Conjugated linoleic acid (CLA) is a generic term used to describe one or a mixture of positional and geometric isomers of 18 : 2 fatty acids containing a conjugated double bond. Ruminant fats are the major source of CLA in the human diet(Reference Lawson, Moss and Givens1, Reference Ritzenthaler, McGuire, Falen, Shultz, Dasgupta and McGuire2) and a number of experiments with various human cell lines and animal models have provided a body of evidence to suggest that cis-9, trans-11-CLA, the major isomer in ruminant-derived foods(Reference Palmquist, Lock, Shingfield, Bauman and Taylor3), exhibits anti-mutagenic actvity(Reference Wahle, Heys and Rotondo4, Reference Yaqoob, Tricon, Burdge, Calder, Williams and Buttriss5). In view of the potential benefits to long-term human health there is considerable interest in developing nutritional strategies to enhance the cis-9, trans-11-CLA content of ruminant milk and meat.
Cis-9, trans-11-CLA is formed in the rumen during metabolism of 18 : 2n-6(Reference Harfoot, Hazlewood and Hobson6) or synthesised endogenously in ruminant tissues from trans-11-18 : 1 via stearoyl-CoA desaturase in ruminant tissues(Reference Palmquist, Lock, Shingfield, Bauman and Taylor3, Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman7, Reference Mosley, Shafii, Moate and McGuire8). Studies in lactating cows have shown that increases in milk fat cis-9, trans-11-CLA content to abomasal infusions of a mixture of fatty acids containing trans-11-18 : 1 are equivalent to proportionately 0·21 of the response to cis-9, trans-11-CLA infusions(Reference Shingfield, Ahvenjärvi, Toivonen, Vanhatalo and Huhtanen9). These findings indicate that enhancing the formation and accumulation of cis-9, trans-11-CLA in the rumen represents the biologically most efficient means to increase the concentration of this fatty acid in ruminant foods.
Incubation of 18 : 2n-6 with pure strains or mixed cultures of rumen bacteria causes cis-9, trans-11-CLA to accumulate in vitro (Reference Kepler, Hirons, McNeill and Tove10, Reference Kemp, White and Lander11). Detailed studies of ruminal cis-9, trans-11-CLA formation and outflow in vivo are limited. Measurements of fatty acids at the duodenum in ruminants have reported relatively minor changes in the flow of cis-9, trans-11-CLA to diets containing plant oils rich in 18 : 2n-6(Reference Duckett, Andrae and Owens12–Reference Kucuk, Hess and Rule14). However, most of these studies have been made in animals offered high-concentrate diets, and there is evidence that the amount of cis-9, trans-11-CLA leaving the rumen in response to plant oils rich in 18 : 2n-6 is greater on diets containing higher proportions of forage(Reference Kucuk, Hess, Ludden and Rule15, Reference Sackmann, Duckett, Gillis, Realini, Parks and Eggelston16). Overall, the available evidence suggests that increases in 18 : 2n-6 intake on high-forage diets could be used as a nutritional strategy for enhancing the supply of cis-9, trans-11-CLA available for absorption. To evaluate this hypothesis, ruminal lipid metabolism and the flow of NEFA at the omasum was determined in lactating cows fed a grass silage-based diet (forage:concentrate ratio 60:40, on a DM basis) supplemented with incremental levels of sunflower-seed oil (SFO) rich in 18 : 2n-6.
Materials and methods
Animals and experimental design
All experimental procedures were approved by the Animal Experiment Committee of MTT Agrifood Research Finland in accordance with the 1985 Use of Vertebrates for Scientific Purposes Act 1985. Four rumen-fistulated multiparous Finnish Ayrshire cows 166 (se 16·2) d postpartum of mean live weight 626 (se 65·2) kg were used to evalute the effects of supplementing the diet with 0, 250, 500 or 750 g SFO/d on ruminal lipid metabolism according to a 4 × 4 Latin square with 14 d experimental periods. Cows were housed in a metabolism unit in individual stalls with continuous access to water and milked in situ at 07.00 and 16.45 hours.
Experimental diets
Cows were offered grass silage supplemented with a cereal-based concentrate (proportionately 0·60 and 0·40 on a DM basis, respectively) at 95 % of ad libitum intake measured over a 14 d interval immediately before the start of the experiment. Silage was produced from secondary growths of mixed timothy (Phleum pratense) and meadow fescue (Festuca pratensis) swards. Grass used to prepare silage was cut and wilted for 6 h before being harvested with a precision-chop forage harvester and treated with a formic acid-based additive (760 g formic acid/kg and 55 g ammonium formate/kg; Kemira Agro Ltd, Helsinki, Finland) applied at a rate of 4·4 litres/t grass.
Concentrate supplements were formulated (g/kg) from rolled barley (522), molassed sugarbeet pulp (250), solvent-extracted rapeseed meal (200) of low glucosinolate content (Raisio Feeds Limited, Raisio, Finland) and a proprietary general-purpose mineral and vitamin supplement (28; Suomen Rehu Limited, Helsinki, Finland). Both the basal diet and SFO were offered as two equal meals at 06.00 and 18.00 hours. Supplements of SFO were mixed with concentrate ingredients just before feeding.
Measurements and sampling
Individual cow intakes were recorded daily, but only measurements collected during the last 5 d of each experimental period were used for statistical analysis. During this period, representative samples of fresh silage and concentrate were composited daily and stored at − 20°C. Chemical composition of silage and concentrates was determined using standard procedures(Reference Shingfield, Jaakkola and Huhtanen17), while the concentration of indigestible neutral-detergent fibre (iNDF) was determined using three cows over 288 h ruminal incubations(Reference Ahvenjärvi, Vanhatalo, Shingfield and Huhtanen18). Samples of rumen fluid (n 8) were collected on the last day of each period from each cow at 1·5 h intervals starting at 06.00 hours. Following removal, pH was measured and samples were filtered through two layers of cheesecloth. Samples of rumen fluid were stored at − 20°C until analysed for volatile fatty acid (VFA) and ammonia-N determinations using standard methods(Reference Shingfield, Jaakkola and Huhtanen19).
