Vitamin D deficiency is known to be a common problem among a variety of population groups, including pregnant women( Reference Saraf, Morton and Camargo 1 ) and dark-skinned people( Reference Yao, Hong and Bandera 2 ). Several studies suggest that an adequate vitamin D status may prevent pregnancy complications and beneficially affect maternal and fetal health( Reference Hypponen, Cavadino and Williams 3 , Reference Zhang, Pan and Guo 4 ). However, as the scientific evidence on this matter is still scarce, the WHO does not recommend vitamin D supplementation in pregnancy as part of routine antenatal care( 5 ).
As the fetus is completely dependent on the vitamin D stores of the mother, low maternal vitamin D concentrations may amongst others influence the developing child by affecting fetal brain development and function (reviewed in Pet et al.( Reference Pet and Brouwer-Brolsma 6 )). Namely, ex vivo studies have revealed the presence of vitamin D receptors in many body tissues, including the placenta( Reference Adams and Hewison 7 ) and the brain( Reference Eyles, Liu and Josh 8 ). Moreover, rodent studies have shown effects of low maternal vitamin D concentrations on offspring brain morphology( Reference Eyles, Brown and Mackay-Sim 9 , Reference Feron, Burne and Brown 10 ), brain physiology( Reference Eyles, Almeras and Benech 11 , Reference Grecksch, Ruthrich and Hollt 12 ), as well as behaviour( Reference Eyles, Rogers and Buller 13 – Reference Kesby, Burne and McGrath 15 ).
Human observational studies on their turn have demonstrated subtle associations between maternal vitamin D status and cognitive function in the child as well, predominantly in Caucasian populations( Reference Hanieh, Ha and Simpson 16 – Reference Strom, Halldorsson and Hansen 21 ). Significant positive associations were reported between first/second trimester maternal serum 25-hydroxyvitamin D (25(OH)D) concentrations and mental/psychomotor scores in 14-month-old Spanish toddlers (n 1820)( Reference Morales, Guxens and Llop 18 ), second trimester 25(OH)D status and language impairment in 5- and 10-year-old Australian children (n 534 and 474)( Reference Whitehouse, Holt and Serralha 19 ), and third trimester 25(OH)D status and language scores in 6-month-old Vietnamese infants (n 960)( Reference Hanieh, Ha and Simpson 16 ). In contrast, no associations were observed of second trimester maternal 25(OH)D status and cord blood 25(OH)D with mental scores among 8-month-old American infants (n 3825)( Reference Keim, Bodnar and Klebanoff 17 ). Moreover, second or third trimester 25(OH)D concentrations were not associated with total intelligence quotient (IQ) scores, tests of scholar achievement, reading and spelling, language impairments or verbal IQ among Danish (n 798)( Reference Strom, Halldorsson and Hansen 21 ), English (n 178)( Reference Gale, Robinson and Harvey 20 ) and American children (n 2987)( Reference Keim, Bodnar and Klebanoff 17 ). The discrepancy between study outcomes may be partly explained by the large heterogeneity between studies, for example differences in timing of blood sampling (1st, 2nd or 3rd trimester), mean maternal 25(OH)D concentrations, cognitive testing procedures, the age of cognitive testing, included covariates and ethnicity. Most studies explored associations of maternal 25(OH)D status in mid- and late gestation with childhood cognition. However, the human brain already starts to develop in the 3rd gestational week where the basic brain structures and central nervous system are already formed after 8 weeks of pregnancy( Reference Stiles and Jernigan 22 ). Therefore, studies on the potential role of early gestation 25(OH)D concentrations in childhood cognition are needed as well.
Thus, the results of human studies are inconclusive and data on the role of early gestation 25(OH)D concentrations in relation to childhood cognition are limited. With this study we aimed to contribute to the current evidence by investigating associations between first trimester maternal 25(OH)D concentrations and child cognitive performance at age 5–6 years in the Dutch multi-ethnic Amsterdam Born Children and their Development (ABCD) mother–child cohort, expecting a better cognitive performance among those exposed to higher intra-uterine 25(OH)D concentrations. As 25(OH)D concentrations were expected to be lower in participants with darker skin colour – leading to the hypothesis that potential associations may be more pronounced in specific ethnic groups – we also conducted stratified analyses based on ethnic background.
