (fig. 1 and supplementary table S1).

Fig. 1. Flowchart describing the study design process.
Table 1 illustrates the findings obtained from the literature search as well as specifications of included studies. It shows the number of canids that were tested in terms of echinococcosis from 1990 until 2015 in various geographical foci of the country (table 1).
Table 1. Results of included studies for definitive hosts.

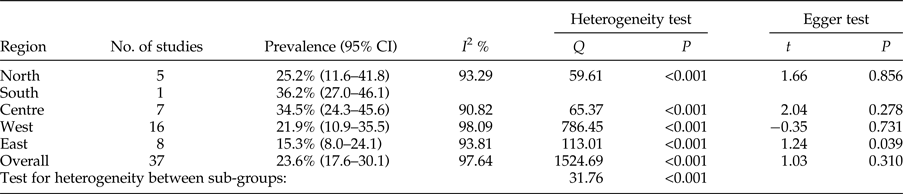
The calculated total prevalence of Echinococcus infection in definitive hosts was 23.6% (95% CI = 17.6–30.1%). Most canids were found to be infected in the south of Iran, 36.2% (95% CI = 27.0–46.1%), but the fewest cases of infection were detected in the east, 15.3% (95% CI = 8.0–24.1%) (table 2). Publication bias was detected using Egger's regression test, but didn't have a significant impact on total prevalence estimates in definitive hosts (P = 0.31) (table 2).
Table 2. Geographical distribution and prevalence of included studies for definitive hosts.
Meta-regression results showed that there is a statistically significant correlation between total prevalence and both year of studies and sample size (P = 0.04 for both) (figs 2 and 3).

Fig. 2. Meta-regression plot of prevalence of Echinococcus granulosus in definitive hosts, based on year of studies.
