INTRODUCTION
Ornithosis, also known as psittacosis, is a zoonotic infection that causes respiratory and sometimes systemic infections in humans. It is caused by the bacterium Chlamydophila (formerly Chlamydia) psittaci. Human infection is transmitted predominantly from birds, but transmission from other animals, and from dust contaminated from bird droppings, has also been reported. Human-to-human transmission is possible but very rarely reported [Reference Ito1]. The route of infection is thought to be inhalation, but direct inoculation via eyes or nose is also possible. Symptoms, usually those of an influenza-like illness initially, generally develop 4–14 days after exposure [Reference Sillis, Longbottom and Palmer2].
In humans the disease usually occurs sporadically, with cases classically being associated with pet psittacine birds such as parrots [Reference Sillis, Longbottom and Palmer2]. Occupational outbreaks in poultry processing plants (turkeys and ducks) have been described in several countries [Reference Anderson, Stoesz and Kaufmann3–5], including incidents in the East of England [Reference Andrews, Major and Palmer6, Reference Newman7]. The two past outbreaks in the East of England were both associated with duck processing, with linked plants affected with an interval of 6 years between incidents [Reference Andrews, Major and Palmer6, Reference Newman7]. Most cases in the second UK outbreak were in the production line, with evisceration, plucking and slaughter being the commoner exposures but some cases occurred in other areas [Reference Newman7]. The route of infection was thought to be inhalation of infective aerosols and dust produced by evisceration and defeathering, hence the association with certain risk areas.
Control of occupational ornithosis in a poultry processing setting is difficult, as infected birds are often asymptomatic, infected birds may only be processed infrequently and diagnosed human cases are rare. In the absence of reliable measures to avoid processing infected birds, prevention of morbidity and mortality due to human ornithosis is mainly achieved through case-finding, with employers advising workers of the risks of ornithosis and the need to seek medical advice.
The UK Health and Safety Executive (HSE) suggests several possible measures for control of ornithosis, including controlling the disease in animals and screening flocks, avoiding dust production, and general precautions against exposure [8]. Personal protective equipment (PPE) is only recommended for on-farm slaughter and after other steps, such as local exhaust ventilation, have been considered. It is therefore generally only used in particularly dusty areas where live, feathered birds are handled and killed. The HSE does recommend using face protection when helping animals to give birth, if there is a risk of splashing from urine or placental fluids.
Following early reports of cases of ornithosis in poultry processing workers in East Anglia, an outbreak investigation was undertaken. This paper describes the investigation's findings including an analytical study, which was undertaken in order to identify risk factors and further control measures beyond the case-finding and education initially employed.
Outbreak
In April 2008 three hospitalized cases of respiratory infection were reported to the public health authorities, all in poultry processing workers from the same plant (plant A) in East Anglia. An outbreak control team (OCT) was convened to investigate and control the outbreak.
A broad case-finding method was used, as many employees did not know the name of their employer. We asked clinicians, two poultry processing employers, and laboratories, to report of any cases of fever and cough in poultry workers in the two affected counties.
A faxed alert was sent to local community and hospital clinicians and microbiology departments on 25 April 2008, with further update faxes (including information on treatment) on 30 April and 14 May. Internal Health Protection Agency (HPA) communications were also sent during May in case there was a wider problem.
For occupational case-finding, we asked the company owning plant A (and a further plant B in the area) to inform staff about the risks of ornithosis through a staff meeting and written information, with appropriate translation. They were also asked to provide their recent sickness records, and regular communications were made with occupational health staff (who also attended the OCT). Furthermore, the occupational health department of another large poultry business was contacted with further telephone checks for possible cases.
Suspected cases were investigated with paired serum samples and antigen detection in sputum (if available). A suspected outbreak case was defined as any person working in the poultry industry, in Norfolk or Suffolk, who reported fever and cough to their employer, primary-care physician or hospital, on or after 1 April 2008. A confirmed outbreak case was any suspected case with serological evidence of infection with C. psittaci, or identification of antigen in sputum by direct immunofluorescence (DIF) (see Methods section).
Case-finding identified 15 suspected outbreak cases, of which nine were confirmed. Of the six unconfirmed cases, one worked at the second plant B, and five worked at two other premises in the area. Four of the six had paired serum samples (both IgG titres <1:16), one a single sample and one was lost to follow-up with no serology taken. One had serological evidence of influenza B infection.
