Rubella vaccination was introduced in 1970 for teenage girls to reduce the risk of infection in pregnancy thereby reducing the incidence of congenital rubella syndrome. The effectiveness of all immunization programmes should be assessed by either the collection of data on subsequent incident infections or by the systematic appraisal of immunity levels in at-risk groups. Following the introduction of rubella vaccination a programme of monitoring immunity to rubella in pregnant women was set up. In addition, this screening programme also served to identify women still at risk of infection in order to offer postnatal vaccination to provide protection in future pregnancies.
Rubella antibody testing has since been absorbed into the wider Infectious Disease in Pregnancy Screening programme. Standards developed to support this programme set an antibody level of 10 IU/ml as consistent with immunity to rubella and initially required that all samples with levels <10 IU/ml be repeated in an alternative assay. This practice was feasible with levels of immunity of 98% when only 2% of samples needed to be re-tested. However, with increasing levels of susceptibility the guidance has recently been changed to allow reports of immunity on a single test [1].
All screening programmes are regularly reviewed to ensure that they remain appropriate. In 2011 the Infectious Disease in Pregnancy Screening Programme undertook such a review of rubella screening requesting discussion and comments from stakeholders [2]. The West Midlands Region Microbiology Laboratory Forum for Antenatal Screening formally responded to this consultation pointing out a marked and increasing level of susceptibility in the antenatal population. As the West Midlands has collected and analysed local data since 2004 it was possible to show that susceptibility was no longer associated only with those maternity units serving inner-city areas with high numbers of immigrant women, known to have a low percentage of immunity, but was now widespread throughout the ‘shires’. The opinion of midwives is that those who are not immune to rubella are now more likely to be UK-born women in their early 20s. Higher levels of susceptibility led to increasing work for the laboratory in the form of repeat testing and on maternity units trying to deliver a postpartum MMR vaccination programme [3]. Because of this, as mentioned previously, the need for repeat testing was re-assessed and is now not required.
Routine data are collected by local antenatal screening coordinators and supplied centrally to Public Health England (PHE; formerly the Health Protection Agency) through the Regional Antenatal and Child Health Screening Teams. In addition, the Shrewsbury and Telford Hospital NHS Trust maternity unit has carried out audits to assess and monitor the uptake of postnatal vaccination. Data from these two sources have been examined to provide information on the age range of those women found to be non-immune.
In the West Midlands, the level of susceptibility has risen from 1·4% in 2004 to 6·9% in 2011 (5588/80834). The range for individual trusts in 2011–2012 was 1·7–12·1% and in the past had been higher in inner-city areas [Reference Boxall, Hodgkiss and Tonks4]. Locally, in Shropshire, the level was 6·9% (376/5413). Since comprehensive laboratory screening information is available for this area it has been possible to study the age range of susceptible women. Those born before 1976 had a level of susceptibility of 1·6% (16/991) and those born between 1977 and 1986 had a level of 2·7% (77/2838) although they comprised 52% of the total antenatal population. Those born since 1986 and offered MMR as toddlers have a non-immunity level of 17·8% (283/1584). Table 1 gives the age distribution of the rubella susceptible women.
Table 1. Distribution of age for rubella susceptible women
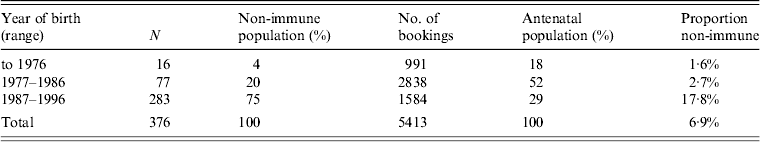
All susceptible women should be offered postpartum MMR vaccine as advised [3]. Therefore, looking at those eligible for MMR vaccine in this group, 75·2% (283/376) of the total were those born between 1987 and 1996.
The data presented here indicate that there is a high level of susceptibility to rubella in the cohort given MMR in their second year (12–15 months) but with no subsequent booster dose. With less rubella circulating in the community there is no natural boost to immunity, therefore levels of antibody are falling below the level considered to be consistent with immunity.
High levels of susceptibility to rubella, particularly in inner-city areas, have historically been explained by a larger proportion of immigrant women who have migrated from areas where vaccination against rubella is not carried out and/or where rubella infection is uncommon. Non-immunity is proportionately higher in these groups but cannot account for all of the observed overall increase [Reference Boxall, Hodgkiss and Tonks4]. However, the data presented in this paper are from an area of the West Midlands with a relatively low level of immigration and is therefore more likely to reflect the situation in UK-born women who had the benefit of MMR vaccination in childhood.