Methods
Participants
This prospective study was conducted using data from the ABCD study, a population-based, multi-ethnic mother–child cohort. The design of the ABCD study has been extensively described by van Eijsden et al.( Reference van Eijsden, Vrijkotte and Gemke 23 ). Women were recruited, between January 2003 and March 2004, during their first antenatal visit to the general practitioner, midwife or gynaecologist. The ABCD study has been approved by the medical ethical committees of the participating hospitals and the Registration Committee of Amsterdam. All mothers gave written informed consent.
Population for analyses
In total, 12 373 pregnant inhabitants of Amsterdam were invited to participate in the study; 8267 women filled out a pregnancy questionnaire; 7863 live-born singleton infants and 132 live-born multiples were delivered. Blood samples were obtained of 4389 women at the first prenatal visit (median 13 weeks gestational age, interquartile range (IQR) 12–14), where 25(OH)D status was determined in 4250 singleton mother–child pairs. A period of 5 years after birth, 6161 mother–child pairs were invited for follow-up measurements. Questionnaires were returned for 4488 pairs of whom 3132 participated in cognitive testing. Data on both 25(OH)D status and cognitive measurements at 5–6 years was available of 1854 participants. Fig. 1 provides a schematic overview of the participant flow.
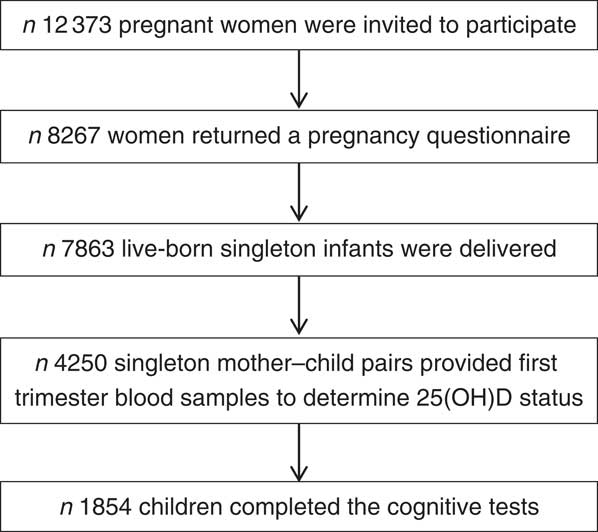
Fig. 1 Participant flow chart of the Amsterdam Born Children and their Development study investigating associations between first trimester 25-hydroxyvitamin D (25(OH)D) status and childhood cognition at age 5–6 years.
Vitamin D status
First trimester 25(OH)D concentrations were determined by enzyme immunoassay (OCTEIA AC-57F1; IDS Ltd) at the Regional Laboratory of Amsterdam. Intra-assay and inter-assay coefficients were 8 and <10 %, respectively( Reference Leffelaar, Vrijkotte and van Eijsden 24 ). Data indicate that the average 25(OH)D concentrations measured with the OCTEIA IDS are approximately −13·7 (sd 1·6) nmol/l lower than the Vitamin D External Quality Assessment Scheme mean (P<0·0001) with a negative difference that becomes larger with increasing 25(OH)D concentrations( Reference Hypponen, Turner and Cumberland 25 ).
Cognitive performance
Domain-specific cognitive performance – including attention, motor fluency and flexibility, and executive function – was assessed by means of computerised tasks of the Amsterdam Neuropsychological Tasks (ANT). The ANT has been extensively used and illustrated elsewhere( Reference Gunther, Herpertz-Dahlmann and Konrad 26 – Reference De Sonneville 29 ) and judged acceptable, which is for instance demonstrated by strong effect sizes in the expected direction for the executive function task( Reference De Sonneville 29 ). Besides, the ANT has been shown to have satisfactory psychometric properties as shown by test–retest correlations ranging from 0·70 to 0·85 (summarised in Koekkoek et al.( Reference Koekkoek, de Sonneville and Wolfs 30 )). Cognitive measurements were conducted in the morning or early afternoon by well-trained researchers according to conscientious procedures. In short, children were individually tested in a quiet room where they first received a verbal task instruction while simultaneously showing an example of the task on the laptop screen. Subsequently, the child completed a practice run to become familiar with the task stimuli and response mode. The actual test trial started once the researcher ascertained that the child understood the task. The cognitive test battery included four tasks, specifically ‘baseline speed’ to measure attention, ‘pursuit’ and ‘tracking’ to measure motor fluency and flexibility, and ‘response organisation objects’ to measure executive function (inherent response inhibition and response flexibility). A more extensive description of these tests can be found in the paper by Finken et al.( Reference Finken, van Eijsden and Loomans 31 ).
