INTRODUCTION
Infection with Salmonella is one of the most commonly reported causes of enteric illnesses in humans in industrialized countries. Between 1996 and 2001 in Ontario, the average yearly incidence of sporadic cases of enteric disease due to Salmonella was 22·6/100 000 people, which was the second highest incidence among the eight reportable pathogens [Reference Lee and Middleton1].
Between 4·5% and 23% of infections in humans worldwide have been attributed to pork [Reference Lee and Middleton1–Reference Hald3]. Although proportionally not as important as poultry and eggs, these statistics place pork as an important animal food-source contributor to salmonellosis, and Salmonella as the most important foodborne pathogen associated with the swine industry in industrialized countries.
While pigs can be infected with many different serovars, clinical disease is reported in the literature mainly in association with S. Choleraesuis, S. Typhisuis, and S. Typhimurium [Reference Fedorka-Cray, Gray, Wray, Wray and Wray4]. Consequently, meat from pigs showing no clinical signs or lesions may be contaminated with Salmonella, and quality assurance throughout the production chain is required to lower the contamination level of the final product [Reference Blaha5]. Due to public health implications, Salmonella surveillance programmes may also have the potential to be used for commercial or trade purposes [Reference Davies and Hueston6]. In Canada, the on-farm implemented Canadian Quality Assurance programme (CQA®), based on Hazard Prevention Critical Control Point principles is currently in place to reduce food safety hazards.
On-farm management procedures have been evaluated as part of the overall effort to decrease Salmonella in the pre-harvest portion of the production chain. Many studies have evaluated farm-level management procedures [Reference van der Wolf7–Reference Nollet10]. Although this approach seems intuitive, there is also a body of evidence suggesting that management procedures at other levels in swine farms may play a role in Salmonella prevalence (or incidence). Geographical location, represented by membership in a specific region, may play a role, since regional farm density may influence the Salmonella herd status [Reference Fedorka-Cray, Gray, Wray, Wray and Wray4, Reference Carstensen and Christensen11]. Within-farm variation plays an important role in the Salmonella status of pigs [Reference Carstensen and Christensen11]. Cohort [Reference Funk, Davies and Nichols12, Reference Beloeil13] and pen differences [Reference Beloeil13, Reference Davies14] were reported as important sources of within-farm variability. However, few studies have examined the nature of the association between the Salmonella status of the pigs and within- and among-farm level covariates simultaneously [Reference Funk, Davies and Gebreyes15]. In Ontario, according to the 2001 Agricultural Census [16], there were 1 807 530 finishing pigs on 3968 swine operations (mean 456). According to the Ontario Pork Producers [17], 4185 producers marketed finishing pigs through that organization in the same year. However, 52·4% of producers marketed less than 500 pigs per year, contributing only 6·6% of marketed pigs. Thus, the Ontario swine industry is of mixed type in a sense that it contains small producers as well as large systems.
The first objective of this study was to identify factors associated with Salmonella status at the farm, pen, and pig level (objective no. 1). The second objective was to explore the nature of variation in the association between pen-level Salmonella status and pen-level covariates, and pig-level Salmonella status and pig- and pen-level covariates using random-coefficient models (objective no. 2). The third objective was to identify the relative importance of factors operating at geographical, farm, and pen level for Salmonella shedding of pigs (objective no. 3).
METHODS
Project description and herd selection
The 80 finisher herds that participated in this study were a convenience and purposive sample of Ontario farms that housed finishing pigs. Although these finishing herds are not a true random sample of Ontario finisher pig herds, all management systems, from single-site farrow-to-finish operations to specialized finisher barns, currently operating in Ontario were represented in the study. Both, small and large herds were included, although on average this study population, with a mean herd size of 1050 finisher pigs (Table 1), was more representative of larger herds within the Ontario swine industry. In addition, all swine-producing regions of Ontario were represented. The study period was between 13 January 2004 and 15 June 2004.
Pen selection
On each farm, five fresh pooled faecal samples were selected from five different pens. Using clean gloves for each pen, samples were collected from five different places within a pen for a total of about 200 g faeces. The sampled pens were purposively selected [Reference Cameron and Salman18] from a room containing pigs closest to market weight. Modifications to this protocol were made on farms that had fewer than five pens per room. In such cases, a total of five pens in more than one room were sampled, and if fewer than five pens were available on any farm, we collected five pooled samples from the available pens. There were nine farms with pigs sampled from two rooms, and three farms with pigs sampled from three rooms. In addition, there were five farms with only one pen sampled, and three farms with 2–4 pens sampled.