It has also been suggested that some of the variation observed between regions could be explained by the different assays being used now that antenatal screening tests are performed locally rather than centrally by the NHS Blood and Transplant (NHSBT). However, all assays must have the same cut-off value, 10 IU/ml [1]. Moreover, in Shropshire, antenatal testing has always been performed locally using the same assay and we have noted a consistent increase in rubella susceptibility, which suggests that the observation is real and not due to assay variations [Reference Boxall, Hodgkiss and Tonks4].
Matthews et al. showed that a higher proportion of women were found to have a level of antibody <10 IU/ml in those born after 1983 (14·0%) compared to those born before (2·2%) [Reference Matthews5]. Recent national data collected by NHSBT have shown that susceptibility to rubella is more likely in younger women and their data show a 68% increase in susceptibility over the 6-year period, 2004–2009 [Reference Byrne6]. Their data showed that for those born before 1985 the level ranged from 1·3% to 2·4% whereas for those born since 1986 the level was 7·5%. NHSBT were able to collect limited information on ethnicity which showed that the ‘non-white’ groups had higher levels of susceptibility. However, the ‘white’ groups still constitute the majority of the population (73% where ethnic status is known) and increasing levels of susceptibility in this group will impact on the ability of maternity units to cope with the delivery of postnatal vaccination.
Pregnant women are currently thought to be protected against rubella infection as there is sufficient immunity in children through the MMR programme to have reduced the level of circulating virus. However, this also has the perverse effect of reducing the likelihood of a natural boost to immunity. It is likely that the MMR programme is effective at preventing childhood rubella but that the level of detectable antibody disappears by the time women reach childbearing age. With low antibody levels in adult women and periods of poor uptake of MMR vaccine there is a risk that a rubella outbreak similar to the recent measles outbreaks will occur. Although the reasons for increased susceptibility are different in that missed vaccinations have more impact than waning antibodies in causing outbreaks of rubella it is clear that a review of vaccination policy is needed.
Importantly, as mentioned, susceptible women need to be offered MMR vaccine postpartum in accordance with current guidelines [3]. A previous study confirmed that delivery of the first dose of vaccine by maternity units before discharge, as recommended, was more successful than delegating vaccination to primary care [Reference Yung, Skidmore and Lord7]. This is now the model used. A local audit performed in 2011–2012 studied the completeness of the offer of vaccination. Of the 376 susceptible women, 21 had moved away and it is assumed that they would have been offered post-delivery vaccination. A further 18 had miscarried or had a termination and a follow-up letter to GPs in these cases does advise that MMR is offered if required. The remaining 337 were offered vaccination. In total, 263 (78%) received MMR prior to discharge, 57 declined, six opted to go to their GP and 11 were undecided about receiving the vaccine.
Although a single dose of MMR vaccine is thought to provide immunity to rubella a second dose is needed to provide immunity to measles and mumps. In an attempt to ensure that the second dose of vaccine is given, as the women receive the first dose of vaccine, in this Trust, they are given a receipt to take to their GP explaining the need for a further dose. We know that 130/376 of the women in this study had had a previous live birth and so should have been offered MMR vaccine. Twenty-six women declined the vaccine in their current pregnancy and were likely to have declined in previous pregnancies. There are several reasons for failure of the intervention in the remaining 104 women. They also may have declined, not received a second dose or not responded to vaccination. However, the fact that a third of the women in the non-immune group had no detectable antibody following the opportunity of vaccination reinforces the need for a review.
As the cohort requiring this intervention increases it is likely that postnatal MMR will become unmanageable and ineffective at a population level. In addition to this group the numbers of non-immune pregnant women will continue to rise as a result of the low uptake of vaccine following the MMR controversy in 1998. Consequences of this can presently be seen in the recent well-publicized measles outbreaks.
It has been widely discussed that the screening programme for rubella, unlike that for hepatitis B, HIV and syphilis, does not result in interventions to reduce mother-to-baby transmission and has no advantages for the current pregnancy.
As stated in our consultation submission, the accumulating evidence should prompt a review of the current guidelines concerning universal MMR vaccination, perhaps introducing a booster dose of MMR for teenagers. This may also lead to a revision of the need for antenatal screening for rubella susceptibility with the emphasis placed on comprehensive investigation of rashes and contacts of rashes in pregnancy.
DECLARATION OF INTEREST
None.



