The efficacy and safety of long-acting injectable risperidone have been evaluated in several trials of patients with schizophrenia or schizoaffective disorder, including a 12-week, double-blind, placebo-controlled study (n=554; Reference Kane, Eerdekens and LindenmayerKane et al, 2003) and a 12-month open-label trial (n=615; Reference Fleischhacker, Eerdekens and KarcherFleischhacker et al, 2003). The effectiveness of long-acting risperidone has also been demonstrated in patients switched from typical and atypical oral antipsychotic medication (Reference Lindenmayer, Eerdekens and BerryLindenmayer et al, 2004; Reference Chue, Eerdekens and AugustynsChue et al, 2005) and from conventional depot antipsychotics (Reference Turner, Eerdekens and JackoTurner et al, 2004). In the 12-week double-blind study by Chue et al (Reference Chue, Eerdekens and Augustyns2005), long-acting risperidone was compared with oral risperidone in patients with schizophrenia. Both treatments were efficacious and well tolerated. According to a non-inferiority analysis, the two treatments showed comparable efficacy in Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987) total scores over the short term. Long-acting risperidone, however, has not been compared with an oral formulation of another atypical antipsychotic such as olanzapine. In this 53-week, open-label, randomised controlled international study (registered with the US National Institutes of Health at http://clinicaltrials.gov as NCT00236457) we compared long-acting risperidone with olanzapine tablets in patients with schizophrenia or schizoaffective disorder.
The objectives of the study were, first, to demonstrate that in the short term long-acting injectable risperidone was at least as effective as (not inferior to) oral olanzapine in patients with schizophrenia or schizoaffective disorder. Non-inferiority would be demonstrated if, at the end of the initial 13-week treatment period, the upper limit of the confidence interval for the difference in mean change from baseline in PANSS total scores was not more than 8 points in favour of oral olanzapine. The second objective was to examine the long-term efficacy and safety of long-acting risperidone and oral olanzapine in these patients.
METHOD
The study protocol and amendments were reviewed by independent ethics committees or institutional review boards. The study was conducted in accordance with the recommendations guiding physicians in biomedical research involving humans contained in the 1989 version of the Declaration of Helsinki and according to the guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2001). All patients or their legal representatives gave their written informed consent to participate in the trial.
Participants
Patients with schizophrenia or schizoaffective disorder were recruited at 48 centres (in Australia, Belgium, France, Germany, Greece, Luxembourg, Poland, Russia, Spain, The Netherlands and the UK). Inclusion criteria included a diagnosis of schizophrenia or schizoaffective disorder (DSM–IV; American Psychiatric Association, 1994); PANSS total score 50 or over at randomisation; age at least 18 years; body mass index (BMI) not exceeding 40 mg/kg2; and the requirement that within the previous 2 months the patient had been hospitalised or required medical intervention for an acute exacerbation of psychosis and had experienced an additional acute exacerbation during the previous 2 years. Exclusion criteria were prior treatment with clozapine or with a depot anti-psychotic within one treatment cycle before screening, and resistance or sensitivity to risperidone or olanzapine. Also excluded were women who were pregnant or breast-feeding or, if of child-bearing age, not using adequate contraception.
Protocol deviations that warranted exclusion from the primary efficacy analysis were:
-
(a) patients who discontinued before week 8 of treatment (to meet International Conference on Harmonisation guidelines);
-
(b) patients who received additional antipsychotic treatment (other than oral risperidone for patients in the risperidone arm or olanzapine in the olanzapine arm) between the end of run-in and the end of the first 13-week period;
-
(c) patients who started treatment with a depot antipsychotic within one treatment cycle before the randomisation visit or who started another depot treatment during the initial 13-week period.
Randomisation
The patients were randomised to receive long-acting risperidone or olanzapine, with stratification factors of psychopathology (PANSS total scores), number of previous psychiatric hospitalisations, BMI and inpatient or out-patient status, using a central dynamic randomisation procedure. Randomisation was based on a minimisation algorithm that used a probability of assignment other than 0.5 to maintain balance of treatment groups within levels of each stratification factor. Constraints on imbalance were defined within each level of each factor; violation of a constraint resulted in adjustment to the treatment assignment probabilities. Randomisation numbers were allocated by an interactive voice response system (IVRS). When a participant was ready to be randomised, the investigator called the IVRS by telephone and entered the person's stratification information. Based on the minimisation algorithm, the IVRS returned the randomisation number of the appropriate box of study medication at the site.
Dosing and delivery of long-acting risperidone
According to the original study protocol, patients in the long-acting risperidone group received 25, 50 or 75 mg of long-acting risperidone. After study initiation, the original clinical trial programme revealed that the 75 mg dose provided no greater benefit than the lower doses and the protocol was amended to restrict doses to 25 or 50 mg of long-acting risperidone. The 64 patients who had already received 75 mg of long-acting risperidone completed the end-point visit and were then withdrawn from this study and invited to enrol in an open-label extension study. Thus, patients receiving 25 or 50 mg of long-acting risperidone were the focus of the analyses reported here.
During week 1 of the study previous antipsychotic treatments were discontinued and replaced with oral risperidone. The dose of oral risperidone was adjusted to 2, 4 or 6 mg according to each patient's clinical response. The initial dose of long-acting risperidone was determined by a protocol-specified conversion scheme: patients who had received 2–4 mg of oral risperidone during week 1 received 25 mg per 14 days of long-acting risperidone and patients who had received 6 mg of oral risperidone during week 1 received 50 mg per 14 days of long-acting risperidone. The dosage of long-acting risperidone could be adjusted during the trial according to each patient's clinical response. Before the protocol was amended and doses were restricted to 25 and 50 mg of long-acting risperidone, patients who had received 6 mg of oral risperidone during week 1 received 75 mg of long-acting risperidone.
Oral risperidone at the week 1 dosage was continued for 3 weeks after the first injection of long-acting risperidone. Oral risperidone supplementation was given when necessary after the initial 3 weeks.