All nine of the confirmed cases worked at plant A, with onset dates between April and August 2008 (Fig. 1). Of these nine, three (the first three) were hospitalized, and 3/9 (33%) had changes consistent with infection on their chest radiograph. Eight of the nine reported cough, 5/9 shortness of breath, 4/9 coryza and 3/9 sore throat. Other symptoms included headache and abdominal pain. Two of the three hospitalized cases were admitted to the intensive care unit, and all recovered.

Fig. 1. Epidemic curve for cases of ornithosis associated with plant A, by week number of onset date, 2008.
Most outbreak cases were in production line workers, but other groups were at risk including an administrator who had visited rather than worked on the production line, and two engineering staff, who became ill shortly after repairing a processing machine that was heavily contaminated with poultry viscera. The attack rate was 4% (nine cases in 225 staff at risk, over 24 weeks).
The case distribution suggested an outbreak related to plant A. We visited this plant and identified a number of possible risk areas where staff were exposed to the viscera and blood of slaughtered birds. Some risk areas identified were outside those where the employer recommended and enforced the usage of PPE. The main initial control measures were based on case-finding and education of staff. Other measures considered were control of infection in birds and use of PPE in staff, but the latter was not initially recommended by the OCT for the reasons outlined in the Introduction.
Therefore we conducted an analytical study of workers at the plant to identify specific risk exposures and areas in order to suggest more specific control measures, including an analysis of the effects of PPE usage.
METHODS
Study design
We conducted a retrospective observational study of employees and agency staff working at plant A on or after 1 April 2008. The study was designed as a cohort, but as overall recruitment was insufficient for a representative cohort and the cases were enriched with known outbreak cases, it was analysed as a case-control study.
Case definitions and population at risk
The population at risk was defined as any person working at plant A on or after 1 April 2008. The company provided a breakdown of the numbers of staff by work area.
A case of ornithosis was defined as a person in the population at risk with either whole cell immunofluorescence (WHIF) suggestive of recent infection, demonstration of Chlamydia spp. antigen by DIF, or symptoms of fever and cough with a rising titre of antibody. The latter two were only used for cases identified through the outbreak investigation.
Controls were individuals recruited from the population at risk that did not meet the case definition.
Recruitment
All staff at plant A were informed about the study and asked about their willingness to participate through a letter sent with their payslips. Human resources staff asked responders to attend at the first recruitment day (17 July 2008), and further staff were recruited on the day by asking attendees to send interested co-workers to the study office. A second recruitment session was held on 22 July, with fewer attenders than the first session. We asked the employers to encourage recruitment through section managers, but it was felt by the end of the second session that all those willing and able to participate had done so.
Study staff, with the help of a translator where necessary, explained the procedure to all participants and obtained signed informed consent. Each participant completed an exposure questionnaire and gave a 5-ml blood sample. Refreshments were offered to those participating.
Additional cases were sought from those confirmed cases identified in outbreak case-finding, who were approached and recruited if consent was given. As confirmed cases with a recent history of relevant illness, these were considered as having evidence of recent infection.
Ethical considerations
Signed, written informed consent was obtained from all participants, with participant information, consent form, exposure form and results letters translated into the three main languages at the plant, Portuguese, Polish and Lithuanian. Subject information stated that participation would have no direct material benefit to the participant and that participation would not in any way compromise the participants employment or health or access to healthcare, and that all records would be handled in strict medical confidence and personal identifiers removed at the earliest possible moment.
The results of blood tests, along with an explanatory letter and contact number for further questions, were distributed to employees through the company, with a copy sent to their general practitioner.
Laboratory testing
Serum samples collected at recruitment were tested for IgG antibodies to Chlamydia spp. by complement fixation assay. Samples with a complement fixation test (CFT) titre of ⩾1:8 were tested further for species-specific antibody by WHIF which detects antibody to three Chlamydiaceae species (Chlamydophila abortus, Chlamydia trachomatis and Chlamydophila pneumoniae). Genus and species antibody were interpreted using the method of Richmond & Caul [Reference Richmond and Caul9]. This method can distinguish between persons without antibody showing no evidence of previous exposure, those with evidence of past exposure to Chlamydia spp. at some time, and those with presumptive evidence of infection in the previous 3 months (from 6 weeks to 14 months prior to testing). Definitive evidence of recent infection depends on paired sera taken at appropriate intervals.