Digesta flow was assessed using LiCoEDTA, Yb-acetate and Cr-mordanted straw as indigestible markers for liquid, and small and large particles, respectively. Coarsely chopped barley straw was soaked in tap water overnight, rinsed with neutral detergent and labelled with Cr(Reference Udén, Colucci and Van Soest20). Cr-mordanted straw containing 79·0 (se 0·09) mg Cr/g DM was administered (40 g/d) twice daily on top of the rumen mat via the cannula at 12 h intervals starting at 18.00 hours on day 7 of each experimental period. LiCoEDTA was prepared using standard procedures(Reference Udén, Colucci and Van Soest20) while Yb-acetate was obtained from a commercial source (Dasico A/S, Birkerød, Denmark). LiCoEDTA (12 g) and Yb-acetate (4 g) were dissolved in 6 litres distilled water and infused via separate lines at 18.00 hours on day 8 into the rumen at a constant rate (4·2 ml/min). Ruminal infusions were made using polyamide tubing (internal diameter 4 mm) that passed through the rumen fistula and a peristaltic pump (Watson-Marlow, High Wycombe, Bucks, UK). Markers were administered to each animal to provide daily doses of 2·9, 1·6 and 1·5 g/d of Cr, Co and Yb, respectively. At the start of marker administration cows received priming doses of CoEDTA, Yb-A and CrEDTA supplying 3·6, 2·5 and 2·2 g Cr, Co and Yb, respectively, to facilitate rapid equilibration of the marker concentrations in the rumen.
Spot samples (500 ml) of digesta entering the omasal canal were collected three times daily at 4 h intervals on day 11 through to day 14 using the omasal sampling device(Reference Huhtanen, Brotz and Satter21) with modifications(Reference Ahvenjärvi, Vanhatalo, Huhtanen and Varvikko22). Sampling started at 06.00 hours and was advanced 1 h each day to cover a 12 h period that was considered representative of the entire feeding cycle. On each occasion, samples were stored at − 20°C immediately after collection. At the end of the study, digesta was thawed at room temperature, composited and separated into large-particle, small-particle and liquid fractions by filtration and centrifugation(Reference Ahvenjärvi, Vanhatalo, Huhtanen and Varvikko22). Each phase was freeze-dried and stored at − 20°C, while subsamples for lipid determinations were stored at − 80°C. Each digesta phase was submitted for the determination of DM, organic matter (OM), N and marker concentrations, while neutral-detergent fibre (NDF) and iNDF were measured in the large-particle and small-particle fractions, and VFA and ammonia-N concentrations were assessed in the fluid phase(Reference Ahvenjärvi, Vanhatalo, Huhtanen and Varvikko22). Based on marker concentrations, the relative proportions of the fluid phase, small-particle and large-particle fractions in true digesta were calculated using the digesta reconstitution technique(Reference France and Siddons23). Thereafter, appropriate amounts of freeze-dried digesta fractions previously stored at − 80°C were weighed to provide a 20 g composite sample and submitted for fatty acid determination.
Whole-tract apparent digestibility coefficients were determined by total faecal collection. Faeces were collected over 96 h starting at 18.00 hours on day 11 of each experimental period. Total faeces excreted were weighed, thoroughly mixed, subsampled (5 %, w/w) and stored at − 20°C before chemical analysis. Urine was separated from faeces by means of a light harness and flexible tubing attached to the vulva. Faecal DM, OM, NDF, iNDF and N content was determined using the same methods applied to feeds, and marker concentrations were determined as for omasal digesta.
Milk yields of individual cows were recorded daily, while only measurements collected during the last 5 d of each experimental period were analysed statistically. Samples of milk for the measurement of fat, crude protein and lactose were collected from each cow over four consecutive milkings starting at 16.45 hours on day 12 of each experimental period and treated with Bronopol preservative (Valio Limited, Helsinki, Finland). Milk fat, crude protein and lactose were determined by near-IR spectroscopy (Milko-Scan 133B analyser; Foss Electric, Hillerød, Denmark) calibrated using milk samples for which reference measurements had previously been made.
Lipid analysis
Fatty acid methyl esters (FAME) of lipid in SFO and freeze-dried samples of silage and concentrate were prepared in a one-step extraction–transesterification procedure using chloroform(Reference Sukhija and Palmquist24) and 2 % (v/v) sulfuric acid in methanol(Reference Shingfield, Ahvenjärvi, Toivonen, Arölä, Nurmela, Huhtanen and Griinari25). Feed fatty acid content was determined using tritridecanoin (T-135; Nu-Chek-Prep, Elysian, MN, USA) as an internal standard. Following the addition of internal standard (13 : 0, N-13A; Nu-Chek-Prep), the pH of omasal digesta was adjusted to 2·0 using 2 m-hydrochloric acid. Lipid in omasal digesta was extracted using a mixture (3:2, v/v) of hexane and isopropanol and NEFA were separated from other lipid classes by solid-phase extraction and transesterified to methyl esters by incubation with 1 % (v/v) sulfuric acid in methanol at 50°C for 30 min(Reference Shingfield, Ahvenjärvi, Toivonen, Arölä, Nurmela, Huhtanen and Griinari25).
Methyl esters were quantified by GLC using a gas chromatograph (6890; Hewlett-Packard, Wilmington, DE, USA) equipped with a flame-ionisation detector, selective quadrupole mass detector (model 5973N; Agilent Technologies Inc., Wilmington, DE, USA) and 100 m fused silica capillary column (CP-SIL 88, Chrompack 7489; Chrompack, Middelburg, The Netherlands) using H2 as the carrier and fuel gas. Total FAME profile in a 2 μl sample at a split ratio of 1:50 was determined using a temperature gradient program(Reference Shingfield, Ahvenjärvi, Toivonen, Arölä, Nurmela, Huhtanen and Griinari25). Isomers of 18 : 1 and 18 : 2 methyl esters were further resolved in a separate analysis under isothermal conditions at 170°C(Reference Shingfield, Ahvenjärvi, Toivonen, Arölä, Nurmela, Huhtanen and Griinari25). Peaks were routinely identified using authentic standards (GLC-463 and GLC-606; Nu-Check-Prep). Identification was verified by GC–MS analysis of FAME and 4,4-dimethyloxazoline (DMOX) fatty acid derivatives under an ionisation voltage of 400 eV, using He as the carrier gas and the same temperature gradient used for routine analysis of FAME. Preparation of DMOX fatty acid derivatives from FAME of selected digesta samples (n 4) was in accordance with procedures outlined elsewhere(Reference Shingfield, Reynolds, Hervás, Griinari, Grandison and Beever26). Electron impact ionisation spectra of DMOX derivatives was used to locate double bonds based on atomic mass unit distances, with an interval of 12 atomic mass units between the most intense peaks of clusters of ions containing n and n-1 carbon atoms being interpreted as cleavage of the double bond between carbon n and n+1 in the fatty acid moiety. Identification was further validated by comparison with an online reference library of DMOX fatty acid derivative electron impact ionisation spectra (http://www.lipidlibrary.co.uk/ms/arch_dm/index.htm). Geometric configuration of polyunsaturated methyl ester double bonds was elucidated based on the elution order of authentic methyl ester standards containing geometric isomers of 9,12–18 : 2 and 9,12,15–18 : 3 (L-8404 and L-6301; Sigma-Aldrich, Helsinki, Finland). A partial gas chromatogram indicating the separation of 18 : 2 methyl esters under isothermal conditions is shown in Fig. 1.