Dosages of oral olanzapine
During week 1 previous medications were discontinued and oral olanzapine was introduced and adjusted to the patients’ optimal dosage of 5–20 mg/day at daily increments of 5 mg. During weeks 2–53 the patients received flexible dosages of 5–20 mg/day of olanzapine.
Assessments of efficacy and safety
The primary measure of efficacy was the change in total score on the PANSS (Structured Clinical Interview) from baseline to end-point (last observation carried forward, LOCF) in the initial 13-week period (short-term outcome). The secondary measures (long-term outcomes) were the changes in PANSS total scores from base-line to month 12 and end-point; changes in PANSS factor scores (positive symptoms, negative symptoms, disorganised thoughts, uncontrolled hostility/excitement and anxiety/depression; Reference Marder, Davis and ChouinardMarder et al, 1997); and changes in scores on the Clinical Global Impression – Severity (CGI–S; Reference GuyGuy, 1976) scale. Quality of life was evaluated by means of the Wisconsin Quality of Life Index (Reference Becker, Diamond and SainfortBecker et al, 1993). The Wisconsin test was designed for patients with severe mental illness and comprises nine dimensions: life satisfaction, occupational activities, psychological well-being, physical health, social relations, economics, activities of daily living, symptoms and the patient's own goals.
Clinical improvement was defined as a 20% or greater reduction in PANSS total scores. Maintenance of effect was assessed by determining the time to significant deterioration in the psychotic condition, defined as hospitalisation for symptom exacerbation; the need for an increased level of care and an increase in CGI–S scores of 2 points or more over a 2-week period; or self-injury, suicidal or homicidal ideation or violent behaviour. Significant psychotic deterioration was assessed in the total group and in patients who were rated as stabilised after 13 weeks of treatment. A patient was considered stabilised if he or she had been on the same dosage for 4 weeks or more, the PANSS total score at week 13 did not exceed 70 and the CGI–S score at weeks 9 and 13 was 3 or below and did not increase between weeks 9 and 13.
Assessments were completed at baseline (randomisation), weeks 5, 9, 13, 25, 37 and 53 and at end-point (last observation carried forward, LOCF). The CGI–S was also completed at weeks 1 and 3 and psychotic deterioration was evaluated at week 3. Adverse events were recorded at each visit. Severity of movement disorders was assessed by means of the Simpson–Angus Rating Scale (SARS; Reference Simpson and AngusSimpson & Angus, 1970) at baseline, at weeks 13, 25, 37 and 53 and at end-point.
Statistical analysis
Differences in changes in PANSS total scores from baseline to end-point (LOCF) in the 13-week study between the two treatment groups were evaluated by an analysis of covariance (ANCOVA) model. Factors included in the model were baseline PANSS score as covariate, randomisation group, the stratification variables (excluding the PANSS factor since it was included as covariate) and investigator nested in country. Because some investigators had only a few patients, pooling of some investigators and countries was required for the fixed-effects ANCOVA model. To avoid pooling, an additional ANCOVA model was performed (for 13 weeks, month 12 visit and end-point) in which country and investigator were treated as random effects. The number and proportion of patients who achieved clinical improvement were tabulated at each assessment point and at endpoint. The 95% CI of the odds ratios of the two treatment groups was obtained at month 12 and at end-point. A logistic model (with logit link function and binomial error structure) was applied with the stratification variables as fixed effects and investigator as random effect.
The number and proportion of participants who experienced a significant deterioration were tabulated at each assessment point and at end-point. A Cox proportional hazards model (controlling for the four stratification variables and stratified by country) was used to obtain the 95% CI of the ratio of the hazards in both treatment groups.
RESULTS
Of the 618 patients who were randomised and treated (318 to long-acting risperidone and 300 to olanzapine), 64 were excluded from the short-term (weeks 1–13) analysis of efficacy because they received injections of 75 mg of long-acting risperidone; 66 (38 risperidone and 28 olanzapine patients) were excluded because of major protocol deviations and 110 (52 risperidone and 58 olanzapine patients) were excluded because of non-adherence to Good Clinical Practice standards at one study site. The principal protocol deviations were use of unapproved concomitant medications and inadequate duration of treatment. Thus the per-protocol short-term sample included 164 patients in the long-acting risperidone group and 214 in the olanzapine group (Fig. 1). For the analysis of long-term treatment (months 1–12) a further 2 patients were excluded because they received 75 mg of long-acting risperidone and 14 patients (7 risperidone and 7 olanzapine) were excluded because of major protocol deviations. Thus the per-protocol long-term sample for the evaluation of efficacy included 155 patients in the long-acting risperidone group and 207 in the olanzapine group (Fig. 1). Safety was evaluated in all randomised participants who received at least one dose of study medication and did not receive a 75 mg injection during the entire trial: 247 in the long-acting risperidone group and 300 in the olanzapine group.