Pig selection
On each farm, we also collected two faecal samples per pen from conveniently selected individual pigs (pig samples) for a total of 10 samples per farm. Using clean gloves for each sample, faecal samples were collected as pigs defecated before the faeces contacted the floor for a total of about 200 g faeces. Pig samples were collected from the same pens as pooled samples. Pig weight was estimated with a measuring tape (Coburn Company, Whitewater, WI, USA) for the two individually sampled pigs and an additional four pigs from the same pen. Signs suggestive of impaired health in the sampled pigs were recorded. Faecal samples were stored on ice during transport, refrigerated at 4°C overnight, and submitted for further processing to the Laboratory Service Division of the University of Guelph (LSD) (Guelph, Ontario, Canada).
Questionnaire
A five-part questionnaire was completed during the farm visit. First, an interview with the producer was used to collect (on a 5-point Likert scale) farm-level information related to the frequency of all-in/all-out procedures, and cleaning practices between batches. Second, photographs or copies of Canadian Quality Assurance protocols, or alternatively an interview with the producer, was used to obtain information related to the use of medications. Third, observation by investigators was used to collect farm-level and room-level information, including the presence of other animal species, building design in relation to the possibility of pigs having contact with the outdoor environment, the number of pigs, the number of pens, the presence of hospital pens, obvious weight and age difference in the sampled room, signs of fighting among pigs in a pen, and type of feed. Fourth, at least two investigators measured pen width and length and assessed pen-level information including position in the barn, the number of pigs in the pen, the possibility of nose-to-nose contact between neighbouring pens, a cleanliness score on a 5-point Likert scale, and signs of disease (yes/no) in pens. Fifth, investigators collected pig-level information as previously described. The questionnaire is available upon request from the corresponding author. In addition, the location of the sampled barn was recorded using a hand-held global positioning system (GPS) receiver (eTrex Legend; Garmin, Olathe, KS, USA) in front of the barn. These location data were then merged with Canadian census data from 2001 at the census-division level (CD) in ArcGIS 8 (ESRI, Redlands, CA, USA). This was used to obtain farm membership in CD and to calculate the density of pigs and pig farms at the CD level.
Laboratory testing
Faecal samples were processed in accordance with the protocol outlined by the Public Health Agency of Canada (MFHPB-20 [19]). Briefly, 25 g mixed faecal material was diluted with 225 ml buffered peptone water (Oxoid, Nepean, ON, Canada), homogenized in a stomacher (Seward Stomacher 400; Seward, Norfolk, UK) for 2 min, and incubated for 24 h at 35°C. In a selective enrichment step, 1-ml aliquots of this suspension were added to 9 ml selenite cystine broth (Becton Dickinson Canada, Oakville, ON, Canada) and incubated for 24 h at 35°C, and added to 9 ml tetrathionate Brilliant Green broth (Becton Dickinson Canada) and incubated for 24 h at 43°C.
Loopfuls of each selective enrichment culture were streaked onto bismuth sulphite agar (Fisher Scientific, Whitby, ON, Canada) and incubated for 24 h at 35°C; and for an additional 24 h at 35°C if needed, and onto Brilliant Green sulpha agar (Oxoid) and then incubated for 24 h at 35°C. Plates were examined for typical Salmonella colonies. Samples were declared negative if colonies were absent. Suspected Salmonella colonies (one colony per sample) were streaked onto MacConkey agar (Oxoid) for purification, incubated for 24 h at 37°C, and inoculated into biochemical media, then incubated for 24 h at 37°C. Biochemical media included triple sugar iron agar, Christensen's urea agar, and lysine iron agar (Fisher Scientific). Suspect isolates were inoculated into nutrient agar slants (Fisher Scientific), incubated for 24 h at 35°C, and submitted to the Laboratory for Foodborne Zoonoses (Public Health Agency of Canada, Guelph, ON, Canada) for further analyses.
Definition of outcome
A pig was classified as Salmonella-positive if it tested positive for Salmonella. As 397 pooled samples were collected from 374 pens, pen samples were aggregated to the pen level, and a pen was classified as positive if any pooled or pig sample from that pen tested positive. A farm was classified Salmonella-positive if any sample tested positive for Salmonella.