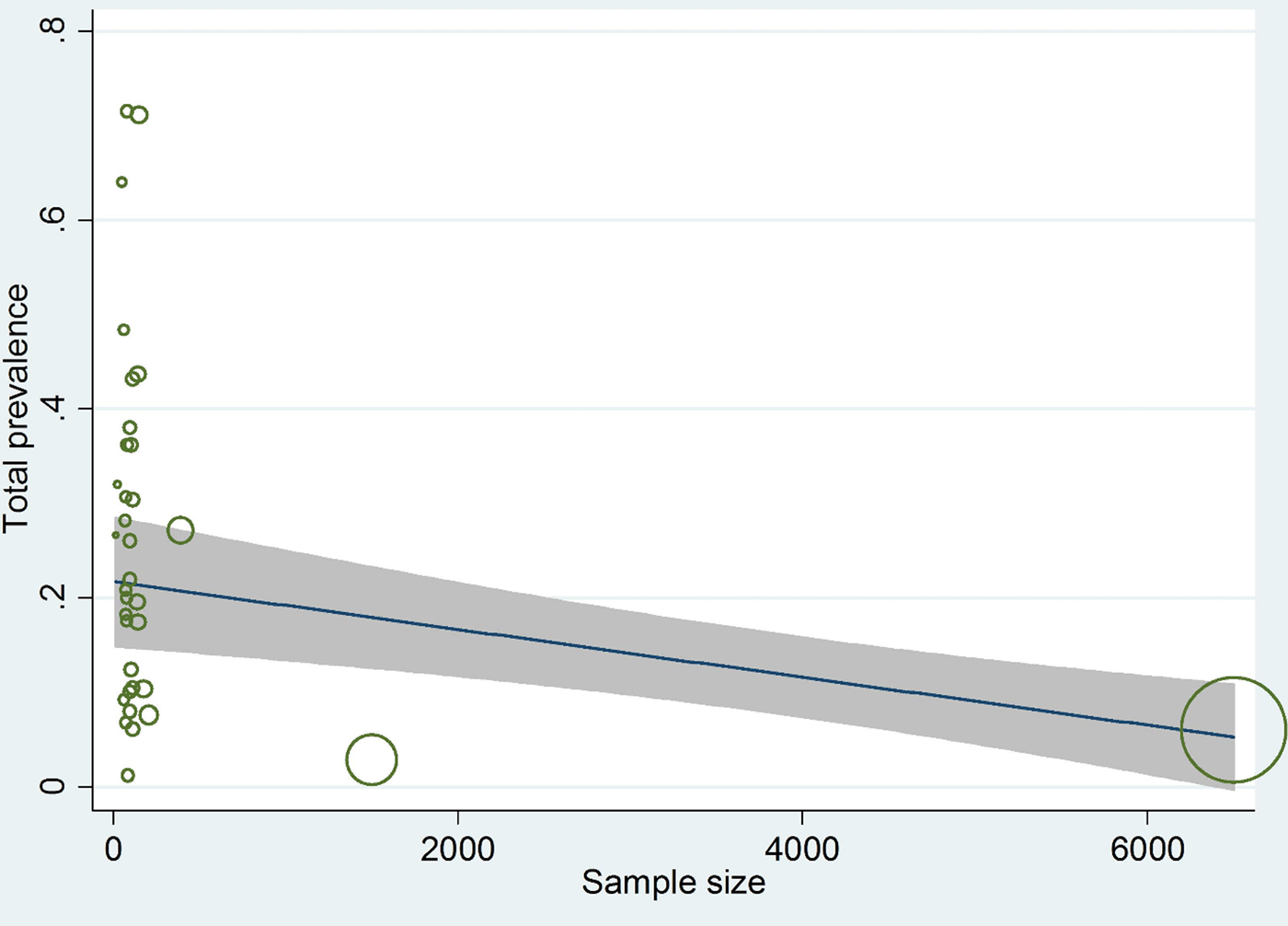
Fig. 3. Meta-regression plot of prevalence of Echinococcus granulosus in definitive hosts, based on sample size.
Animal intermediate hosts
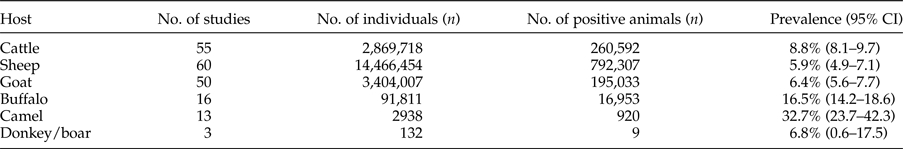
In total, 90 studies in the field of CE prevalence in animal intermediate hosts were gathered (fig. 1 and supplementary table S2). Database results, number of studies and positive cases are summarized in table 3.
Table 3. Results of included studies for animal intermediate hosts.
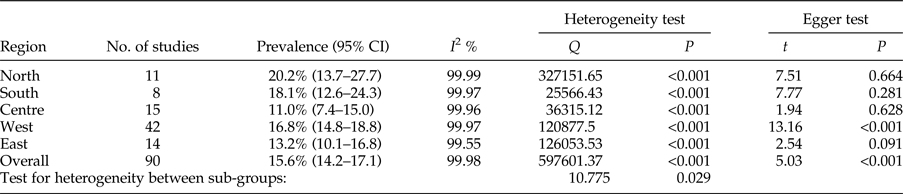
The total prevalence of infection in animal intermediate hosts was assessed as 15.6% (95% CI = 14.2–17.1%). In herbivores, the highest and lowest prevalence rates were observed in the north, 20.2% (95% CI = 13.7–27.7%), and in central Iran, 11.0% (95% CI = 7.4–15.0%), respectively (table 4). Egger's regression test revealed statistically very significant publication bias (P < 0.001) (table 4).
Table 4. Geographical distribution and prevalence of included studies for animal intermediate hosts.
In compliance with the meta-regression, there was a remarkable relationship between weighted prevalence and sample size (P = 0.050), although prevalence didn't show any substantial correlation with year of studies (P = 0.29) (figs 4 and 5).