Covariates
Information on age (years) and sex of child (boy/girl, n(%)), birth order (first/second/third/fourth/fifth or more, n (%)), birth weight (g), gestational age at birth (weeks), ethnicity based on birth country of the grandmother of the child categorised into three groups ((1) Caucasian: Dutch and other Caucasian populations; (2) African: Creole Surinamese, Antillean and Ghanaian; (3) other: Hindu, Turkish, Moroccan and other non-Caucasian), n (%)), maternal education years after primary school (<6/6–10/>10 years, n (%)), breast-feeding duration (weeks), use of vitamin D drops in infancy (no/yes), game-time child at age 5–6 (<1/≥1 h, n (%)), television-time child at age 5–6 (<1/≥1 h, n (%)), maternal age (years), smoking during pregnancy (yes/no, n (%)), alcohol consumption during pregnancy (yes/no, n (%)) and physical activity level during pregnancy (no, low, moderate, vigorous (n, %)) was collected by means of questionnaires completed by the mothers during pregnancy, 3 months after delivery and when children were 5–6 years old. Pre-pregnancy BMI (weight/height2) was calculated using measures of self-reported height and weight. Winter/summer season was defined based on the date of blood sampling. Blood samples collected in June, July, August, September, October and November were defined as ‘summer’ months; samples collected in December, January, February, March, April and May were defined as ‘winter’ months( Reference Brouwer-Brolsma, Vaes and van der Zwaluw 32 ).
Statistical analyses
Participant characteristics are reported over maternal serum 25(OH)D quartiles as means and standard deviations, or numbers and percentages. Moreover, columns displaying the characteristics of the whole ABCD study population as well as the total subsample were included. Medians and IQR were used to report skewed variables. Differences between quartiles were analysed by means of ANOVA (normally distributed continuous variables), Kruskal–Wallis (skewed continuous variables) and χ 2 test (categorical variables). Linearity of the associations between maternal vitamin D status and childhood cognitive performance was investigated using restricted cubic spline regression, which was conducted with three knots located at the 1st, 5th and 9th deciles of 25(OH)D status. Multivariable regression analyses were used to quantify the strength of the associations between maternal 25(OH)D status and child cognitive performance at age 5 years. Analyses were adjusted for age, sex of child, date of blood sampling using a sinusoidal function( Reference Sachs, Shoben and Levin 33 ) (model 1), birth order, birth weight, gestational age at birth, ethnicity, maternal education years after primary school, breast-feeding duration, vitamin D drops, game-time, television-time, maternal age, pre-pregnancy BMI, smoking during pregnancy, alcohol consumption during pregnancy and physical activity level during pregnancy (model 2). Potential interactions of maternal 25(OH)D status with ethnicity, sex and age were analysed by including a cross-product term in the fully adjusted model, and by analysing the data per ethnic group (i.e. Caucasian, African, other), sex (i.e. boy, girl) and age-group (i.e. <5·6 years, ≥5·6 years). A two-sided P value of ≤0·05 was considered to be statistically significant. Except for the restricted cubic spline analyses, all statistical analyses were performed using the statistical package SAS, version 9.3 (SAS Institute Inc.). Restricted cubic spline analyses were performed using R version 2.15.