Data management
Data were entered into an Access 2000 database (Microsoft Corporation, Redmond, WA, USA) and imported to SAS 9.1 (SAS Institute, Cary, NC, USA) for further data management and descriptive statistics. Inferential statistical analyses were performed in Stata 8 (StataCorp, College Station, TX, USA). In addition, variance component pig-level models were fitted using SAS 9.1 and MLwiN 2.0 (Institute of Education, London, UK). The means of ratio and ordinal variables at the pen level were calculated from assessments obtained from all investigators and used as pen-level variables.
Statistical analysis of farm-level data for objective no. 1
The association between the covariates and Salmonella status of the farm was evaluated in contingency tables and univariable logistic regression models by the likelihood ratio test and Fisher's exact test as required. Linearity of continuous variables with the logit of Salmonella status was assessed by evaluating quadratic polynomials [Reference Dohoo, Martin and Stryhn20]. The multivariable logistic regression model was fitted from variables that had P values <0·10 in a univariable analysis. The significance of two-way interactions was assessed. The best model from the final candidate models was selected on the basis of the smallest value for Akaike's Information Criterion (AIC). The model was evaluated using the Hosmer–Lemeshow goodness-of-fit statistic and by assessing the residual and influence statistics.
Statistical analysis of pen-level and pig-level data for objective no. 1
The association between pen-level Salmonella status and pen- and farm-level covariates, and between pig-level Salmonella shedding and pig-, pen-, and farm-level covariates, were assessed using a logistic regression model with farm as a random effect. This model was fitted as a generalized latent linear mixed model (gllamm; random-intercept model). Factors residing at all applicable levels of a hierarchy were evaluated in univariable and multivariable random-intercept models in a sequence identical to the one described for the farm-level analysis. Significance of the association was tested by a likelihood ratio test. The best final model was determined on the basis of the AIC criterion.
Statistical analysis of pen-level and pig-level data for objective no. 2
The nature of variation in the association between the pen-level Salmonella status and pen-level covariates, and between pig Salmonella shedding and pig- and pen-level covariates (centred at the mean or median value) was assessed in univariable models in four steps. The logistic regression model with a random intercept at the farm-level was fitted and fixed effects were evaluated by a Wald test. Then, the same model was fitted with a random intercept and random slope for that coefficient [random-coefficient model; see equation (1)], and a fixed effect was evaluated by a Wald test; the statistical significance of the random variation in the slope was assessed by a likelihood ratio test, and the fit was also assessed by AIC. Models that did not converge in gllamm were refitted in proc glimmix and the model with the lower pseudo-AIC was selected. Finally, farm-specific estimates of the association from the random-coefficient models were plotted and visually inspected.
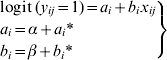
where α and β are the fixed effects of the model (the intercept and the slope, respectively), which are expected values of the population of intercepts and slopes. The variable a i* represents the difference between the intercept for the ith herd and the overall intercept α, and b i* represents the difference between the slope for the ith herd and the overall slope β.
Statistical analysis of pig-level data for objective no. 3
Variance of Salmonella shedding in finishing pigs was partitioned using a threshold logistic method [Reference Snijders and Bosker21]. A random effects model with intercept as the only fixed term (empty model) and with the random effects of CD, farm nested within CD, and pen nested within farm was fitted using three different estimation methods. The first method was first-order penalized quasi-likelihood (PQL) fitted in proc glimmix (SAS), the second was maximum likelihood (ML) fitted in gllamm using adaptive quadrature (Stata), and the third was Monte Carlo Markov Chain (MCMC) (MLwiN) based on a burn-in period of 5000 iterations, and with inferences based on an additional 100 000 iterations. We used three logistic regression models with the intention to compare whether they would agree in identifying the proportion of variance at different hierarchical levels. A similar approach has been reported previously [Reference Dohoo22]. The statistical significance of each applicable level was tested by a likelihood ratio test when the model was fitted by the maximum-likelihood method.
RESULTS
Descriptive statistics
In this study, 38/80 (47·5%) farms, 87/374 (23·3%) pens, and 91/799 (11·4%) pigs tested positive for Salmonella. Pigs were sampled from only 371 pens, because on one farm, three pens were eliminated from the collection of individual pig samples since the pigs appeared to be younger than the rest of the sampled pigs. Descriptive statistics of variables used in the univariable and multivariable models at different levels are summarized in Tables 1–3. A total of 12 observers participated in the collection of pen-level information. However, one observer scored 70·2% of pens, another scored 47·2% of pens, and the third scored 42·3% of pens.