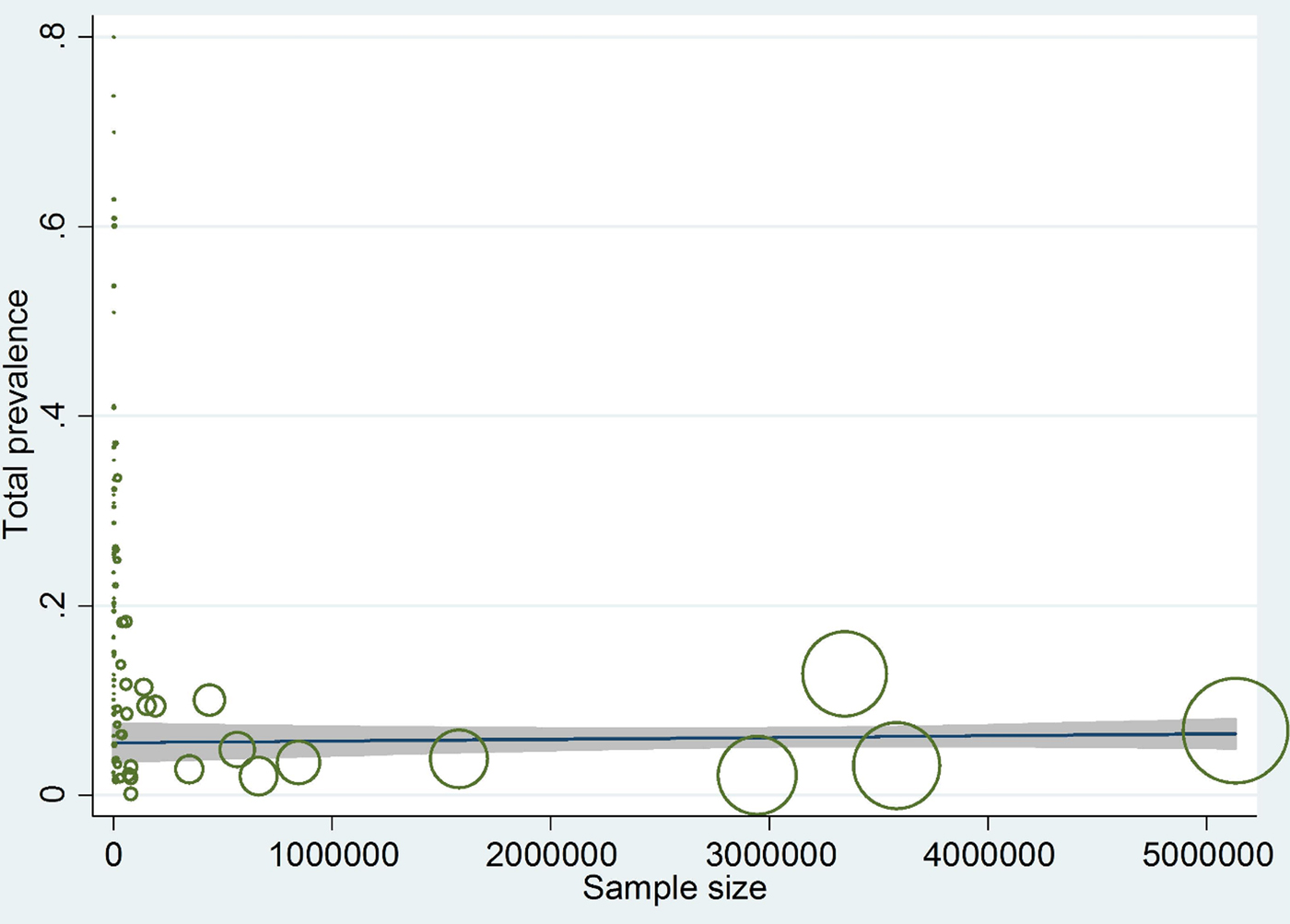
Fig. 4. Meta-regression plot of prevalence of Echinococcus granulosus in animal intermediate hosts, based on sample size.

Fig. 5. Meta-regression plot of prevalence of Echinococcus granulosus in animal intermediate hosts, based on year of studies.