Cases recruited as part of outbreak case-finding were diagnosed using paired serum samples tested for complement-fixing antibodies (CFT) against Chlamydia spp., or direct detection of Chlamydia spp. antigen where sputum was available [Reference Richmond and Caul9]. A rising titre of antibodies to Chlamydia spp., using CFT, with a highest titre of at least 1:32, was considered serological evidence of recent infection.
One outbreak case sputum sample (DIF positive) was tested by ArrayTube DNA microarray to determine the infecting genotype of C. psittaci [Reference Sachse10].
Exposure assessment
Clinical details and exposures to work areas, processes and other animal contact were assessed using a self-completed questionnaire. All participants recruited at the two sessions were assisted by study staff and a Polish translator. Cases who did not attend recruitment sessions were asked to complete an exposure questionnaire by post (with a stamped addressed return envelope). Questions included job type, duration of employment, area of work, work tasks, use of protective equipment, exposure to birds or lambs outside work, and underlying health status. Protective equipment questions included use of filtering face piece (FFP3) masks, goggles, gloves, and aprons. Staff tended to rotate between areas of work and work tasks, and could undertake different work tasks in the same area, hence the division in exposures between work task and area.
Site visits identified circumstances where staff could be exposed to infectious materials (blood or viscera) which sprayed onto their face, for example where hand-held eviscerating tools were used; similarly, staff could be directly exposed to these materials if they touched their face while their hands (with or without gloves) were covered with blood or viscera. As these were potential routes of infection, separate questions were included on each exposure and coded as dichotomous variables.
We asked about exposures at the plant between 1 April and 5 June 2008. The dates correspond to the time period in which recent infections could theoretically be diagnosed with a single serum sample. Where contemporaneous information had been collected on symptoms for outbreak cases, this was used in preference to study questionnaire responses, as it was less likely to be affected by lack of recall. Early outbreak cases were asked about their exposures in the month prior to onset, even if this was before 1 April 2008.
Statistical analysis
Questionnaires were entered into EpiData [Reference Lauritsen11] and the resulting data imported into Stata v. 10 [12]. A case-control analysis was used due to the nature of the recruitment strategy. Univariable analysis, for recent infection was performed for each exposure variable (‘don't know’ or blank responses coded as zero) using the cctable command [Reference Desve and Makary13]. Continuous variables were converted into categorical groups for analysis, based on the distribution of the variable.
Logistic regression was performed using variables significant at P < 0·25, with separate analyses being performed for area exposures, task exposures and general exposures before a final model was constructed from these sub-models. Possible confounders such as age, language spoken, and time working in the industry, were analysed separately and significant and plausible variables were added to the final model.
Use of PPE was considered separately from the regression model due to small cell sizes and an expectation that it would be an effect modifier for certain risk areas. Odds ratios (OR) for the main area exposures were stratified by PPE usage to determine any protective or other effect.
Where there were small cell sizes and collinearity, and where plausible, explanatory variables were merged into broader categories.
Veterinary investigation
The company reviewed their recent records on reported illness in flocks and carcass defect, and mortality records for the previous 6 months. Farm staff were asked to check for signs of dead wild birds in the vicinity of the housed birds, and the biosecurity measures for preventing access to barns by wild birds. Agreement on the testing of birds for ornithosis could not be reached by the OCT and was not undertaken.
Site visits
The processing areas of the plant were inspected during three site visits (May, June, December) involving the HPA, HSE (the enforcing authority) and a microbiologist. From these an overall risk assessment for exposure was made, based on the likely presence of infectious material and the nature of exposure.
RESULTS
Site visits
Processing
Birds, mainly ducks, were raised in 90 farms in two adjacent counties, with around 6% being free range and the remainder housed in barns. They were processed at two plants, A and B. There was no change in the type of birds being processed in the month before the onset of the first cases.