Fig. 1 Partial gas chromatogram indicating the separation of 18 : 2 isomers obtained under isothermal conditions (170°C) in omasal digesta of cows fed grass silage-based diets supplemented with sunflower-seed oil. Identification was based on electron impact ionisation spectra obtained by GC–MS analysis of fatty acid methyl esters and 4,4-dimethyloxaline derivatives and the elution of an authentic standard (L-8404; Sigma-Aldrich, Helsinki, Finland) containing a mixture of 9,12 geometric 18 : 2 isomers. Peak identification : 1, trans-16-18 : 1; 2, 19 : 0; 3, cis-15-18 : 1; 4, trans-9, trans-14-18 : 2; 5, trans-11, trans-15-18 : 2; 6, trans-10, trans-14-18 : 2 (tentative identification); 7, unresolved trans-10, trans-15-18 : 2 and trans-9, trans-12-18 : 2; 8, trans-11, trans-14-18 : 2; 9, methyl-11-cyclohexyl-11 : 0; 10, trans-9, cis-15-18 : 2; 11, cis-9, trans-12-18 : 2; 12, cis-16-18 : 1; 13, 18 : 2 (double-bond position and geometry undetermined); 14, trans-9, cis-12-18 : 2; 15, trans-11, cis-15-18 : 2; 16, cis-7-19 : 1; 17, cis-9, cis-12-18 : 2.
The distribution of CLA isomers in omasal digesta was determined by HPLC using four silver-impregnated silica columns (ChromSpher 5 lipids, 250 × 4·6 mm, 5 μm particle size; Varian Ltd, Walton-on-Thames, Surrey, UK) coupled in series and 0·1 % (v/v) acetonitrile in heptane as the mobile phase(Reference Shingfield, Ahvenjärvi, Toivonen, Arölä, Nurmela, Huhtanen and Griinari25). Isomers were identified using an authentic CLA methyl ester standard (O-5632; Sigma-Aldrich) and chemically synthesised trans-9, cis-11-CLA(Reference Shingfield, Reynolds, Lupoli, Toivonen, Yurawecz, Delmonte, Griinari, Grandison and Beever27). Identification was verified by cross-referencing with the elution order reported in the literature(Reference Delmonte, Kataoka, Corl, Bauman and Yurawecz28) using cis-9, trans-11-CLA as a landmark isomer.
Statistical analysis
Intake, milk production, omasal flow and digestibility data were subjected to ANOVA using the mixed linear model procedure of Statistical Analysis Systems software package version 8.2 (SAS Institute, Inc., Cary, NC, USA) with a model that included the random effect of cow and fixed effects of period and treatment. Sums of squares for treatment effects were further separated using orthogonal contrasts into single degree of freedom comparisons to test for the significance of linear, quadratic and cubic components of the response to experimental treatments.
Measurements of rumen fermentation characteristics were initially subjected to ANOVA for repeated measures using a mixed linear model that evaluated the effects of sampling time, cow, period, treatment and interactions between sampling time and treatment assuming an autoregressive order 1 covariance structure. Treatment and sampling time interactions were not significant (P < 0·10) for measured rumen fermentation characteristics and therefore rumen fermentation data were reduced to daily means and analysed using the same statistical model applied to other experimental data. Least-square means with their standard errors are reported and treatment effects were declared significant at P < 0·05.
Results
Diet composition
Silage was of high quality both in terms of nutritive value and fermentation characteristics and had the following composition (g/kg DM, unless otherwise stated): DM (g/kg fresh weight), 206; OM, 916; N, 20·3; NDF, 539; iNDF, 105; digestible OM, 650; pH 4·00; lactic acid, 47; acetic acid, 18; water-soluble carbohydrate, 42; soluble N (g/kg total N), 611; ammonium-N (g/kg total N), 72. Concentrate contained (g/kg DM, unless otherwise stated): DM (g/kg fresh weight), 887; OM, 921; N, 26·9; NDF, 214; iNDF, 60·6. Silage contained 16·2 g fatty acids/kg DM with relatively high amounts (g/kg DM) of 16 : 0 (2·82), 18 : 2n-6 (2·89) and 18 : 3n-3 (8·41), whilst 16 : 0 (3·55), cis-9-18 : 1 (4·55) and 18 : 2n-6 (5·88) predominated in concentrates (total fatty acids 16·9 g/kg DM). SFO was rich in 18 : 2n-6 (597), but also contained 16 : 0 (56·0), 18 : 0 (39·7) and cis-9-18 : 1 (184), with a total fatty acid content of 901 g/kg.
Animal performance
Supplements of SFO decreased linearly (P < 0·001) silage DM intake, causing a reduction in NDF, N and 18 : 3n-3 ingestion, but had no effect on total DM and OM intake (Table 1). Incremental amounts of SFO in the diet resulted in linear increases (P < 0·001) in 14 : 0, 16 : 0, cis-9-16 : 1, 18 : 0, cis-9-18 : 1, cis-11-18 : 1, 18 : 2n-6, 20 : 0, 22 : 0, 24 : 0 and total fatty acid intake (Table 1). Supplementing the diet with SFO was associated with a linear increase (P < 0·01) in milk yield and lactose secretion, but had no effect (P>0·05) on milk fat and protein output, due to significant (P < 0·001) reductions in milk protein content (Table 2).
Table 1 Effect of incremental levels of sunflower-seed oil in the diet on nutrient intake (Mean values with their standard errors)

OM, organic matter; NDF, neutral-detergent fibre; pdNDF, potentially digestible neutral-detergent fibre.