Results
Participant characteristics stratified by serum 25(OH)D quartile are shown in Table 1. In this population, the mean 25(OH)D concentration was 60 (sd 31) nmol/l of which 38 and 18 % of the women had a status below 50 and 30 nmol/l, respectively. Compared with women with higher 25(OH)D concentrations, women exhibiting the lowest 25(OH)D concentrations were significantly younger, more likely to smoke during their pregnancy, had a higher pre-pregnancy BMI and less education years. Women with the lowest 25(OH)D concentrations were also less likely to consume alcohol during their pregnancy and to physically exercise. Children exposed to the lowest intra-uterine 25(OH)D concentrations had a significantly lower birth weight, were less likely to be the first child in the family, had a shorter breast-feeding duration, and were less likely to have received vitamin D drops/supplements after birth compared with children exposed to higher intra-uterine 25(OH)D concentrations. Whereas 87–98 % of the mother–child pairs in the second, third, or fourth 25(OH)D quartile were of Caucasian origin, only 44 % of the mother–child pairs in the first 25(OH)D quartile were Caucasian. Moreover, compared with the total ABCD-study sample, the subsample used for the analyses on 25(OH)D status and childhood cognition was somewhat higher educated, more likely to be exposed to alcohol during pregnancy and vitamin D drops after birth, and more likely to be Caucasian. Crude analyses furthermore suggest poorer motor fluency and flexibility with higher 25(OH)D concentrations as shown by, for example, a larger mean distance (mm) between mouse cursor and the actual target on the pursuit for children exposed to higher intra-uterine 25(OH)D concentrations (online Supplementary Table S1).
Table 1 Amsterdam Born Children and their Development (ABCD)-study population characteristics by quartiles (Q) of maternal 25-hydroxyvitamin D status (nmol/l)Footnote * (Numbers and percentages; mean values and standard deviations)
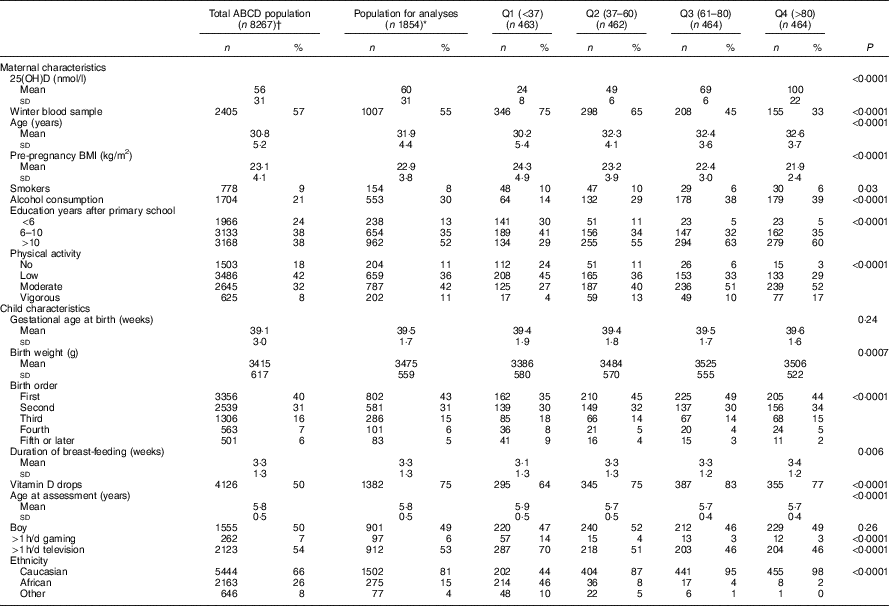
* Differences between quartiles were analysed by means of ANOVA (normally distributed continuous variables), Kruskal–Wallis (skewed continuous variables), or χ 2 test (categorical variables). Missing’s for the population for analyses: pre-pregnancy BMI, n 111; alcohol during pregnancy, n 1; physical activity, n 1; gestational age, n 4; birth weight, n 10; birth order, n 1; duration of breast-feeding, n 22; gaming, n 144; television, n 136.
† Data are selected based on the mothers returning a pregnancy questionnaire (n 8267); within this ‘total ABCD population’, vitamin D status was determined in n 4250. Missing for the population returning the pregnancy questionnaire: pre-pregnancy BMI, n 784; alcohol during pregnancy, n 4; smoking during pregnancy, n 12; physical activity, n 7; gestational age, n 394; birth weight, n 475; birth order, n 1; duration of breast-feeding, n 2109; age at assessment, n 5156; sex, n 5156; gaming, n 4348; television, n 4335; ethnicity, n 13.