Table 1. Farm-, pen-, and pig-level variables recorded in a study of Salmonella shedding in 80 Ontario finishing pig herds, 2004
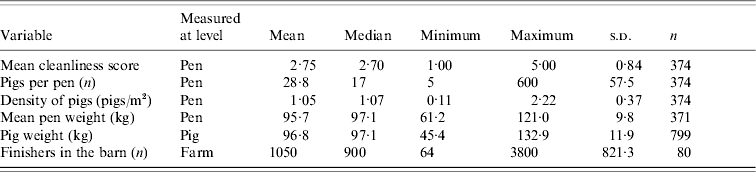
Table 2. Prevalence of dichotomous farm-level variables describing the farms participating in a study of Salmonella shedding in Ontario, 2004

* Barn was considered to be completely closed if built so that possibility of direct contact between pigs and wildlife, birds, and other domestic animals was minimized.
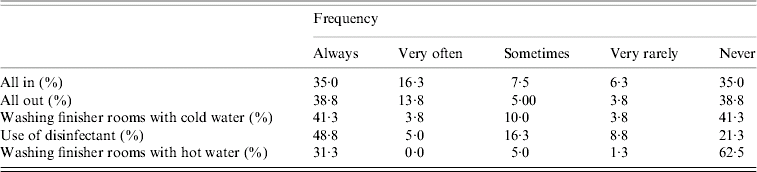
Table 3. Management of farms involved in a study of Salmonella shedding in Ontario, 2004
Farm-, pen-, and pig-level associations for objective no. 1
Table 4 presents coefficients and their 95% confidence interval (CI) on the logit scale for management procedures that were univariably associated with the Salmonella status of farms, pens, and pigs when evaluated in three separate sets of analyses. In addition, Table 5 shows coefficients and their 95% CI on the logit scale for management procedures that were multivariably associated with the Salmonella status of farms, pens, and pigs when evaluated in three separate sets of analyses. At the farm level, a completely closed barn relative to completely or partly open barns; and mash and liquid finisher rations relative to a pelleted finisher ration decreased the log odds of Salmonella farm positivity in the final multivariable model. In contrast, a larger number of finisher pigs in the barn, and a higher frequency of disinfection between batches both increased the log odds (Table 5). At the pen level (Table 5), the log odds of Salmonella positivity also increased with a higher frequency of washing with cold water, and with having only finisher pigs on the site. These same factors were also associated with pig-level Salmonella shedding (Table 5).
Table 4. Univariable associations between management factors and Salmonella status of farms, pens, and pigs in Ontario, 2004, represented by the log odds ratios from logistic (farm-level) and random-intercept logistic (pen-level, pig-level) regression models
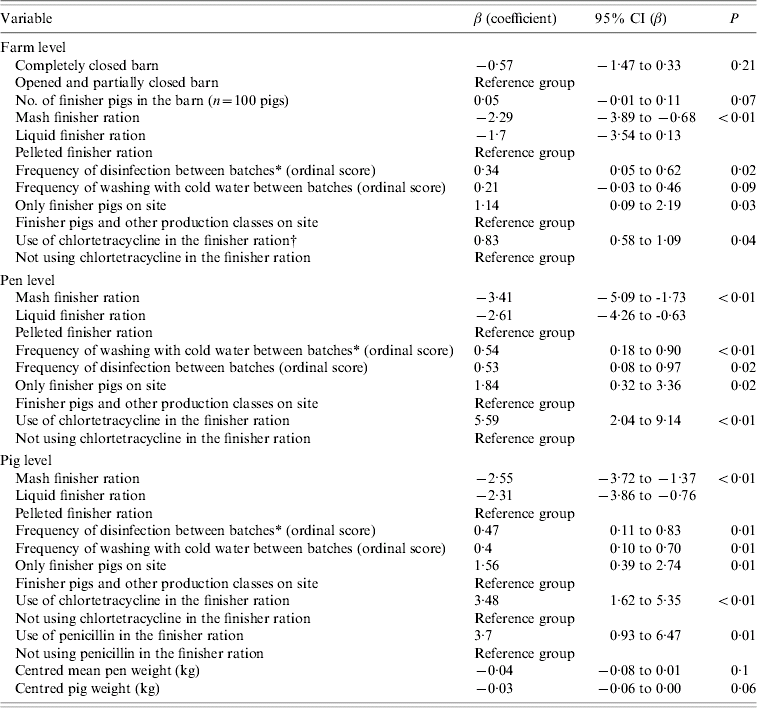
* Batch is defined as a new group of animals coming into the facility (room or a barn) after the previous group had been shipped to market.
† P value is based on two-sided Fisher's exact test.