* Significance of linear responses to sunflower-seed oil in the diet. Quadratic and cubic responses to sunflower-seed oil were not significant (P>0·05).
† sem for n 16 measurements; error degrees of freedom 6.
Table 2 Effect of incremental levels of sunflower-seed oil in the diet on milk yield and composition (Mean values with their standard errors)
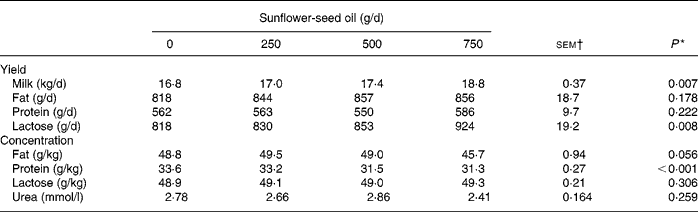
* Significance of linear responses to sunflower-seed oil in the diet. Quadratic and cubic responses to sunflower-seed oil were not significant (P>0·05).
† sem for n 16 measurements; error degrees of freedom 6.
Rumen fermentation
Inclusion of SFO in the diet had no effect on rumen pH or VFA content, but tended (P = 0·09) to decrease rumen ammonia-N concentrations (Table 3). Supplements of SFO modified rumen fermentation patterns, causing a shift towards propionate at the expense of acetate (Table 3). Effects on rumen fermentation characteristics were relatively minor in response to 250 or 500 g SFO/d with the main changes occurring to diets supplemented with 750 g SFO/d.
Table 3 Effect of incremental levels of sunflower-seed oil in the diet on rumen fermentation characteristics (Mean values with their standard errors)

* Significance of linear (L), quadratic (Q) and cubic (C) responses to sunflower-seed oil in the diet.
† sem for n 16 measurements; error degrees of freedom 6.
Nutrient flow entering the omasal canal
Increasing levels of SFO in the diet had no effect on OM, N or non-ammonia-N flow, but altered that of NDF and potentially digestible NDF in a quadratic or cubic manner. Flow of OM, NDF and potentially digestible NDF was lower (P < 0·05) for diets supplemented with 500 compared with 750 g SFO/d, and the amounts of non-ammonia-N and NDF entering the omasum were reduced (P < 0·05) on diets supplemented with 500 g SFO/d compared with the control diet (Table 4). Incremental amounts of SFO decreased linearly (P < 0·05) the flow of VFA and increased linearly (P < 0·001) the amount of fatty acids at the omasum (Table 4). Fatty acid flow at the omasum exceeded dietary intake for all diets, with an indicated net synthesis of 131, 160, 125 and 233 g/d on diets supplemented with 0, 250, 500 and 750 g SFO/d, respectively. Addition of SFO in the diet modified the flow of fatty acids entering the omasal canal, responses characterised by linear or quadratic increases (P < 0·05) in the flow of 12 : 0, 14 : 0, 16 : 0, 18 : 0, 10-keto-18 : 0, 18 : 1, 18 : 2, CLA and long-chain SFA (20 : 0–30 : 0), and a decrease in 18 : 3n-3 at the omasum (Table 4). Flows of cis- (Δ9–16) and trans- (Δ4–16) 18 : 1 leaving the rumen were all increased (P < 0·05) in a linear dose-dependent manner to SFO (Table 5). Trans-11 was the predominant 18 : 1 isomer in omasal digesta, accounting for 49, 45, 46 and 56 % of total trans-18 : 1, in cows fed 0, 250, 500 and 750 g SFO/d, respectively.
Table 4 Effect of incremental levels of sunflower-seed oil in the diet on the flow of nutrients at the omasum (Mean values with their standard errors)

OM, organic matter; NDF, neutral-detergent fibre; pdNDF, potentially digestible neutral-detergent fibre; NAN, non-ammonia-N; VFA: volatile fatty acid; CLA, conjugated linoleic acid.
* Significance of linear (L), quadratic (Q) and cubic (C) responses to sunflower-seed oil in the diet.
† sem for n 16 measurements; error degrees of freedom 6.
‡ Sum of 18 : 2 fatty acids excluding isomers of CLA.
Table 5 Effect of incremental levels of sunflower-seed oil in the diet on the flow of 18 : 1 fatty acids at the omasum (Mean values with their standard errors)
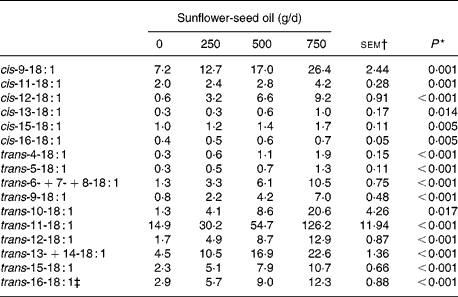
* Significance of linear responses to sunflower-seed oil in the diet. Quadratic and cubic responses to sunflower-seed oil were not significant (P>0·05).
† sem for n 16 measurements; error degrees of freedom 6.
‡ Contains cis-14-18 : 1 as a minor component.
Supplements of SFO increased linearly (P < 0·05) omasal 18 : 2n-6- and trans-9, cis-12-18 : 2 flow, had no effect (P>0·05) on trans-11, trans-15-18 : 2, and reduced in a quadratic manner (P < 0·05) the amount of trans-11, cis-15-18 : 2 at the omasum (Table 6). Furthermore, SFO supplements increased linearly (P < 0·01) ruminal cis-9, cis-11-CLA, cis-9, trans-11-CLA, trans-9, trans-11-CLA, cis-10, cis-12-CLA, trans-10, cis-12-CLA, trans-10, trans-12-CLA and trans-8, trans-10-CLA outflow, but had no effect on the flow of other CLA isomers at the omasum (Table 6). On all diets, cis-9, trans-11 was the major conjugated 18 : 2 isomer entering the omasal canal, accounting for 66, 78, 83 and 80 % of total CLA flow, in cows fed 0, 250, 500 and 750 g SFO/d, respectively. Omasal digesta was devoid of trans-7, cis-9-CLA, trans-9, cis-11-CLA and cis-10, trans-12-CLA.
Table 6 Effect of incremental levels of sunflower-seed oil in the diet on the flow of 18 : 2 fatty acids at the omasum (Mean values with their standard errors)

CLA, conjugated linoleic acid.