Restricted cubic splines for first trimester serum 25(OH)D status and childhood cognitive function that were adjusted for all available covariates suggested linearity (online Supplementary Fig. S1). In unadjusted linear regression analyses, the associations of serum 25(OH)D with motor fluency and flexibility, represented by the tasks pursuit and tracking, reached statistical significance. After adjustment for age, sex of the child and date of blood sampling, these associations attenuated and became non-significant. In contrast, adjustment for age, sex of the child and date of blood sampling, substantially strengthened the associations between serum 25(OH)D and the cognitive domains attention (i.e. baseline speed) and executive function (i.e. response organisation objects part 1). After adjustment for all available covariates the associations remained, showing a significantly faster reaction time, faster response speed and better response speed stability per 1 nmol/l increase in 25(OH)D, β −0·30 (sd 0·14), β −0·58 (sd 0·21) and β −0·45 (sd 0·17), respectively (Table 2). Additional adjustment for the number of errors somewhat attenuated the associations for response organisation objects part 1, showing β −0·49 (sd 0·19) (P=0·01) for response speed and β −0·38 (sd 0·16) (P=0·02) for response speed stability (data not shown in tables).
Table 2 Linear regression analyses showing the associations between 25-hydroxyvitamin D status and child cognition at age 5–6 years (β-Coefficients and standard deviations)
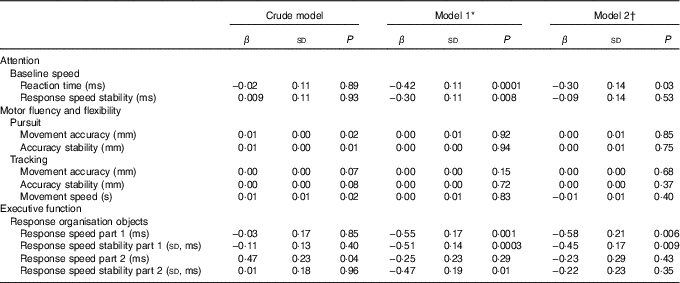
* Model 1 is adjusted for age, sex of child and date of blood sampling.
† Model 2 is adjusted for model 1, demographics and child’s characteristics (i.e. birth order, birth weight, gestational age at birth, ethnicity (Caucasian, African, other), maternal education years after primary school (<6, 6–10, >10), breast-feeding duration (weeks), vitamin D drops (no/yes), game-time (<1 h/≥1 h), television-time (<1 h/≥1 h) and maternal characteristics (i.e. age, pre-pregnancy BMI, smoking during pregnancy (yes/no), alcohol consumption during pregnancy (yes/no) and physical activity level during pregnancy (no, low, moderate, vigorous).
Associations between first trimester 25(OH)D concentrations and cognitive performance at age 5–6 years were most pronounced in mother–child pairs of African origin (Table 3). Specifically, each nmol/l increase in 25(OH)D status was associated with a −1·95 (sd 0·94) ms faster response speed in participants of African origin, whereas this was only −0·52 (sd 0·22) ms among those with Caucasian origin (P for interaction: 0·12). Moreover, stratified analyses also suggested a significant association between serum 25(OH)D and attention in those of African origin (i.e. baseline speed, reaction time, β −2·06 (sd 0·70)), whereas no such association was observed in those of Caucasian origin (i.e. baseline speed, reaction time, β 0·17 (sd 0·14)) (P for interaction: 0·0002). Interaction analyses and stratification for age and sex did not suggest substantial differences in the associations for these two factors (online Supplementary Table S2).