Plant A processed about 90 000 birds a week, and was the only plant processing the free-range birds. Birds delivered by lorry are hung on a moving rail on the outside wall of the plant building (hanging area), then are electrocuted by water bath and enter the plant building. In the first room (killing/defeathering room) the throats are cut and blood drained, and are defeathered and waxed by machines. From here they are eviscerated using further line machinery (automated evisceration room) then pass to the liver sort room where further evisceration and inspection takes place, and then beyond to be trussed and packed.
Area risk assessment
The areas judged to be of highest risk for ornithosis, based on likely routes of exposure and amount of infective material, were the hanging, killing and defeathering, automated evisceration and liver sort rooms. The truss, portion and packing rooms were assessed as low to medium risk.
Case-control study
We recruited 63 (28%) of 225 employees, which was about one third of the usual number of staff present at plant A during a normal day (n = 180). Fifty-nine out of 63 were recruited at the two site visit dates, and three further participants (reported via case-finding) were recruited by telephone and post 3 weeks after the site visits. Staff recruited were broadly representative of the plant as a whole (Table 1), although the proportion working on the production line was lower in participants than for all employees.
Table 1. Main roles of staff recruited compared to all staff at plant A
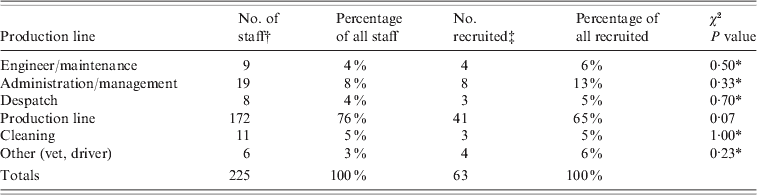
* Fisher's exact test.
† Based on a staff breakdown sent by the company.
‡ Main role reported in exposure questionnaire.
Clinical
Nine (14%) participants out of 63 met the ornithosis case definition. Six (67%) of the nine were symptomatic cases reported through outbreak case-finding.
Four of the nine cases had evidence of Chlamydia antigen in sputum by DIF, and 5/9 had WHIF evidence of recent infection or a rising CFT titre (Table 2). Sixteen (25%) out of 63 participants had some evidence of infection with C. psittaci. Table 2 summarizes the laboratory results and symptoms for study cases.
Table 2. Summary of investigations and symptoms for study participants fitting case definition (n = 9)

WHIF, Whole cell immunofluorescence; DIF, direct immunofluorescence; n.d., not done.
* Serology on sample taken after study recruitment, as symptomatic after study.
In the nine cases the commonest symptoms were fever and cough (67%), sore throat (44%), headache or coryza (33%), shortness of breath (22%), and gastrointestinal symptoms (22%, diarrhoea or abdominal pain). Red eyes and muscle pain were reported in 1/9 (11%) of cases.
Univariable analysis
Table 3 shows the exposure and possible confounder variables by case status, significant at P < 0·1. The work areas of risk were the automated evisceration and killing areas, with exposure to either a significant risk factor for recent infection (OR 7·6, P = 0·021). Having direct facial contact with giblets and blood, through self-touching or spray, were significant risk factors for recent infection.
Table 3. Odds ratios for exposures and possible confounder variables significant at P < 0·1
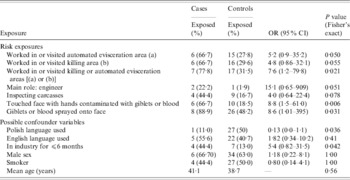
OR, Odds ratio; CI, confidence interval.
Contact with other farms, lambing, having birds at home, smoking, pregnancy and age group were not significantly associated with recent infection. In participants not judged as having had a recent infection, the prevalence of antibody to Chlamydia spp. was higher in employees working at the plant for >6 months, but this was not statistically significant (21% vs. 0%, P = 0·11).
Multivariable analysis
Table 4 shows the variables included in the final logistic regression model. A combined area risk (killing or autoevisceration areas) and one action (contact with viscera or blood) were independent risk factors. Recent employment in the poultry industry remained independently associated with the outcome although it was not significant at P < 0·05.
Table 4. Exposures in logistic regression model, outcome recent infection

OR, Odds ratio; CI, confidence interval.