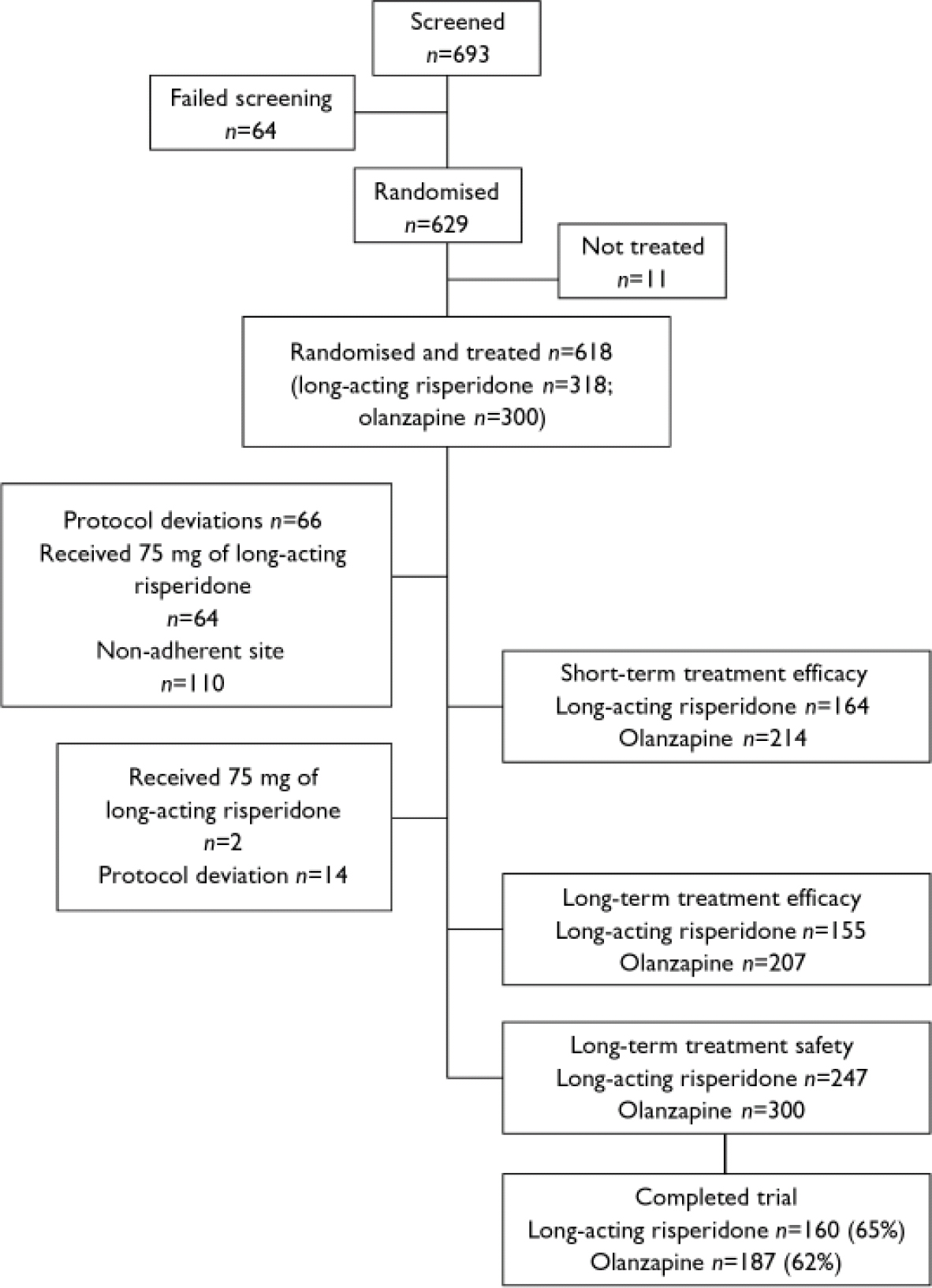
Fig. 1 Patient disposition.
Patient characteristics and disposition
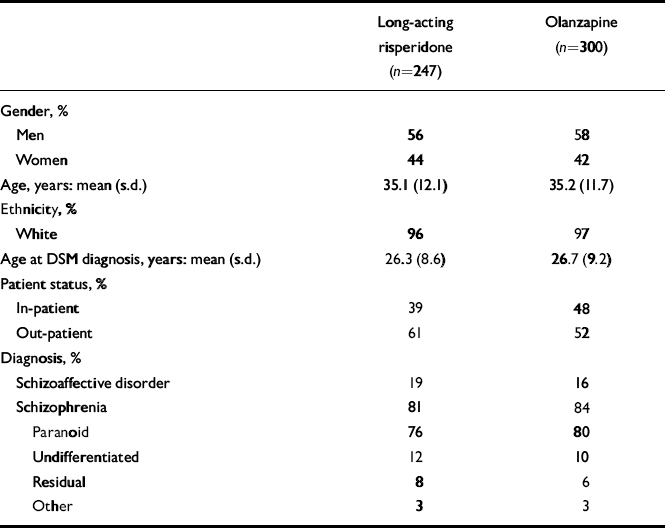
Background characteristics of the two patient groups were similar (Table 1). Of the 547 patients who were randomised, received at least one dose of study medication and did not receive a 75 mg injection during the entire trial, 347 (63%) completed the 12-month trial. These included 160 (65%) of the long-acting risperidone group and 187 (62%) of the oral olanzapine group (Fig. 1, Table 2).
Table 1 Patient characteristics
| Long-acting risperidone (n=247) | Olanzapine (n=300) | |
|---|---|---|
| Gender, % | ||
| Men | 56 | 58 |
| Women | 44 | 42 |
| Age, years: mean (s.d.) | 35.1 (12.1) | 35.2 (11.7) |
| Ethnicity, % | ||
| White | 96 | 97 |
| Age at DSM diagnosis, years: mean (s.d.) | 26.3 (8.6) | 26.7 (9.2) |
| Patient status, % | ||
| In-patient | 39 | 48 |
| Out-patient | 61 | 52 |
| Diagnosis, % | ||
| Schizoaffective disorder | 19 | 16 |
| Schizophrenia | 81 | 84 |
| Paranoid | 76 | 80 |
| Undifferentiated | 12 | 10 |
| Residual | 8 | 6 |
| Other | 3 | 3 |
Table 2 Study completion and reasons for discontinuation

| Long-acting risperidone (n=247) % | Olanzapine (n=300) % | |
|---|---|---|
| Completed | 65 | 62 |
| Discontinued | 35 | 38 |
| Consent withdrawal | 18 | 7 |
| Insufficient response | 9 | 11 |
| Non-adherence | 2 | 8 |
| Adverse events | 3 | 4 |
| Other | 3 | 8 |
Medication dosage and duration
The patients received a mean of 20.3 injections (s.d.=8.8) of long-acting risperidone. The mean modal dosage was 40.7 mg per 14 days (s.d.=12.1) and the mean duration of treatment with long-acting risperidone was 274 days (s.d.=124.2). A modal dosage of 25 mg per 14 days was received by 37% of the patients and 50 mg per 14 days was received by 63%. Oral risperidone supplementation was received by 98% of the patients during the 3 weeks after the first injection of long-acting risperidone (as per protocol). Oral risperidone supplementation was received by 54 patients (25%) during months 2–3 at a mean modal dosage of 2.2 (s.d.=0.7) mg/day, and by 14–16% patients during the remainder of the trial. When a dosage increase in long-acting risperidone was deemed necessary, additional coverage with oral risperidone was required during the first 3 weeks of the higher dosage.