Table 3 Linear regression analyses showing associations between 25-hydroxyvitamin D status and cognitive performance at age 5–6 years stratified by ethnicityFootnote * (β-Coefficients and standard deviations)
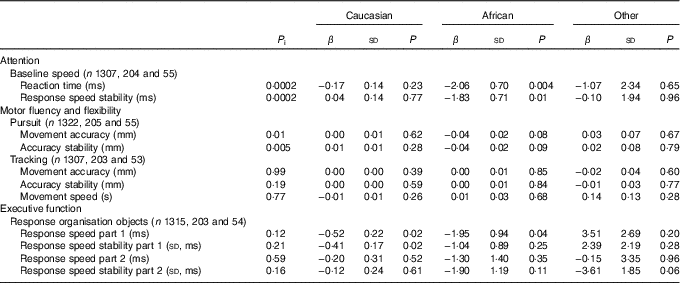
P i, P for interaction.
* The models are adjusted for age and sex of child, date of blood sampling, demographics and child’s characteristics (i.e. birth order, birth weight, gestational age at birth, maternal education years after primary school (<6, 6–10, >10), breast-feeding duration (weeks), vitamin D drops (no/yes), game-time (<1 h/≥1 h), television time (<1 h/≥1 h)), maternal characteristics (i.e. age, pre-pregnancy BMI, smoking during pregnancy (yes/no), alcohol consumption during pregnancy (yes/no), physical activity level during pregnancy (no, low, moderate, vigorous).
Discussion
In this Dutch multi-ethnic mother–child cohort, higher first trimester 25(OH)D status was significantly associated with a better childhood attention and executive functioning – but not motor fluency and flexibility – at age 5–6 years. Stratified analyses pointed towards more pronounced associations of first trimester 25(OH)D status with childhood attention and executive function among participants of African origin. Although statistically significant, the associations observed for the total population are very modest. For instance, for executive function a 10 nmol/l higher 25(OH)D status was associated with a 6-ms faster response speed, which is only 0·6 % of the average response speed on this task (i.e. 666 ms). However, when looking at the associations observed in the subsample of African origin, a 10-nmol/l higher 25(OH)D status was associated with an 20 ms faster response speed, which is 3 % of the average response time on this task in this subpopulation. Considering the low 25(OH)D concentrations in this subgroup (i.e. 29 (sd 19) nmol/l of which 87 % of the women had a 25(OH)D status below <50 nmol/l), an average 20 nmol/l increase in 25(OH)D status in this subgroup would be recommended in order to meet the current recommendations as formulated by the Institute of Medicine( Reference Ross, Manson and Abrams 34 ). As our data suggest that a 20-nmol/l increase in 25(OH)D may equal a 6 % (40 ms) faster response speed, we feel that this result may suggest a relevant role for maternal vitamin D status in childhood cognition among those with 25(OH)D concentrations far below the currently recommended status levels. However, as our data are observational it may be evident that future studies are warranted to confirm our findings.
To the best of our knowledge, this study belongs to one of the largest prospective cohort studies on the potential impact of maternal 25(OH)D status on childhood cognitive performance so far. Another major study in this field was conducted as part of the US Collaborative Perinatal Project (CPP), including 3146 up to 3587 mother–child pairs with data on 25(OH)D status and child cognitive development and achievement collected at the age of 8 months, and 4 and 7 years( Reference Keim, Bodnar and Klebanoff 17 ). In contrast to our study, the US CPP did not point towards a potential benefit of higher 25(OH)D concentrations during gestation. Whereas the variation in 25(OH)D status in the two studies was rather similar, the US CPP used a paper-pencil IQ test to assess childhood cognition while the ABCD population was tested used computerised testing procedures. Moreover, in the US CPP, blood was predominantly (i.e. 86 %) sampled in the second trimester of gestation (median 21 (IQR 7·3) weeks), whereas blood samples in the ABCD population were obtained in the first trimester. As basic brain structures are formed in the first trimester, it may be postulated that our study points towards a specific critical window for exposure. This hypothesis is supported by previous data from the Spanish INfancia y Medio Ambiente Project (n 1820), which showed significant associations between higher early second trimester 25(OH)D concentrations and better mental and psychomotor scores. Alternatively, three somewhat smaller prospective cohort studies also showed significant associations between higher maternal or cord blood 25(OH)D concentrations and better cognitive performance, while in these studies blood was drawn at 18 weeks of gestation( Reference Whitehouse, Holt and Serralha 19 ), 32 weeks of gestation( Reference Hanieh, Ha and Simpson 16 ) or at delivery (cord blood)( Reference Zhu, Tong and Hao 35 ). Thus, these data on their turn suggest that sufficient 25(OH)D concentrations seem to be important throughout the whole pregnancy, which is also very plausible as the brain continues to develop during pregnancy where in the third trimester even the so-called brain-growth-spurt initiates. Aforementioned findings highlight the need for future studies in which 25(OH)D concentrations are measured at various time points during gestation.