PPE and effect modification
Fifty-three out of 63 participants gave information on PPE usage. Of these, 27/53 (51%) reported useing FFP3 masks sometimes or always, 8/53 (15%) always, and 13/53 (24%) reported useing goggles sometimes or always. Wearing of FFP3 masks was more common in staff exposed to those areas where this was recommended by the employer (yard, hanging, killing), with 20/24 (83%) using the masks sometimes or always.
The association between recent infection and self-inoculation was modified by reported use of eye protection and respiratory protection, with a significantly lower OR in those reporting some use of eye protection compared to no use (bold values in Table 5). Similarly, the OR for visiting the two high-risk areas was lower in those reporting some use of FFP3 masks compared to no use (OR 1·6 vs. 30), but this was not statistically significant.
Table 5. Risk of recent infection with main exposures, stratified by use of personal protective equipment (n = 53)

CI, Confidence interval.
∞, Not calculable as infinite.
Microbiology
Genotyping identified the infecting C. psittaci as genotype C, a genotype found commonly in ducks [Reference Laroucau14].
Control measures
Following the site visits and epidemiological investigation, the OCT advised the plant to implement a number of control measures.
Respiratory protection with FFP3 rated facemasks was recommended for all workers on the production line, with observance reinforced for workers exposed to the higher risk activities or areas identified in the univariate analysis and on the site visit.
We also advised that staff be made aware of both respiratory protection usage and the need to ensure prompt medical attention if they become ill. Methods recommended were through induction, workplace training, a health alert card, and reminders of the risk of ornithosis for staff reporting illness.
Further cases following advice
The initial outbreak was declared closed after two incubation periods (56 days) had elapsed following the onset of the last case. However, a further six cases from the plant were reported in November 2008. After the first case was notified to the health protection unit, we contacted the plant to remind them of previous HPA advice on PPE given in August. The company had implemented advice on staff education and on PPE, but had not fully enforced PPE usage. The cases occurred mainly in new workers recruited for Christmas.
In response, the company immediately made wearing of FFP3 masks compulsory in all primary areas (killing, hanging, autoevisceration), with non-compliance a disciplinary matter. No further cases occurred in production line workers after this was implemented. One case did occur in a senior manager who was aware of the advice but had elected not to use PPE.
DISCUSSION
This was an occupational outbreak of ornithosis in a duck processing plant, with an attack rate of 4% over a half-year period. Exposure to the killing/defeathering and automated evisceration areas, and contact with viscera or blood were the main risk factors. Recent employment was also a significant risk factor in univariable analysis. PPE (goggles and FFP3 masks) reduced the effect of exposure to risk areas. No illness was detected in birds.
The risk areas from the epidemiological study were a subset of those identified as high risk from the site visit. This risk assessment also identified the liver sorting and hanging areas as high risk, but neither these, nor the areas assessed as low or medium risk, were significant risk factors.
The killing/defeathering area was a potential source of infective material from both feathers (contaminated by faeces) and blood. Killing and defeathering areas have been identified as high risk in some studies [Reference Tiong4, Reference Hedberg15], but not in others [5, Reference Andrews, Major and Palmer6]. This variation may be due to staff factors – slaughtering is a skilled role with lower turnover, so workers may be immune from prolonged exposure – or to the arrangement and nature of processes within the plant.
The processes in the automated evisceration area, involving pneumatic and mechanical methods for removing bird organs, were likely to cause exposure to aerosols containing bird viscera which could be inhaled. Contact with evisceration areas has been identified as a risk factor for ornithosis in other processing plant outbreaks [Reference Anderson, Stoesz and Kaufmann3, 5, Reference Andrews, Major and Palmer6, Reference Hedberg15]. In the one outbreak [Reference Tiong4] where it was not a significant factor, evisceration was mechanical.
Direct contact with viscera or blood was a significant risk factor in this study in both univariable and multivariable analyses. The killing/defeathering and liver sort areas are both areas where such direct contact is likely. In the one study where this exposure was examined [Reference Anderson, Stoesz and Kaufmann3], facial splattering with animal tissues was not a significant factor, but those with higher contact between hands and arms with poultry had a higher seroprevalence.