Human intermediate hosts
In this section, 33 studies were included (fig. 1 and supplementary table S3). Requisite data were extracted and studies were evaluated in terms of residence and gender risk factors (table 5). More people were infected in rural territories, with a prevalence of 4.1% (95% CI = 1.6–7.8%), than in urban areas. Additionally, mostly females were affected with CE, 4.5% (95% CI = 2.9–6.5%). However, prevalence was not significantly associated with residence or gender (P = 0.172; P = 0.718) (table 5 and supplementary tables S4 and S5).
Table 5. Results of included studies for human hosts.

The overall prevalence of cystic echinococcosis in human hosts of Iran was calculated as 4.2% (95% CI = 3.0–5.5%). Geographically, the highest prevalence was found in the south, with 5.8% (95% CI = 3.2–9.2%), while the lowest prevalence was appointed to the central regions of the country, with 2.2% (95% CI = 1.3–3.4%) (table 6). Based on Egger's regression, publication bias did not have a considerable effect on total prevalence in human hosts (P = 0.4) (table 6).
Table 6. Geographical distribution and prevalence of included studies for human hosts.
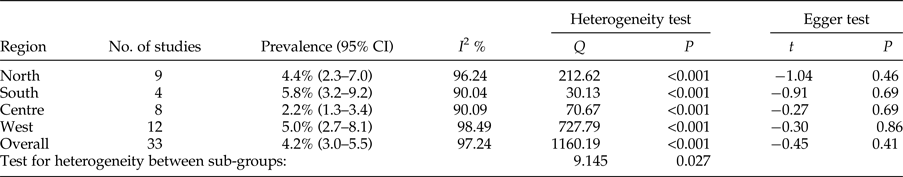
Meta-regression between prevalence and both sample size and year of studies showed no significant correlation (P = 0.424; P = 0.994) (figs 6 and 7).
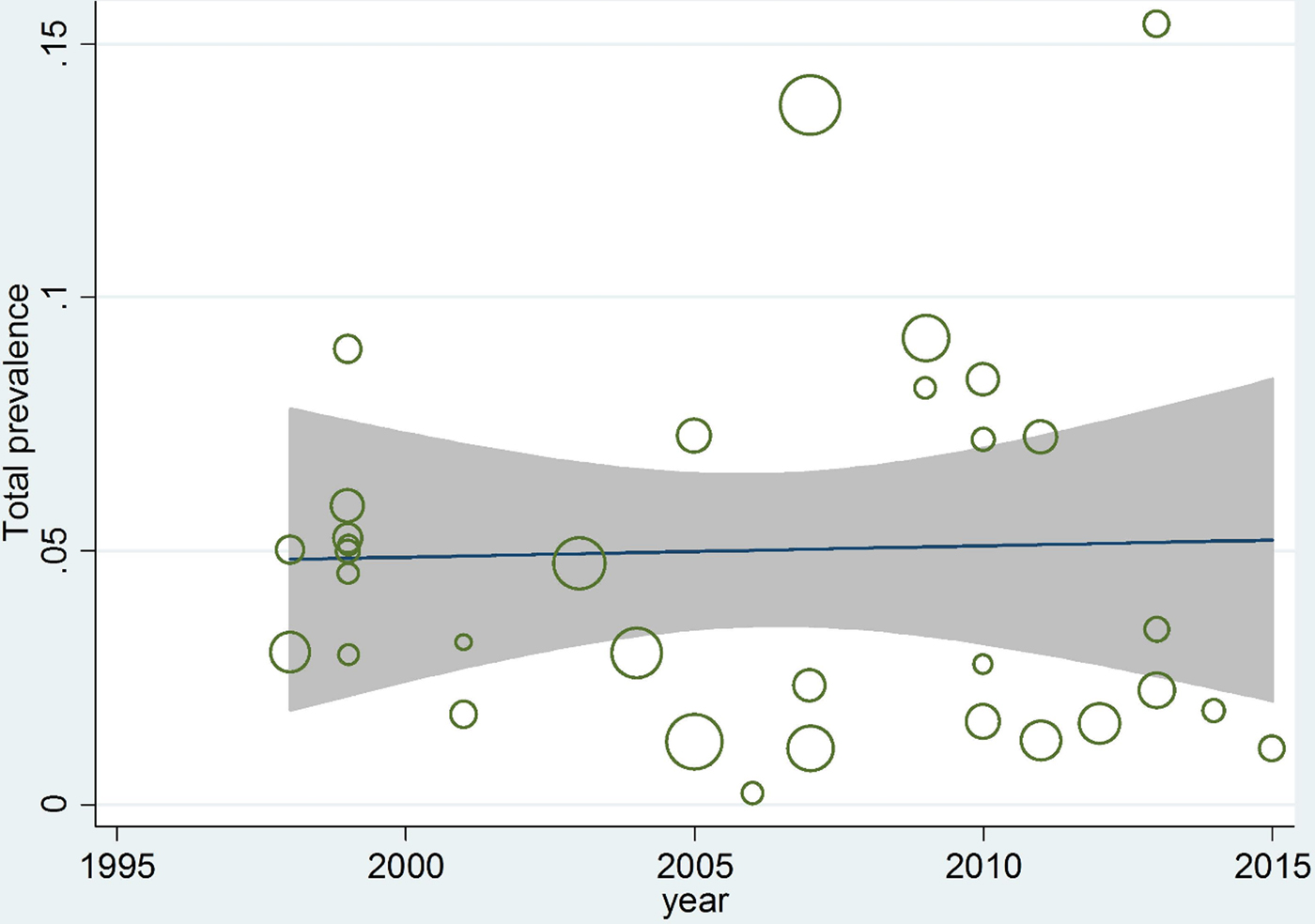
Fig. 6. Meta-regression plot of prevalence of Echinococcus granulosus in human hosts, based on year of studies.
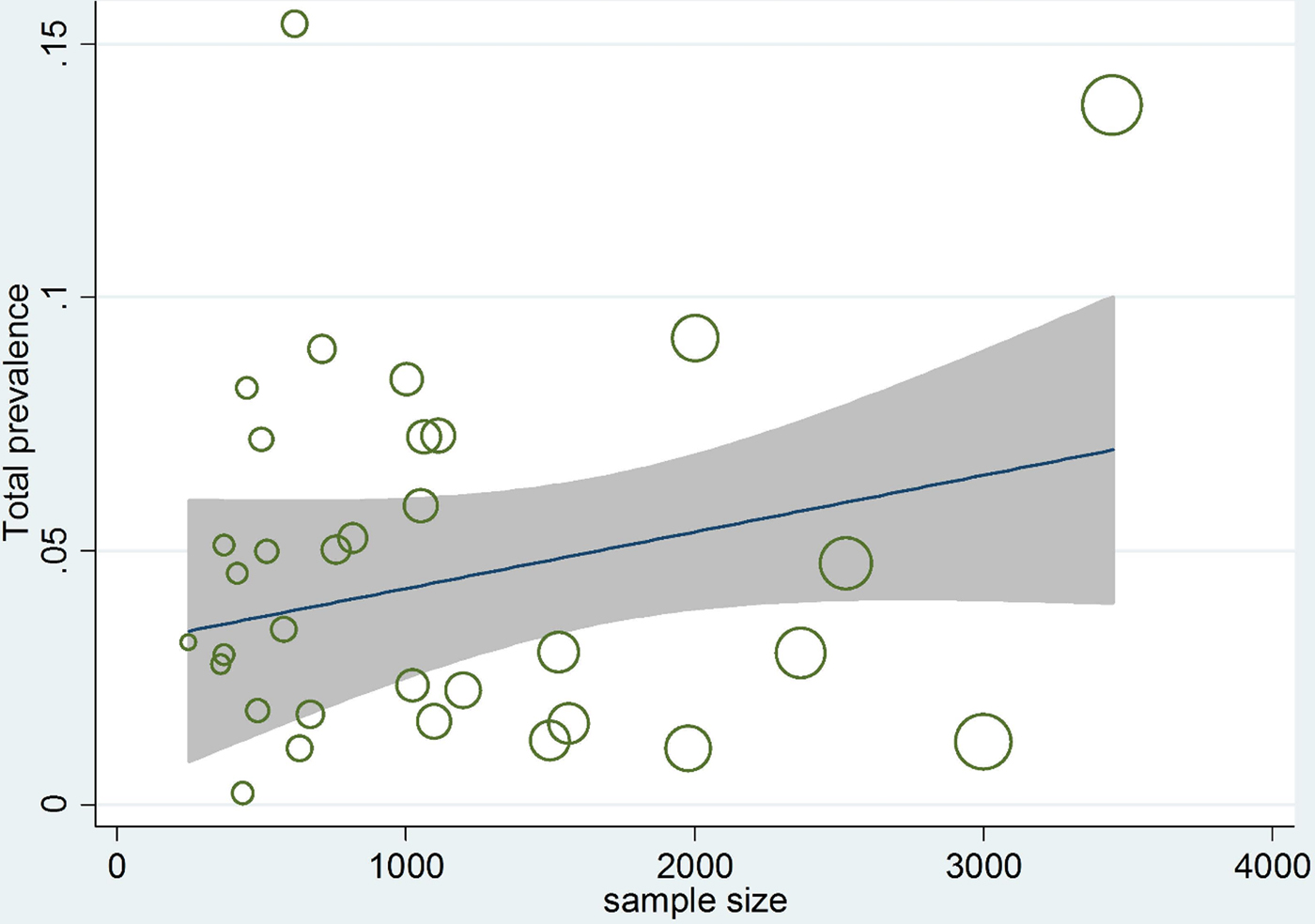
Fig. 7. Meta-regression plot of prevalence of Echinococcus granulosus in human hosts, based on sample size.
Discussion