* Significance of linear (L) and quadratic (Q) responses to sunflower-seed oil in the diet. Cubic responses to sunflower-seed oil in the diet were not significant (P>0·05).
† sem for n 16 measurements; error degrees of freedom 6.
Supplements of SFO also altered the flow of branched-chain and odd-chain fatty acids at the omasum, responses characterised by linear or quadratic increases (P < 0·05) in the amount of 11-cyclohexyl-11 : 0, 15 : 0, 15 : 0 anteiso, 3,7,11,15-tetramethyl-16 : 0, 23 : 0, 27 : 0 and 29 : 0 at the omasum (Table 7). Flows of unsaturated fatty acids at the omasum were lower than their respective intake, while increasing amounts of SFO in the diet increased linearly (P < 0·05) the extent of ruminal cis-9-18 : 1, 18 : 2n-6 and 18 : 3n-3 metabolism (Table 8).
Table 7 Effect of incremental levels of sunflower-seed oil in the diet on the flow of odd-chain and branched-chain fatty acids at the omasum (Mean values with their standard errors)

* Significance of linear (L) and quadratic (Q) responses to sunflower-seed oil in the diet. Cubic responses to sunflower-seed oil in the diet were not significant (P>0·05).
† sem for n 16 measurements; error degrees of freedom 6.
Table 8 Effect of incremental levels of sunflower-seed oil in the diet on apparent ruminal biohydrogenation of unsaturated fatty acids (Mean values with their standard errors)
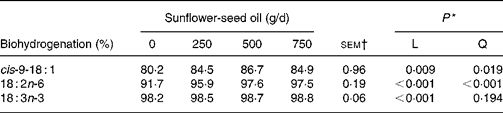
* Significance of linear (L) and quadratic (Q) responses to sunflower-seed oil in the diet. Cubic responses to sunflower-seed oil in the diet were not significant (P>0·05).
† sem for n 16 measurements; error degrees of freedom 6.
Nutrient digestibility
Inclusion of SFO in the diet had no effect (P>0·05) on apparent ruminal and total-tract OM and N digestibility coefficients or on the relative proportion of nutrient digestion occurring in the rumen and intestines, but decreased in a quadratic manner (P < 0·05) ruminal digestibility of potentially digestible NDF and whole-tract NDF digestibility (Table 9).
Table 9 Effect of incremental levels of sunflower-seed oil in the diet on rumen and whole tract apparent digestibility coefficients (Mean values with their standard errors)

OM, organic matter; NDF, neutral-detergent fibre; pdNDF, potentially digestible neutral-detergent fibre.
* Significance of linear (L) and quadratic (Q) responses to sunflower-seed oil in the diet. Cubic responses to sunflower-seed oil in the diet were not significant (P>0·05).
† sem for n 16 measurements; error degrees of freedom 6.
Discussion
Animal performance
Plant oils rich in PUFA typically depress DM intake in lactating cows, an effect that has been attributed to the effects of unsaturated fatty acids on rumen fermentation and ruminal OM digestion coupled with a tendency for plant lipids to shift the site of nutrient digestion from the rumen to the intestines(Reference Jenkins29, Reference Bateman and Jenkins30). Even though SFO at the highest level of inclusion in this experiment decreased ruminal digestion of potentially digestible NDF, DM intake was not altered with no evidence of an increase in the proportion of nutrient digestion occurring in the intestine. Previous studies have also shown that plant oils rich in 18 : 2n-6 have no adverse effect on DM intake of diets containing proportionately 0·60 forage DM when incorporated at rates of between 37 and 60 g/kg diet DM(Reference Kalscheur, Teter, Piperova and Erdman31, Reference Bell, Griinari and Kennelly32).
Supplementing the diet with up to 50 g SFO/kg diet DM had no effect on milk fat yield consistent with the previous findings in cows fed high-forage diets containing 37 g SFO/kg diet DM(Reference Kalscheur, Teter, Piperova and Erdman31). In contrast, other studies have shown that milk fat synthesis is decreased in response to 50 g SFO/kg diet DM supplements(Reference Roy, Ferlay, Shingfield and Chilliard33) or 60 g safflower-seed oil/kg diet DM(Reference Bell, Griinari and Kennelly32), with the extent of diet-induced milk-fat depression being dependent on the relative proportions of starch and fibre in the diet(Reference Roy, Ferlay, Shingfield and Chilliard33). In the present study, SFO supplements increased linearly the flow of trans-10, cis-12-CLA and trans-10-18 : 1 at the omasum from 0·09 to 0·40 and 1·3 to 20·6 g/d, respectively, but had no effect on milk fat synthesis. Trans-10, cis-12-CLA is a known inhibitor of milk fat synthesis in the lactating cow(Reference Bauman and Griinari34), but the marginal increases in omasal flow of this biohydrogenation intermediate in response to 750 g SFO/d would be predicted(Reference deVeth, Griinari, Pfeiffer and Bauman35) to induce minimal reductions (4·3 %) in milk fat yield. Furthermore, milk fat secretion was also independent of increases in ruminal trans-10-18 : 1 outflow consistent with direct evidence based on abomasal infusions that this biohydrogenation intermediate has no role in the regulation of milk fat synthesis in lactating cows(Reference Lock, Tyburczy, Dwyer, Harvatine, Destaillats, Mouloungui, Candy and Bauman36). Overall, milk fat responses to SFO in cows fed grass silage-based diets are consistent with the view that alterations in ruminal lipid metabolism characterised as a shift towards trans-10-18 : 1 at the expense of trans-11-18 : 1 are a prerequisite for diet-induced milk-fat depression when plant oils are fed(Reference Bauman and Griinari34, Reference Shingfield and Griinari37).
Rumen fermentation
Supplementation of SFO had no effect on rumen pH, but at the highest level of inclusion was associated with a shift in rumen fermentation towards propionate at the expense of acetate, consistent with responses to soyabean oil reported in non-lactating cows fed hay-based diets(Reference Bateman and Jenkins30). In other studies, supplements of plant oils rich in 18 : 2n-6 have been reported to have no effect on rumen VFA profiles in cows fed maize silage-based diets(Reference Kalscheur, Teter, Piperova and Erdman31) or increase the molar proportions of propionate, but reduce that of butyrate in grain-fed cattle(Reference Hristov, Kennington, McGuire and Hunt38). The present data indicate that in addition to the composition of the basal diet, the amount of supplemental lipid is also an important determinant of the extent of changes in rumen fermentation patterns that can be expected when plant oils are fed.