The mean dose of olanzapine during months 1–12 was 14.6 mg/day (s.d.=4.6) and the duration of treatment was 285 days (s.d.=119.7). Most patients (62%) received modal doses of 15 mg (24%) or 20 mg (38%).
Concomitant medications
Concomitant medications were received by 85% of patients in the long-acting risperidone group and 80% of patients in the olanzapine group. These included sedatives or hypnotics, taken by 65 and 53% respectively; antidepressants, taken by 43 and 34%; antiparkinsonian drugs, taken by 37 and 18%; anticonvulsants, taken by 21 and 19%; and muscle relaxants, by 11 and 10% respectively.
Medication adherence
Medication adherence was high. In the risperidone group the mean injection interval was 14.2 days (range 13–16) and in the olanzapine group the mean time off drug per patient was 0.7 days (s.d.=3.7, range 0–52).
Efficacy
Short-term outcome (weeks 1–13)
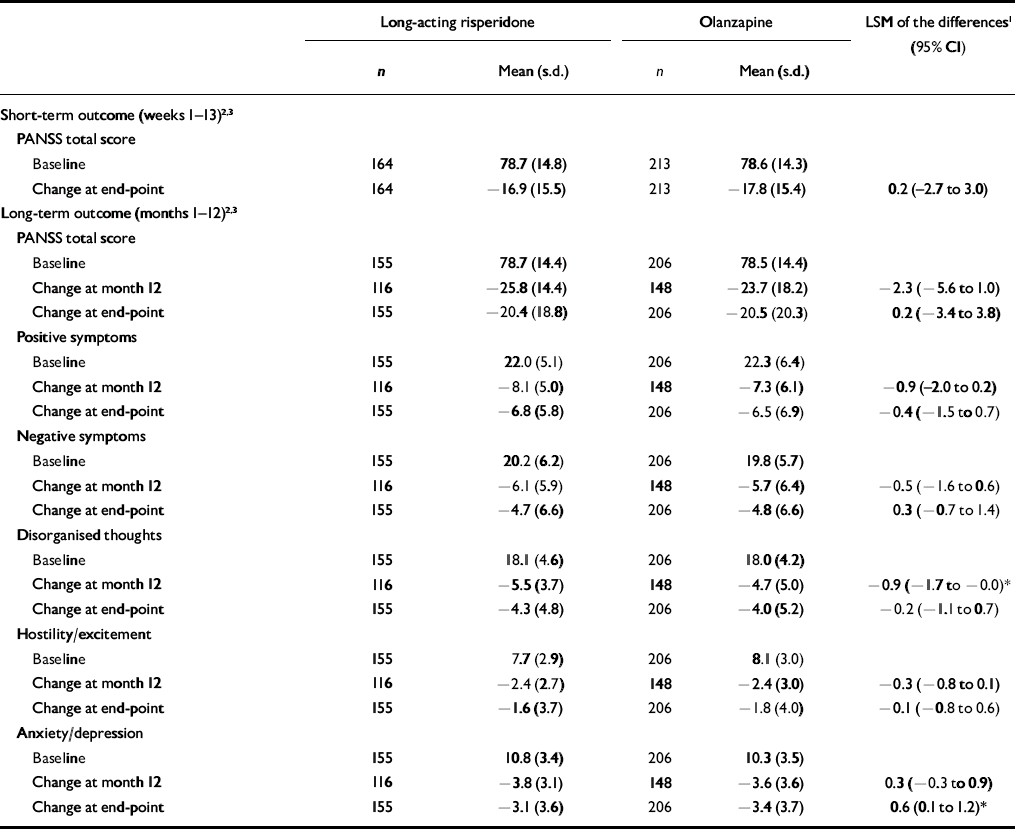
The upper limit of the PANSS 95% CI (score of 3.0) was well below the non-inferiority margin (score of 8), demonstrating the primary end-point that long-acting risperidone was at least as effective as olanzapine (Table 3).