This meta-analysis deals with the prevalence of CE in animal and human populations of Iran. With a synanthropic life cycle among dogs and domestic livestock, E. granulosus sensu lato infection has been distributed around the globe. Data from various provinces of Iran were acquired from online documented literature. Altogether, we searched nine databases for related articles. In the case of carnivore definitive hosts of CE, 37 papers were found for CE. The estimated total prevalence of infection in definitive hosts was calculated as 23.6% (95% CI = 17.6–30.1%). The gold-standard experiment for detecting infection in canine species is post-mortem examination (intestinal scraping) or sedimentation, while copro-antigen enzyme-linked immunosorbent assay (ELISA) is the method of choice in mass-screening studies (Deplazes et al., Reference Deplazes, Gottstein, Eckert, Jenkins, Ewald and Jimenez-Palacios1992; Eckert, Reference Eckert2003; Duscher et al., Reference Duscher, Prosl and Joachim2005; Barnes et al., Reference Barnes, Deplazes, Gottstein, Jenkins, Mathis, Siles-Lucas, Torgerson, Ziadinov and Heath2012). There have been many studies on the prevalence of canine CE globally. In Africa, very high rates of infection have been detected, particularly in the northern and eastern regions, ranging from 3% in Tunisia to 66% in Uganda (Lahmar et al., Reference Lahmar, Sarciron, Rouiss and Mensi2008; Inangolet et al., Reference Inangolet, Biffa, Opuda-Asibo, Oloya and Skjerve2010). Also, countries neighbouring Iran show a relatively heavy worm burden in dogs, with 49.5% in Iraq (Saeed et al., Reference Saeed, Kapel, Saida, Willingham and Nansen2000), 29.5% in Jordan (Al-Qaoud et al., Reference Al-Qaoud, Abdel-Hafez and Craig2003), 28.2% in Kazakhstan (Stefanic et al., Reference Stefanic, Shaikenov, Deplazes, Dinkel, Torgerson and Mathis2004), 20.1% in Uzbekistan (Torgerson et al., Reference Torgerson, Oguljahan, Muminov, Karaeva, Kuttubaev, Aminjanov and Shaikenov2006), 19% in Kyrgyzstan (Ziadinov et al., Reference Ziadinov, Mathis, Trachsel, Rysmukhambetova, Abdyjaparov, Kuttubaev, Deplazes and Torgerson2008) and 15.2% in Tajikistan (Torgerson et al., Reference Torgerson, Oguljahan, Muminov, Karaeva, Kuttubaev, Aminjanov and