Rumen lipid metabolism
Measurements of ruminal metabolism of dietary unsaturated fatty acids are typically based on the comparison of intake with fatty acid flow at the duodenum. Fatty acids entering the duodenum are derived from NEFA adhered to food particles and microbial cells, lipids in rumen bacteria and protozoa, desquamated endothelial cells and bile(Reference Noble and Christie39, Reference Doreau and Ferlay40). Sampling at the omasum has the advantage of reducing the contribution of endogenous secretions in digesta and therefore provides a more direct assessment of ruminal fatty acid outflow. In the present experiment, the effects of SFO in the diet on ruminal lipid metabolism were assessed based on the flow of NEFA at the omasum using procedures optimised for the methylation of this fraction(Reference Shingfield, Ahvenjärvi, Toivonen, Arölä, Nurmela, Huhtanen and Griinari25), which was considered more important to understanding the effects of SFO on the formation of specific biohydrogenation intermediates, rather than using alternative transesterification procedures for the methylation of total fatty acids that increase the risk of isomerisation of unsaturated fatty acids or synthesis of methoxy-artifacts(Reference Kramer, Fellner, Dugan, Sauer, Mossoba and Yurawecz41).
Comparison of fatty acid intake and flows of NEFA at the omasum indicated a net synthesis of fatty acids in the rumen on all diets. These measurements are consistent with minimal or insignificant metabolism of long-chain fatty acids by rumen microbes or absorption of fatty acids across the rumen epithelium or omasum(Reference Noble and Christie39) with the implication that SFO in the diet had no apparent inhibitory effects on microbial de novo fatty acid synthesis. Based on an extensive evaluation of 113 mean treatment values of fatty acid flow at the duodenum, proportionately 0·80 of ingested fatty acids were reported to be recovered, with evidence that the extent of negative balances was associated with diets containing more than 50 g fatty acids/kg DM(Reference Doreau and Ferlay40, Reference Doreau and Chilliard42) with no clear association with dietary lipid composition. More recent studies have estimated net losses(Reference Loor, Ueda, Ferlay, Chilliard and Doreau43) or increases in fatty acids at the duodenum in cows fed diets containing SFO(Reference Lock and Garnsworthy13, Reference Kalscheur, Teter, Piperova and Erdman31). Furthermore, fatty acid flows at the omasum have also been shown to exceed fatty acid ingestion in cows fed diets containing soyabean oil(Reference Lundy, Block, Bridges, Bertrand and Jenkins44). Discrepancies in estimates of fatty acid synthesis in the rumen in response to plant oils rich in 18 : 2n-6 are difficult to reconcile, but must to some extent reflect differences in experimental techniques used to estimate post-ruminal DM flow and the fatty acid content of feed ingredients and digesta.
Supplements of SFO modified ruminal lipid metabolism causing an increase in the flow of 18 : 0, cis-18 : 1, trans-18 : 1, 18 : 2, CLA and total fatty acids at the omasum. Previous studies have shown that incubation of increasing amounts of soyabean oil containing 56·5 g 18 : 2n-6/100 g fatty acids with mixed rumen microbes reduced the rate of lipolysis and extent of 18 : 2n-6 metabolism in vitro (Reference Beam, Jenkins, Moate, Kohn and Palmquist45). Even though the flow of TAG, phospholipids, sphingolipids and sterol lipids at the omasum were not determined, a mean marginal recovery of C18 fatty acids in SFO as non-esterified 18 : 0 at the omasum of 73·3 %, coupled with increased accumulation of 18 : 1 and 18 : 2 biohydrogenation intermediates, indicate that biohydrogenation rather than lipolysis is rate limiting for ruminal metabolism of SFO unsaturated fatty acids in vivo.
Incubation of trilinolein(Reference Noble, Moore and Harfoot46) or soyabean oil(Reference Beam, Jenkins, Moate, Kohn and Palmquist45) with strained rumen contents has shown that biohydrogenation of 18 : 2n-6 is slower and less complete as the concentration of added lipid substrate increases. In contrast, supplementing the diet with incremental amounts of SFO in the present experiment was associated with linear increases in apparent ruminal cis-9-18 : 1, 18 : 2n-6 and 18 : 3n-3 metabolism from 80·2, 91·7 and 98·2 % to 84·9, 97·5 and 98·8 %, respectively. However, these estimates are more apparent than true, because they are based on NEFA and the contribution of esterified lipids to total fatty acid flow at the omasum is not accounted for. Ruminal 18 : 2n-6 and 18 : 3n-3 biohydrogenation based on measurements of fatty acid flow at the duodenum varies between 70 and 95 % and 85 and 100 %, respectively(Reference Doreau and Ferlay40). Even though ruminal biohydrogenation of dietary unsaturated fatty acids is overestimated based on the flow of NEFA at the omasum, the relative changes to incremental amounts of SFO in the diet can be considered more reliable, since TAG are extensively hydrolysed in the rumen(Reference Harfoot, Hazlewood and Hobson6, Reference Doreau and Ferlay40, Reference Doreau and Chilliard42) and plant oils in the diet enhance the incorporation of unsaturated fatty acids as NEFA in cytoplasmic droplets, but have no effect on diacylglycerol, phospholipid or sterol ester content of rumen bacteria(Reference Bauchart, Legay-Carmier, Doreau and Gaillard47). Extensive evaluation of published studies implied that the amount or source of 18 : 2n-6 in the diet has little influence on the extent of ruminal 18 : 2n-6 biohydrogenation in vivo (Reference Doreau and Ferlay40). However, evidence from this and earlier studies(Reference Lock and Garnsworthy13, Reference Sackmann, Duckett, Gillis, Realini, Parks and Eggelston16) tends to suggest that ruminal 18 : 2n-6 metabolism is more extensive when the amount of SFO in the diet is increased.