Table 3 Positive and Negative Syndrome Scale total and factor scores in patients receiving long-acting risperidone or olanzapine
| Long-acting risperidone | Olanzapine | LSM of the differences1 (95% CI) | |||
|---|---|---|---|---|---|
| n | Mean (s.d.) | n | Mean (s.d.) | ||
| Short-term outcome (weeks 1-13)2,3 | |||||
| PANSS total score | |||||
| Baseline | 164 | 78.7 (14.8) | 213 | 78.6 (14.3) | |
| Change at end-point | 164 | -16.9 (15.5) | 213 | -17.8 (15.4) | 0.2 (-2.7 to 3.0) |
| Long-term outcome (months 1-12)2,3 | |||||
| PANSS total score | |||||
| Baseline | 155 | 78.7 (14.4) | 206 | 78.5 (14.4) | |
| Change at month 12 | 116 | -25.8 (14.4) | 148 | -23.7 (18.2) | -2.3 (-5.6 to 1.0) |
| Change at end-point | 155 | -20.4 (18.8) | 206 | -20.5 (20.3) | 0.2 (-3.4 to 3.8) |
| Positive symptoms | |||||
| Baseline | 155 | 22.0 (5.1) | 206 | 22.3 (6.4) | |
| Change at month 12 | 116 | -8.1 (5.0) | 148 | -7.3 (6.1) | -0.9 (-2.0 to 0.2) |
| Change at end-point | 155 | -6.8 (5.8) | 206 | -6.5 (6.9) | -0.4 (-1.5 to 0.7) |
| Negative symptoms | |||||
| Baseline | 155 | 20.2 (6.2) | 206 | 19.8 (5.7) | |
| Change at month 12 | 116 | -6.1 (5.9) | 148 | -5.7 (6.4) | -0.5 (-1.6 to 0.6) |
| Change at end-point | 155 | -4.7 (6.6) | 206 | -4.8 (6.6) | 0.3 (-0.7 to 1.4) |
| Disorganised thoughts | |||||
| Baseline | 155 | 18.1 (4.6) | 206 | 18.0 (4.2) | |
| Change at month 12 | 116 | -5.5 (3.7) | 148 | -4.7 (5.0) | -0.9 (-1.7 to -0.0)* |
| Change at end-point | 155 | -4.3 (4.8) | 206 | -4.0 (5.2) | -0.2 (-1.1 to 0.7) |
| Hostility/excitement | |||||
| Baseline | 155 | 7.7 (2.9) | 206 | 8.1 (3.0) | |
| Change at month 12 | 116 | -2.4 (2.7) | 148 | -2.4 (3.0) | -0.3 (-0.8 to 0.1) |
| Change at end-point | 155 | -1.6 (3.7) | 206 | -1.8 (4.0) | -0.1 (-0.8 to 0.6) |
| Anxiety/depression | |||||
| Baseline | 155 | 10.8 (3.4) | 206 | 10.3 (3.5) | |
| Change at month 12 | 116 | -3.8 (3.1) | 148 | -3.6 (3.6) | 0.3 (-0.3 to 0.9) |
| Change at end-point | 155 | -3.1 (3.6) | 206 | -3.4 (3.7) | 0.6 (0.1 to 1.2)* |
Long-term outcomes (months 1–12)
Significant improvements in PANSS total and factor scores from baseline to month 12 and end-point were seen in both groups of patients (Table 3, Fig. 2). Among the patients who completed the long-term trial, significantly greater improvement on one PANSS factor score (disorganised thoughts, P<0.05) was seen in patients receiving long-acting risperidone than in those receiving oral olanzapine (Table 3). At end-point, significantly greater improvement in anxiety/depression was seen in the olanzapine group.

Fig. 2 Changes in Positive and Negative Syndrome Scale (PANSS) total scores (a) and scores on the five PANSS factors ((b), positive symptoms; (c), negative symptoms; (d), disorganised thoughts; (e) hostility/excitement; (f) anxiety/depression) from week 5 to end-point.
Clinical improvement (20% minimum reduction in PANSS total scores) was achieved by significantly more patients receiving long-acting risperidone than those receiving oral olanzapine at month 12 (91 v. 79%; P<0.001), based on a logistic regression model controlling for in-patient/out-patient status, BMI, number of previous hospitalisations and investigator. At end-point, 79% of patients in the long-acting risperidone group and 73% in the olanzapine group achieved clinical improvement (P=0.057; Fig. 3). Similar reductions in the overall severity of illness (CGI–S score) were seen in the long-acting risperidone and olanzapine groups: mean CGI–S scores at baseline were 3.1 (s.d.=0.8) in the long-acting risperidone group and 3.3 (s.d.=0.9) in the olanzapine group, and mean changes at end-point were –1.1 (s.d.=1.2) and –1.3 (s.d.=1.2) respectively. The proportions of patients who were rated as ‘not ill’ or ‘mildly ill’ increased respectively from 19 and 17% at baseline to 82 and 76% at month 12 and 66 and 67% at end-point. Mean scores on the patient version of the Wisconsin Quality of Life Index were similar in the two treatment groups at baseline: 0.40 (s.d.=0.85) and 0.38 (s.d.=0.92). Patients’ quality of life improved from baseline to end-point on all sub-scale ratings. Clinically meaningful improvements (score changes >0.5 points) were seen in three domains in both treatment groups: occupational activities, psychological well-being and symptoms/outlook.
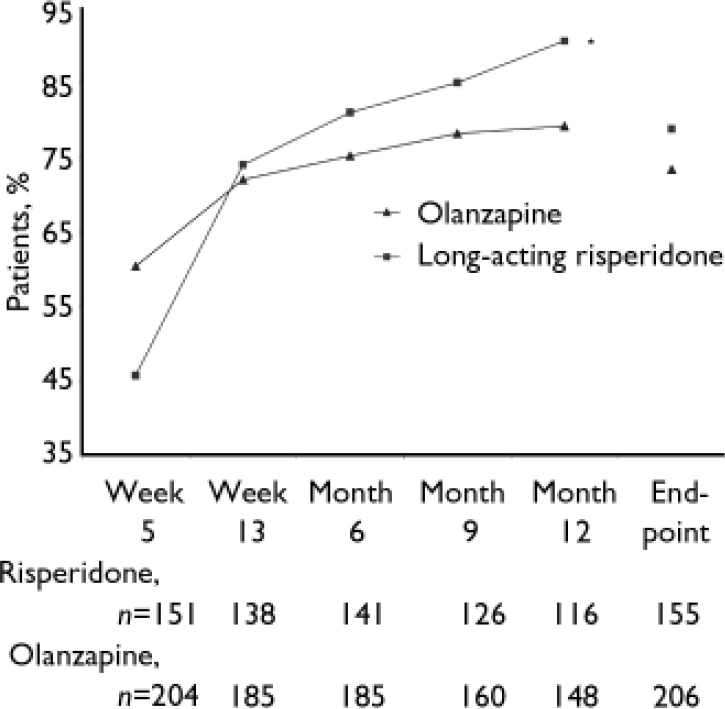
Fig. 3 Percentages of patients with clinical improvement (minimum 20% reduction in Positive and Negative Syndrome Scale total scores) from week 5 to end-point. *P=0.001 v. olanzapine.