Shaikenov2006). The range limits of rates of infection in Europe and the Americas are between 0.2–77.1% and 1.8–79.2%, respectively, and may reach rates greater than even 100% in Australia (Carmena & Cardona, Reference Carmena and Cardona2013). Three groups of risk factors contribute to disease dispersion in dogs, including biological (age of dog, worm burden and immunity), environmental (climate variations) and human behavioural elements (dog management and principles of slaughtering). Two outcomes are expected from appraising the prevalence and mean abundance of CE infection in carnivorous hosts: (1) to carefully monitor the control programmes; and (2) to evaluate the propagation, dynamics of transmission and status of human infection in a particular region (Craig et al., Reference Craig, Rogan and Campos-Ponce2003; Romig et al., Reference Romig, Omer, Zeyhle, Huttner, Dinkel, Siefert, Elmahdi, Magambo, Ocaido, Menezes, Ahmed, Mbae, Grobusch and Kern2011; Barnes et al., Reference Barnes, Deplazes, Gottstein, Jenkins, Mathis, Siles-Lucas, Torgerson, Ziadinov and Heath2012).
Among 90 records obtained from the literature review, weighted prevalence was evaluated as 15.6% (95% CI = 14.2–17.1%) in animal intermediate hosts. Infection identification was similar to that of definitive hosts, relying mostly upon animal post-mortem inspection in abattoirs (Carmena & Cardona, Reference Carmena and Cardona2013). According to scientific reports in Africa, highest prevalences of hydatidosis in sheep (0.2–68%), goats (0.2–65%) and camels (1.9–61%), were recorded (Bardonnet et al., Reference Bardonnet, Piarroux, Dia, Schneegans, Beurdeley, Godot and Vuitton2002; Njoroge et al., Reference Njoroge, Mbithi, Gathuma, Wachira, Gathura, Magambo and Zeyhle2002; Sissay et al., Reference Sissay, Uggla and Waller2008). In Asia, the greatest rate of infection among production animals was found in sheep intermediate hosts (0.1–82.6%) (Yu et al., Reference