Measurements of fatty acid flow at the omasum in response to incremental amounts of SFO in the diet supports earlier in vitro and in vivo studies(Reference Harfoot, Hazlewood and Hobson6) demonstrating that the major pathway of ruminal 18 : 2n-6 biohydrogenation involves the formation of cis-9, trans-11-CLA and trans-11-18 : 1 as major intermediates with 18 : 0 being the final endproduct. Increases in ruminal outflow of trans-11-18 : 1 to SFO were approximately ten-fold higher compared with cis-9, trans-11-CLA. Differences in the flow of specific biohydrogenation intermediates indicate that the metabolism of cis-9, trans-11-CLA to trans-11-18 : 1 occurs at a faster rate than the conversion of trans-11-18 : 1 to 18 : 0 and that the final reduction of trans-18 : 1 intermediates is rate limiting for complete ruminal biohydrogenation of non-esterified 18 : 2n-6 in vivo.
Inclusion of SFO in the diet as a source of 18 : 2n-6 enhanced linearly the flow of cis-9, trans-11-CLA leaving the rumen. A mean marginal increase in the amount of cis-9, trans-11-CLA leaving the rumen of 198 mg cis-9, trans-11-CLA/g SFO per kg diet DM is markedly higher than corresponding values of 11·0 and 39·8 based on measurements of fatty acids at the duodenum in steers(Reference Sackmann, Duckett, Gillis, Realini, Parks and Eggelston16) or lactating cows(Reference Loor, Ueda, Ferlay, Chilliard and Doreau43) fed high-concentrate diets. Several factors including amount of oil supplement, composition of the basal diet(Reference Palmquist, Lock, Shingfield, Bauman and Taylor3), sampling site, choice of marker system(Reference Ahvenjärvi, Vanhatalo, Shingfield and Huhtanen18) and procedures for handling, storage and analysis of digesta lipids(Reference Kramer, Fellner, Dugan, Sauer, Mossoba and Yurawecz41) may contribute to variation in post-ruminal cis-9, trans-11-CLA outflow to increases in dietary 18 : 2n-6 supply. Studies in sheep have shown that increases in the proportion of forage in the diet enhance cis-9, trans-11-CLA flow at the duodenum when supplements of soyabean oil are fed(Reference Kucuk, Hess, Ludden and Rule15). In contrast, increases in the forage:concentrate ratio of diets containing SFO from 12:88 to 36:64 were reported to have no effect on the flow of cis-9, trans-11-CLA at the duodenum in steers(Reference Sackmann, Duckett, Gillis, Realini, Parks and Eggelston16). The present measurements indicated that cis-9, trans-11-CLA was the most abundant isomer in digesta, accounting for between 67 and 83 % of total CLA, consistent with previous determinations of the CLA isomer profile of omasal digesta(Reference Shingfield, Ahvenjärvi, Toivonen, Arölä, Nurmela, Huhtanen and Griinari25). Analysis of lipids in duodenal digesta have shown that the relative abundance of cis-9, trans-11-CLA is considerably lower, accounting for between 14 and 31 % of total CLA content(Reference Duckett, Andrae and Owens12, Reference Piperova, Sampugna, Teter, Kalscheur, Yurawecz, Ku, Morehouse and Erdman48, Reference Loor, Ueda, Ferlay, Chilliard and Doreau49). It is possible that cis-trans and trans-cis isomers of CLA entering the omasal canal are further metabolised during the passage of digesta into the duodenum. However, previous studies have reported estimates of ruminal biohydrogenation based on the collection of omasal digesta samples(Reference Lundy, Block, Bridges, Bertrand and Jenkins44) consistent with those derived from fatty acid flows at the duodenum(Reference Doreau and Ferlay40). No direct comparisons of sampling sites have been made, but the available evidence does not support extensive metabolism of fatty acids during transit from the omasum to duodenum. Further studies are required to establish the role of the omasum and abomasum on the supply of fatty acids available for absorption in ruminants. There is also clear evidence that the choice of catalyst used for the transesterification of lipids can alter the isomer profile and amount of CLA recovered in digesta(Reference Kramer, Fellner, Dugan, Sauer, Mossoba and Yurawecz41). It is therefore probable that at least part of the variation in the distribution of CLA isomers and changes in post-ruminal cis-9, trans-11-CLA flow to 18 : 2n-6 in the diet may arise from differences in the methods used for the extraction and transesterification of digesta lipids.
Data from the present experiment also confirmed trans-10, cis-12-CLA as a minor intermediate of ruminal 18 : 2n-6 metabolism on grass silage-based diets. Previous studies have also shown that the flow of trans-10, cis-12-CLA at the duodenum is increased in response to plant oils rich in 18 : 2n-6(Reference Duckett, Andrae and Owens12, Reference Kramer, Fellner, Dugan, Sauer, Mossoba and Yurawecz41), with evidence that ruminal formation of trans-10, cis-12-CLA is further promoted when supplements containing 18 : 2n-6 are included in high-concentrate diets(Reference Kucuk, Hess, Ludden and Rule15, Reference Sackmann, Duckett, Gillis, Realini, Parks and Eggelston16). Supplements of SFO also enhanced the flow of trans-8, trans-10 and several other geometric isomers of 9,11- and 10,12-CLA at the omasum. Recent studies have also shown that cis-9, cis-11-CLA, trans-9, cis-11- and trans-9, trans-11-CLA are intermediates of 18 : 2n-6 metabolism in vitro (Reference Wąsowska, Maia, Niedźwiedzka, Czauderna, Ramalho Ribeiro, Devillard, Shingfield and Wallace50, Reference Wallace, McKain, Shingfield and Devillard51). Furthermore, abomasal infusion studies in lactating cows have established that trans-9, trans-11-CLA(Reference Perfield, Lock, Griinari, Sæbø, Delmonte, Dwyer and Bauman52) and trans-10, trans-12-CLA(Reference Sæbø, Sæbø, Griinari and Shingfield53) alter milk fatty acid composition and decrease milk fat desaturase indices demonstrating the potential physiological importance of intermediates produced during ruminal 18 : 2n-6 metabolism.
Omasal flow of other CLA and non-conjugated 18 : 2 isomers were decreased or independent of SFO in the diet. The occurrence of these fatty acids can be attributed to 18 : 3n-3 metabolism in the rumen and serve to highlight the diversity of specific fatty acid intermediates formed during ruminal biohydrogenation of dietary unsaturated fatty acids. Previous studies have also shown that one or more of these biohydrogenation intermediates accumulates during incubation of 18 : 3n-3 with mixed rumen microbes or pure cultures(Reference Harfoot, Hazlewood and Hobson6, Reference Wąsowska, Maia, Niedźwiedzka, Czauderna, Ramalho Ribeiro, Devillard, Shingfield and Wallace50) or are enhanced in duodenal digesta in response to diets containing linseed oil as a source of 18 : 3n-3(Reference Loor, Ueda, Ferlay, Chilliard and Doreau43, Reference Loor, Ueda, Ferlay, Chilliard and Doreau49).