Maintenance of effect
The proportion of patients with significant deterioration in psychotic symptoms was 0.6% in the long-acting risperidone group and 2.0% in the olanzapine group at week 3; these respective proportions rose to 3% and 2% at week 13 and at month 12 and to 10% and 9% at end-point. The time to first deterioration was comparable in the two groups (hazard ratio 1.38, 95% CI 0.82–2.33). Among the 179 patients who were stabilised at week 13, significant deterioration was noted in 3% of both the long-acting risperidone group and the olanzapine group at month 12, and in 5% and 6% respectively at end-point. The time to first deterioration was comparable in the two groups (hazard ratio 1.37, 95% CI 0.47–3.99).
Safety
Adverse events
Treatment-emergent adverse events reported by 5% or more of patients in either group are listed in Table 4. Adverse events resulted in treatment discontinuation for 7 patients in the long-acting risperidone group (3%) and 11 patients in the olanzapine group (4%). Serious adverse events were reported by 23% of the patients in the long-acting risperidone group and 21% of the olanzapine group (Table 4).
Table 4 Adverse events reported by at least 5% of patients and serious adverse events reported by at least 2% of patients in either group

| Long-acting risperidone % | Olanzapine % | |
|---|---|---|
| Adverse events1 | ||
| Psychosis | 29 | 25 |
| Insomnia | 22 | 14 |
| Depression | 20 | 14 |
| Anxiety | 14 | 16 |
| Agitation | 10 | 5 |
| Headache | 8 | 5 |
| Hyperkinesia | 8 | 3 |
| Extrapyramidal disorder | 7 | 4 |
| Rhinitis | 7 | 6 |
| Weight increase | 6 | 9 |
| Somnolence | 5 | 7 |
| Tremor | 5 | 2 |
| Injury | 5 | 2 |
| Serious adverse events2 | 23 | 21 |
| Psychosis | 12 | 12 |
| Suicide attempt | 4 | 3 |
| Anxiety | 3 | 2 |
| Injury | 2 | 1 |
Adverse events related to extrapyramidal symptoms were reported by 25% of the long-acting risperidone group and 15% of the olanzapine group (P<0.05; Table 5). Only one patient (in the long-acting risperidone group) discontinued treatment because of an extrapyramidal adverse event (hyperkinesia). Severity of extrapyramidal symptoms was mild in both treatment groups. Median scores on the SARS – scores range from 0 (no symptom) to 4 (extreme) – were 0 at all time points in both treatment groups. At end-point, SARS total scores ranged from 0 to 1.5 in the long-acting risperidone group and from 0 to 1.7 in the olanzapine group. New-onset tardive dyskinesia was reported in two patients in each treatment group.
Table 5 Adverse events related to extrapyramidal symptoms reported in the two patient groups

| Long-acting risperidone (n=247) % | Olanzapine (n=300) % | |
|---|---|---|
| Hyperkinesia | 8 | 4 |
| Extrapyramidal disorder | 8 | 4 |
| Tremor | 7 | 3 |
| Hypertonia | 4 | 3 |
| Tardive dyskinesia | 1 | 1 |
| Dystonia | 1 | 0 |
| Involuntary muscle contractions | 1 | <1 |
| Oculogyric crisis | 1 | <1 |
| Dyskinesia | <1 | <1 |
| Tetany | <1 | 0 |
| Hypokinesia | 0 | <1 |
Treatment-emergent sexual side-effects were reported by 3% of the patients in each treatment group. The most common of these were non-puerperal lactation (in five patients in the long-acting risperidone group and two patients in the olanzapine group) and impotence (in two patients in each group). One patient in each group discontinued because of a sexual side-effect. Glucose-related adverse events were reported in 2% of patients in both the long-acting risperidone and olanzapine groups. These included diabetes mellitus in one patient in each group; hyperglycaemia in four patients in each group; and hypoglycaemia in one patient in the olanzapine group. No clinically relevant change in mean laboratory test values was seen in either treatment group.
Deaths
Eight patients died during the study or soon after its termination, two in the long-acting risperidone group and six in the olanzapine group. Causes of death were accident (n=1) and oesophageal cancer (n=1) in the long-acting risperidone group, and cardiac insufficiency/circulatory insufficiency (n=1), status epilepticus/myocardial ischaemia, (n=1) myocardial infarction (n=1), pneumonia (n=1), and suicide (n=2) in the olanzapine group.
Body weight
Body weight increased by 1.7 kg in the long-acting risperidone group and by 4.0 kg in the olanzapine group (P<0.05; Fig. 4). Body weight increases of 7% or more were seen in 20% of the long-acting risperidone group and 36% of the olanzapine group; decreases of 7% were seen in 6% of patients in both groups. Body mass index increased by 0.6 kg/m2 in the long-acting risperidone group and by 1.4 kg/m2 in the olanzapine group (P < 0.05). Six patients discontinued because of weight gain, one in the risperidone group and five in the olanzapine group.