Yu, Wang, Wu, Ma, Liu, Liu, Zhao, Morishima and Kawanaka2008; Pednekar et al., Reference Pednekar, Gatne, Thompson and Traub2009); furthermore, among Iran's neighbours, CE prevalence in sheep ranged from 7.5% in Pakistan (Latif et al., Reference Latif, Tanveer, Maqbool, Siddiqi, Kyaw-Tanner and Traub2010) to 64.2% in Kyrgyzstan (Torgerson et al., Reference Torgerson, Ziadinov, Aknazarov, Nurgaziev and Deplazes2009), while cattle and goat infections were 1.8–45.5.% and 5.5–11.1%, respectively (Torgerson et al., Reference Torgerson, Oguljahan, Muminov, Karaeva, Kuttubaev, Aminjanov and Shaikenov2006; Latif et al., Reference Latif, Tanveer, Maqbool, Siddiqi, Kyaw-Tanner and Traub2010; Guo et al., Reference Guo, Kubo, Kudo, Nibe, Horii and Nonaka2011). In Europe, sheep (0–75%), and to a lesser extent cattle (0–41.5%) and goats (0–25.7%), were more involved and are considered to be the main intermediate hosts (Varcasia et al., Reference Varcasia, Canu, Lightowlers, Scala and Garippa2006, Reference Varcasia, Canu, Kogkos, Pipia, Scala, Garippa and Seimenis2007; European Food Safety Authority, 2015). The same situation exists in the Americas (Cardona & Carmena, Reference Cardona and Carmena2013; Carmena & Cardona, Reference Carmena and Cardona2013). Many risk factors may be involved in disease spatial distribution, such as farm hygiene practices and breeding conditions, environmental elements and strain/genotype of hydatid cysts, as well as socio-cultural beliefs (Eckert & Deplazes, Reference Eckert and Deplazes2004). Hydatid infection in livestock not only leads to the need to regulate disposal of infected viscera and dead animals, and to international restrictions on export of infected products, but the infection also detracts from the quality and quantity of livestock products (milk, meat, wool and feathers) and reduces birth rates. Hence, requisite control measures should be taken into account (Torgerson, Reference Torgerson2003).