Dietary inclusion of SFO increased the flow of cis- (Δ9–16) and trans- (Δ4–16) 18 : 1 isomers at the omasum. With the exception of trans-11-18 : 1(Reference Kemp, Lander and Gunstone54), formation of 18 : 1 metabolites during the metabolism of dietary unsaturated fatty acids is not well established. In attempting to identify the origins of 18 : 1 biohydrogenation intermediates, it is important to recognise that SFO contains significant amounts of cis-9-18 : 1, and that the rates of trans-18 : 1 reduction to 18 : 0 vary according to the position of the double bond, being higher for trans (Δ8–10) isomers than trans (Δ5–7) or trans (Δ11–13)(Reference Kemp, Lander and Gunstone54). Further work has also shown that metabolism of cis-9-18 : 1 in vitro results in the production of trans-18 : 1 isomers with double bonds from Δ4–16(Reference Mosley, Powell, Riley and Jenkins55) with evidence that once trans-18 : 1 intermediates accumulate further conversion to a number of positional isomers may occur(Reference Proell, Mosley, Powell and Jenkins56). Nevertheless, through consideration of the flow of 18 : 2 fatty acids at the omasum and probable reduction products coupled with the changes in the profile of 18 : 1 fatty acids in omasal digesta to SFO supplementation, it is possible to deduce that metabolism of 18 : 2n-6 in the rumen involves the formation of cis- (Δ10–12) and trans- (Δ8–12) 18 : 1 intermediates in vivo (Fig. 2). The present data confirm and extend earlier studies investigating the formation of fatty acid metabolites liberated during in vitro incubations of 18 : 2n-6 with pure cultures or mixed rumen microbes(Reference Harfoot, Hazlewood and Hobson6, Reference Wąsowska, Maia, Niedźwiedzka, Czauderna, Ramalho Ribeiro, Devillard, Shingfield and Wallace50, Reference Wallace, McKain, Shingfield and Devillard51, Reference Hudson, Morvan and Joblin57).

Fig. 2 Putative pathways of 18 : 2n-6 metabolism in the rumen. →, Production of major biohydrogenation intermediates of 18 : 2n-6; ⇢, formation of minor fatty acid metabolites liberated during ruminal biohydrogenation of 18 : 2n-6.
Inclusion of plant oils rich in 18 : 2n-6 is a well-established nutritional strategy for enhancing milk fat cis-9, trans-11-CLA content(Reference Palmquist, Lock, Shingfield, Bauman and Taylor3, Reference Roy, Ferlay, Shingfield and Chilliard33). Supplementing grass silage-based diets with SFO enhanced ruminal cis-9, trans-11-CLA and trans-11-18 : 1 outflow with no evidence of a shift in biohydrogenation towards trans-10-18 : 1 at the expense of trans-11-18 : 1 that can occur on high-starch rations based on maize silage or low-forage diets(Reference Roy, Ferlay, Shingfield and Chilliard33). These observations appear to account for the relatively high enrichment of milk fat cis-9, trans-11-CLA content reported over an extended period of time in cows fed mixed forage diets containing 60 g safflower-seed oil/kg diet DM(Reference Bell, Griinari and Kennelly32).
SFO in the diet increased the flows of 15 : 0 iso, 15 : 0 anteiso and 17 : 0 anteiso, but had no effect on ruminal outflow of other odd-branched fatty acids. Since SFO was devoid of these fatty acids, alterations in the flow of specific odd-branched-chain fatty acids can be attributed to the effects of additional lipid in the diet on microbial de novo fatty acid synthesis. Odd-branched-chain fatty acids incorporated into bacterial lipids are derived from the elongation of branched-chain Co-A precursors. Leucine and isoleucine are deaminated and decarboxylated to yield isovaleryl-Co A and 2-methylbutyryl Co A that serve as substrates for microbial synthesis of iso and anteiso fatty acids, respectively(Reference Vlaeminck, Fievez, Cabrita, Fonseca and Dewhurst58). Variations in the relative abundance of odd-branched-chain fatty acids of rumen bacteria are considered to be more dependent on the specificity of inherent fatty acid synthetase rather than the availability of Co-A precursors(Reference Vlaeminck, Fievez, Cabrita, Fonseca and Dewhurst58). A number of studies have established that the fatty acid profiles of rumen bacteria are species specific(Reference Harfoot, Hazlewood and Hobson6, Reference Vlaeminck, Fievez, Cabrita, Fonseca and Dewhurst58, Reference Harfoot and Christie59), such that measurements of odd-branched-chain fatty acids in digesta or milk may reflect changes in ruminal microbial populations. No direct measurements of microbial community were made in the present study, but the changes in the flow of several odd-branched-chain fatty acids at the omasum may imply that SFO induced alterations in the relative abundance of certain bacterial populations. It appears reasonable to assume that populations of rumen bacteria most sensitive to the toxic effects of 18 : 2n-6 would be decreased in response to incremental amounts of SFO in the diet.
Conclusions
Supplementing grass silage diets with SFO had no effect on DM intake, milk fat or protein yield, but increased milk yield and at the highest level of inclusion shifted rumen fermentation towards propionate at the expense of acetate. SFO altered ruminal lipid metabolism, leading to dose-dependent increases in the flow of 18 : 0, trans-18 : 1, cis-18 : 1, 18 : 2n-6 and several positional and geometric isomers of CLA at the omasum. Cis-9, trans-11 was the most abundant isomer of CLA while trans-11 was quantitatively the most important 18 : 1 biohydrogenation intermediate in omasal digesta. It is concluded that supplementing grass silage-based diets with plant oils rich in 18 : 2n-6 increases ruminal outflow of trans-11-18 : 1 and cis-9, trans-11-CLA in lactating cows.
Acknowledgements
This research was supported by the Finnish Ministry of Agriculture and Forestry. The authors gratefully acknowledge the staff of the Animal Nutrition Metabolism Unit for the diligent care of experimental animals and assistance during sample collection and the assistance of Minna Aalto and Piia Kairenius during sample lipid analysis.