Before continuing to the final conclusion of our research, first, some strengths and limitations need to be discussed. A major strength of this study relates to the multi-ethnic origin of the population, where stratified analyses showed that the link between maternal 25(OH)D status and child cognitive performance was most pronounced in those of African origin. Although the African-subsample was rather small (n 205), it may be postulated that this modification effect relates to genetic differences between the different ethnic populations. However, our data also indicate that participants of African origin had much lower 25(OH)D concentrations than Caucasian participants. Given findings of Zhu et al.( Reference Zhu, Tong and Hao 35 ) and Hanieh et al.( Reference Hanieh, Ha and Simpson 16 ), which suggest an optimal 25(OH)D cord blood concentration somewhere between 30–50 nmol/l, the modification effect observed in our study may also reflect a threshold effect of a minimally required 25(OH)D status for optimal brain development. More specifically, with a mean 25(OH)D concentration of 68 (sd 28) nmol/l in the Caucasian subsample, the 25(OH)D concentrations in the Caucasian subsample may already be optimal for fetal brain development and hence limit the possibility to detect potential adverse influences of insufficient 25(OH)D concentrations. Other strengths include the extensive neuropsychological test battery used and the possibility to adjust for many potentially relevant covariates. Conversely, limitations of our study are that not all recruited mother–child pairs participated in the cognitive assessment at age 5–6 years; thus, selection bias may have affected the observed associations. Moreover, given the sample size of our population we were only able to stratify our data according to white, dark and other skin colour based on country of birth of the grandmother, while more specific stratified analyses (i.e. differentiating between Caucasian, African, Moroccan, Turkish, Asian, etc.) would have been even more informative. In addition, using birth country of the child’s grandmother as a proxy for skin colour may have led to misclassification as children of an ‘African’ mother, for instance, may have a Caucasian father and vice versa. Finally, although we observed an association between 25(OH)D status and executive function as measured with the response organisation object part 1, we did not observe an association between 25(OH)D and the response organisation object part 2. Although we do not have a definite explanation for this discrepancy, this difference may relate to the fact that the response organisation object part 2 included reversed reaction patterns (inhibition) and therefore required a more complex cognitive strategy than the response organisation object part 1. Hence, it may be that this second part of the test was too difficult for this relatively young population and as such did not provide useful data for our analyses.
In this study we showed significant associations between low maternal vitamin D concentrations during pregnancy and a poorer attention and executive function, particularly among participants of African origin. No association was observed between 25(OH)D and motor fluency and flexibility. To substantiate this finding, further study on this potential effect is warranted, for instance by investigating vitamin D in relation to more robust measures of brain development and function (e.g. imaging and electroencephalography), exploring vitamin D supplementation effects on epigenetic signatures related to brain development and function, and by examining aforementioned associations in various ethnic groups that are sufficiently powered. If future studies confirm a beneficial vitamin D supplementation effect with respect to brain development and function of the developing child, the benefit of vitamin D supplementation during gestation can be easily communicated as soon as pregnant women call in for their first antenatal visit.
Acknowledgements
The authors thank all participants, principal investigators and collaborators of the ABCD study.
This study has been supported by the Netherlands Organization for Health Research and Development (ZonMw), The Hague.
E. M. B.-B. analysed the data and wrote the paper. E. J. M. F. provided input to the draft and had primary responsibility for the final content. T. G. M. V was involved in project conception, development of the overall research plan, study oversight and contributed to the finalisation of the manuscript. All authors read and approved the final manuscript.
E. M. B.-B. reports to have filed a patent related to vitamin D and cognitive executive function. T. G. M. V has nothing to disclose. E. J. M. F. reports to have filed a patent related to vitamin D and cognitive executive function.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518001319