Using eye protection to reduce the risk of infection through direct contact with blood or viscera, and respiratory protection to reduce the risk from aerosols are plausible explanations to support our observations. Previously published outbreak reports, although suggesting PPE as a preventive measure, have not identified evidence supporting its effectiveness. One UK outbreak found no association with the use of glasses or gloves [Reference Newman7], but there is no clear evidence of PPE effectiveness in this context.
In the UK the HSE does not require use of respiratory PPE in poultry processing plants other than in areas where there is irritant dust [16], but does suggest considering use for on-farm slaughter where local exhaust ventilation cannot be achieved [8]. In this plant this was translated into a recommendation to wear FFP3 masks in the yard, hanging and killing areas. PPE usage was higher in this group but compliance was not complete. It was recognized that wearing FFP3 masks could become hot and uncomfortable for employees, so in conjunction with enforcing usage the employers improved air-conditioning measures in production areas.
Outbreak cases, including those in the second incident, were mostly in newer workers. This is consistent with findings in other outbreaks [Reference Tiong4, Reference Newman7]. Longer-term work may result in seroconversion by exposure, but provide relative protection against acute disease [Reference Tiong4]. We found a higher seroprevalence in longer-term workers, but this was not statistically significant.
The main limitations of this study were the difficulty in serological diagnosis, the relatively low participation rate, and the multiple overlapping exposures.
Diagnosis of ornithosis using immunological methods was sometimes inconclusive. Only two of the four DIF-positive cases with serological results showed evidence of recent infection. One was DIF positive but the study serum sample had a titre of <1:8, despite being taken 10 weeks after disease onset. This case was identified and treated soon after onset; it is possible that the early antibiotic treatment modified the immune response to ornithosis. Nucleic acid techniques may be more sensitive and timely than serology for diagnosing recent cases [Reference Heddema17]. Further developments in diagnostic methods for ornithosis in animals and humans would be very helpful in the investigation of future incidents.
The use of recent infection as an outcome is likely to have underestimated cases, given the low sensitivity of serological diagnosis. This should not have affected the direction of any associations, but would decrease the power of the study to detect them. The exposure period assessed by the study may not have matched the window for serological diagnosis using the WHIF technique, again reducing power but not biasing the results. Last, it is possible that controls with evidence of past immunity might have been less susceptible to infection than those without. Although some studies show an increased risk in newer employees, there is no clear evidence of serological protection in this setting, and again if this effect did occur its main effect would be to have decreased the study's power.
Although only around a third of plant employees participated, they were broadly representative of the main roles. Recruitment was difficult as workers could only be sequentially spared from the production line, and participation depended on the encouragement of line managers. Finally, this limited participation and the low attack rate meant that the study was analysed using case-control methods, despite the recruitment initially aiming at a cohort design. Thus controls were not randomly selected from the population at risk, as would have been the case had the initial design been of a case-control study.
The source of infection in the poultry is unclear. Wild birds may be a natural reservoir [Reference Sillis, Longbottom and Palmer2], and this outbreak, in common with others [Reference Andrews, Major and Palmer6, Reference Newman7, Reference Gaede18] started in the spring, when bird migration may change disease ecology. Biosecurity measures should prevent transmission from wild birds to confined poultry, but free-range birds may be more at risk. It is interesting that all confirmed cases occurred in staff at the only plant processing free-range birds. Further microbiological investigation of birds and their environment, comparing confined and free-range poultry, may help determine the likely source of infection.
Our study provides some evidence that PPE can protect poultry workers against ornithosis in high-risk areas where aerosols of bird viscera are generated. Although the study was limited in power and involved a low number of cases, this tentative evidence, coupled with the circumstantial cessation of further cases following PPE enforcement, suggests that PPE should be recommended where there are human cases of ornithosis associated with a processing plant. We have developed step-up and step-down guidance for such situations, in conjunction with the enforcing authority, in recognition that universal and constant usage of PPE in all processing plants may neither be desirable nor practical.
ACKNOWLEDGEMENTS
The authors thank the members of the incident management team; staff and management at the company involved (not named for confidentiality reasons); and Dr Konrad Sachse (Friedrich-Loeffler-Institut, Jena, Germany) for nucleic acid testing of sputum material. The work was funded entirely by the Health Protection Agency, which is a non-governmental departmental body funded by the UK government.
DECLARATION OF INTEREST
None.