In total, 33 articles related to humans as the aberrant intermediate hosts of hydatidosis were gathered, resulting in a 4.2% (95% CI = 3.0–5.5%) pooled prevalence of hydatid cyst infection. The infection rate was higher in rural regions, with 4.1% (95% CI = 1.6–7.8%); we also found that 4.5% (95% CI = 2.9–6.5%) of women were infected, having more positive cases than men. The greatest rate of prevalence is frequently correlated with temperate regions. Human hydatidosis is an endemic infection in some parts of the world, including in Asia, Australia, South America, North Africa and the Mediterranean littoral (Kilic et al., Reference Kilic, Cangir, Bulut and Akay2003; Moro & Schantz, Reference Moro and Schantz2006b, Reference Moro and Schantz2009; Yang et al., Reference Yang, Sun and Li2006). In these regions, the incidence rate of human CE was reported to be fewer than 50 cases/100,000 inhabitants (Mandal & Mandal, Reference Mandal and Mandal2012). Accordingly, in a recent systematic review (Budke et al., Reference Budke, Carabin, Ndimubanzi, Nguyen, Rainwater, Dickey, Bhattarai, Zeziulin and Qian2013), annual rates of incidence were limited to 0–32 cases/100,000 individuals in hospital-based studies, representing an optimistic hospitalization estimation of 32 cases every year. Besides, the prevalence of hydatid infection reached 1–7% in cross-sectional community-based studies. Consistent with our result, women showed elevated CE prevalence rates (prevalence proportion ratio: 1.35) in this review and hence are considered more prone to infection than men, probably due to traditional household tasks in endemic lands, such as dealing with dogs, gardening and food preparation. Based on Budke et al. (Reference Budke, Carabin, Ndimubanzi, Nguyen, Rainwater, Dickey, Bhattarai, Zeziulin and Qian2013), with regard to imaging techniques, the three top prevalence rates of hepatopulmonary CE were assigned to China.
Our findings about the prevalence of cystic echinococcosis in Iran were affected by some limitations, comprising: (1) lack of a modulus question form to obtain needed information in most human studies; (2) lack of an appropriate, identical-examination method with high specificity and sensitivity; and (3) absence of studies in some regions of the country. These restrictions may bias the reported epidemiologic aspects of CE infection.
In Iran, it has been estimated that a financial burden of up to US$232.3 million per annum is inflicted by CE, divided into human and animal cases with losses of US$93.39 million and US$132 million, respectively. Also, the surgical cost of human cases was estimated at US$1539 (Fasihi Harandi et al., Reference Fasihi Harandi, Budke and Rostami2012). Disease control, reduction of infection prevalence in animals, and hence interrupting the transmission cycle, are not only decisive practices to lower the incidence rate of human hydatidosis, but also lead to an economically improved society. Therefore, given the information extracted from the current systematic review and meta-analysis, safety precautions and management programmes in cases of animal and human cystic echinococcosis should be devised.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X17000463
Acknowledgements
The authors would like to thank all staff of the Department of Medical Parasitology and Mycology of Urmia University of Medical Sciences, Urmia, Iran.
Authors’ contributions
S.K. conceived the study; S.K. and H.K. designed the study protocol; M.F. and S.K. searched the literature; P.K. and S.A. extracted the data; H.K. analysed and interpreted the data; H.M. wrote the manuscript; S.K., H.M. and A.A. critically revised the manuscript. All authors read and approved the final manuscript.
Financial support
This study received financial support from the Student Research Committee of Urmia University of Medical Sciences, Urmia, Iran (grant no: 1394-01-42-2157).
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical standards
This study was approved by the Ethical Committee of Urmia University of Medical Sciences, Urmia, Iran (no.: Ir.UMSU.rec.1395.242).