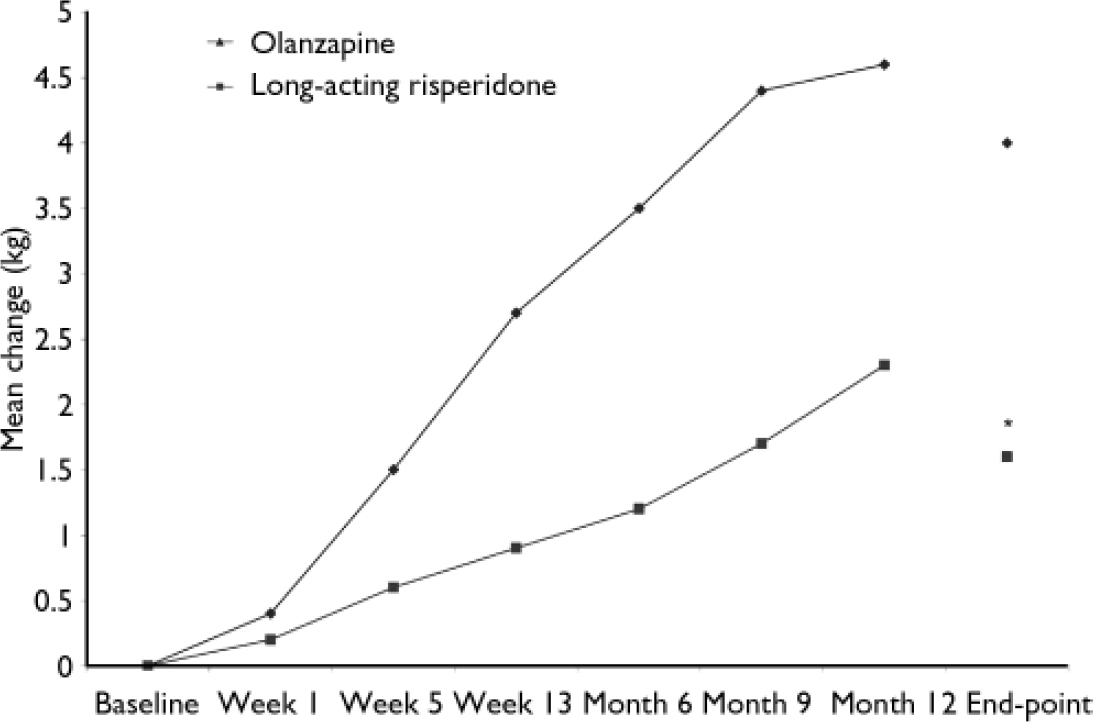
Fig. 4 Changes in body weight from baseline to month 12 and end-point in patients receiving long-acting risperidone or olanzapine. *P<0.05 v. olanzapine.
Patients receiving 75 mg long-acting risperidone
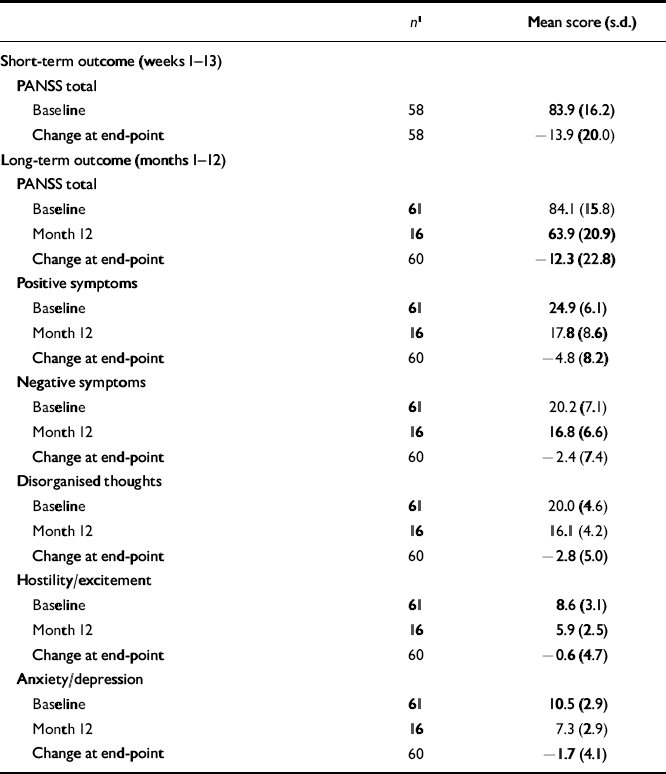
The PANSS total and factor scores and adverse events in patients receiving 75 mg of long-acting risperidone are reported in Tables 6 and 7. In these patients, who had received 6 mg (the highest dose) of oral risperidone during week 1, mean PANSS total and factor scores at baseline were substantially higher than in patients receiving 25 or 50 mg of long-acting risperidone. More adverse events were also reported in this group.
Table 6 Positive and Negative Syndrome Scale total and factor scores in patients receiving 75 mg of long-acting risperidone
| n 1 | Mean score (s.d.) | |
|---|---|---|
| Short-term outcome (weeks 1-13) | ||
| PANSS total | ||
| Baseline | 58 | 83.9 (16.2) |
| Change at end-point | 58 | -13.9 (20.0) |
| Long-term outcome (months 1-12) | ||
| PANSS total | ||
| Baseline | 61 | 84.1 (15.8) |
| Month 12 | 16 | 63.9 (20.9) |
| Change at end-point | 60 | -12.3 (22.8) |
| Positive symptoms | ||
| Baseline | 61 | 24.9 (6.1) |
| Month 12 | 16 | 17.8 (8.6) |
| Change at end-point | 60 | -4.8 (8.2) |
| Negative symptoms | ||
| Baseline | 61 | 20.2 (7.1) |
| Month 12 | 16 | 16.8 (6.6) |
| Change at end-point | 60 | -2.4 (7.4) |
| Disorganised thoughts | ||
| Baseline | 61 | 20.0 (4.6) |
| Month 12 | 16 | 16.1 (4.2) |
| Change at end-point | 60 | -2.8 (5.0) |
| Hostility/excitement | ||
| Baseline | 61 | 8.6 (3.1) |
| Month 12 | 16 | 5.9 (2.5) |
| Change at end-point | 60 | -0.6 (4.7) |
| Anxiety/depression | ||
| Baseline | 61 | 10.5 (2.9) |
| Month 12 | 16 | 7.3 (2.9) |
| Change at end-point | 60 | -1.7 (4.1) |
Table 7 Adverse events reported in at least 5% of patients receiving 75 mg of long-acting risperidone (n=71)

| Adverse event | % |
|---|---|
| Psychosis | 44 |
| Insomnia | 28 |
| Anxiety | 25 |
| Depression | 14 |
| Suicide attempt | 10 |
| Agitation | 7 |
| Extrapyramidal disorder | 11 |
| Hyperkinesia | 11 |
| Headache | 10 |
| Tremor | 6 |
| Rhinitis | 10 |
| Pharyngitis | 7 |
| Constipation | 6 |
| Back pain | 6 |
| Flu-like symptoms | 6 |
| Weight increase | 6 |
| Amenorrhoea | 6 |
DISCUSSION
Risperidone and olanzapine have been shown to be effective and generally well tolerated both in short-term (Reference Marder and MeibachMarder & Meibach, 1994; Reference Tollefson, Beasley and TranTollefson et al, 1997) and long-term (Reference Csernansky, Mahmoud and BrennerCsernansky et al, 2002; Reference Beasley, Sutton and HamiltonBeasley et al, 2003) trials of patients with schizophrenia and schizoaffective disorder. Three recent large studies have compared the oral formulations of the two agents. Conley & Mahmoud (Reference Conley and Mahmoud2001) reported that the efficacy and safety of risperidone and olanzapine were generally similar in their double-blind, 8-week study. The only significant between-group differences were the greater improvements in the risperidone-treated patients on two of the five PANSS factors (positive symptoms and anxiety/depression) among patients who completed the trial, and the greater weight gain in the olanzapine-treated patients. Risperidone and olanzapine were among the five atypical antipsychotics evaluated in the double-blind Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial of patients with schizophrenia (Reference Lieberman, Stroup and McEvoyLieberman et al, 2005). Time to treatment discontinuation (the primary outcome measure) was significantly longer in patients receiving olanzapine than risperidone (9.2 v. 4.8 Kaplan–Meier estimated median months, P<0.01). However, similar improvements in PANSS total scores were seen in patients treated with risperidone and olanzapine at month 18, both in the total group (Reference Lieberman, Stroup and McEvoyLieberman et al, 2005) and in patients whose treatment had been switched to one of these two antipsychotics after discontinuing their previous treatments (Reference Stroup, Lieberman and McEvoyStroup et al, 2006). There was some suggestion that olanzapine was not as well tolerated as risperidone: substantial differences were noted in the proportions of patients who discontinued treatment because of intolerability (10% of the risperidone patients v. 19% of the olanzapine patients; P<0.05) and 2% of the risperidone group v. 9% of the olanzapine group discontinued because of weight gain or metabolic effects (P<0.001, comparing all five treatment groups) (Reference Lieberman, Stroup and McEvoyLieberman et al, 2005). The 12-month results of the large, international open-label Intercontinental Schizophrenia Outpatient Health Outcomes (IC–SOHO) study have been published recently (Reference Dossenbach, Arango-Davila and IbarraDossenbach et al, 2005): similar proportions of patients in the risperidone and olanzapine groups responded to treatment during the 12 months (74 and 81%) or had relapsed (9 and 8%) (response and relapse were defined according to patient scores on the Clinical Global Impression–Schizophrenia scale).
In their meta-analysis of studies of atypical antipsychotics, Davis et al (Reference Davis, Chen and Glick2003) reported that the effect sizes of risperidone and olanzapine (compared with conventional antipsychotics) were similar (0.25 and 0.21 respectively) and highly significant (P<0.001). This analysis included data from 22 risperidone trials and 14 olanzapine trials.
The primary efficacy result of our trial was that in the short term (weeks 1–13) long-acting injectable risperidone was as effective as oral olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder, an expected outcome given the previous findings of short-term studies of the oral formulations of the two agents.
Efficacy of the two treatments
Significant reductions in PANSS total and factor scores were seen in the analyses of the short-term and long-term data in both treatment groups. Patients receiving long-acting risperidone demonstrated significant benefits over treatment with olanzapine on two outcomes: clinical improvement (at least 20% reduction in PANSS total score) at month 12 and at end-point, and improvement on a PANSS factor at month 12 (disorganised thoughts). According to the patients’ ratings in both treatment groups, quality of life was improved from baseline to end-point.
Long-term outcomes
Figure 2 shows that the improvements with long-acting risperidone and olanzapine in PANSS total scores and scores on three of the five factors start to diverge at months 9–12, suggesting more positive long-term responses to long-acting risperidone than to olanzapine. A similar trend was evident in the data on clinical improvement (at least 20% reduction in PANSS total score). These results seem to be in line with those of a previous study (Reference Hogarty, Schooler and UlrichHogarty et al, 1979), which reported comparable relapse rates with depot and oral antipsychotics (fluphenazine decanoate and fluphenazine hydrochloride) during the first year of treatment (39 and 35% respectively), but substantially lower rates with the depot medication than with the oral formulation during the second treatment year (8 and 42% respectively).
The high medication adherence rates in this 1-year controlled study are note-worthy. The mean time off drug was 0.7 days (s.d.=3.7) in the oral olanzapine group, a substantially higher rate than reported in 1-year and 2-year studies of adherence rates in patients with schizophrenia receiving oral antipsychotics (Reference Gilmer, Dolder and LacroGilmer et al, 2004; Reference Weiden, Kozma and GroggWeiden et al, 2004). Thus, application of our findings to the real-world effectiveness of the two medications will need to take into account the impact of medication adherence rates on treatment outcome.
Tolerability
A high proportion of the patients completed the 1-year trial (65% of the long-acting risperidone group and 62% of the olanzapine group). Both treatments were safe and well tolerated. Few patients (7 in the risperidone group and 11 in the olanzapine group) withdrew from treatment because of an adverse event. The incidence of extrapyramidal adverse events was higher in the long-acting risperidone group than in the olanzapine group at baseline, but by months 9–12 the rates were comparable in the two groups (this does not appear to be a result of differential withdrawal rates). New-onset tardive dyskinesia (reported in two patients in each treatment group) was a rare event. Increases in body weight and BMI were significantly lower in the long-acting risperidone group than in the olanzapine group.
Implications
In patients with schizophrenia or schizoaffective disorder, long-acting risperidone and olanzapine tablets were efficacious and well tolerated over the 12-month duration of this study. The efficacy results suggest that in the long term patients might benefit more from treatment with long-acting risperidone than with oral olanzapine, but longer-term comparative data will help to confirm these observations.
Acknowledgements
The study was supported by Johnson & Johnson Pharmaceutical Research and Development. The authors thank Ilse Van Hove, MSc (Johnson & Johnson Pharmaceutical Research and Development, Beerse, Belgium) for completing the statistical analyses.














eLetters
No eLetters have been published for this article.