Table 5. Multivariable models for associations between management factors and Salmonella status of farms, pens, and pigs in Ontario, 2004, represented by the log odds ratios from logistic (farm-level) and random-intercept logistic (pen-level, pig-level) regression models
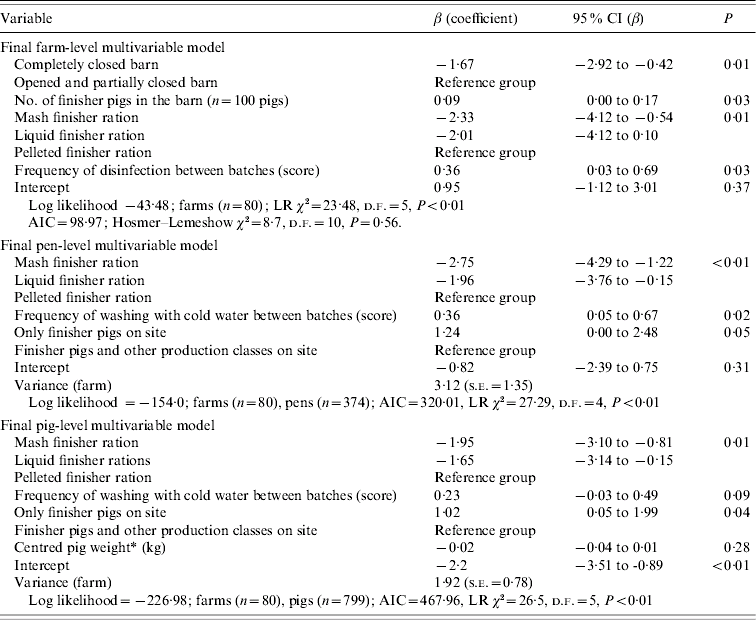
* Forced into the final multivariable model.
The nature of variation for pen- and pig-level associations for objective no. 2
At the pen level, the fixed effect for the cleanliness score, mean pen weight, number of pigs in a pen, and pig density in a pen were not associated with the likelihood of Salmonella pen positivity either in random-intercept (P>0·11), or in random-coefficient models (P>0·22). Out of these covariates, only the random effect of slope for the cleanliness score was statistically significant (P=0·02), and the inclusion of this random coefficient improved the fit of the model as evaluated by AIC.
At the pig level, the fixed effect of weight and mean pen weight were associated with Salmonella shedding in individual pigs in the random-intercept models (Table 4); as an individual pig weight or a mean pen weight increased, the likelihood of shedding decreased. In addition, the random coefficient for pig weight was not significant (P=0·97) relative to the random-intercept model and had higher AIC than the random-intercept model, suggesting a lack of random variation around the estimated negative association between shedding and pig weight in farms. The fixed effects of other variables measured at the pen-level were not associated with Salmonella shedding in either the random-intercept models (P>0·30), or random-coefficient models (P>0·30). Similarly to the results of pen-level analyses, only the cleanliness score had a significant random slope effect (P=0·02).
Proportion of variation for objective no. 3
Estimates of variance, standard error of variance, and proportion of variance for Salmonella shedding at each level are presented in Table 6. In the model estimated by the maximum- likelihood method, pen (P<0·01) and farm (P<0·01) were statistically significant, but CD was not (P=0·48).
Table 6. Variance components and proportion of variance for the pig-level Salmonella shedding due to the Census division-, farm- and pen-level clustering in Ontario, 2004 based on three statistical estimation techniques

Est., Estimate; s.e., standard error; Prop., proportion.
DISCUSSION
Clustering of Salmonella positivity has been studied previously [Reference Nollet10, Reference Funk, Davies and Nichols12–Reference Davies14]; however, the question of clustering of Salmonella shedding at multiple levels has not been explored simultaneously. To address this question, we took the multilevel model approach of Dohoo et al. [Reference Dohoo22].
In this study, variance estimates for Salmonella shedding calculated by the three estimation methods differed numerically, but agreed in the relative variability (i.e. ordering) of these three levels. Geographical location represented by CD membership was the least variable level in our analysis, suggesting that location of the farm was not an important contributor to the Salmonella shedding status of the pigs in our study. This finding was in accordance with the absence of spatial clustering in these data as reported elsewhere [Reference Poljak23], although it does not preclude the possibility of different findings if a larger geographical scale was considered. Density of pig farms in the area has been suggested as a potentially important factor for Salmonella status of the farms [Reference Fedorka-Cray, Gray, Wray, Wray and Wray4, Reference Carstensen and Christensen11]. In our study, neither pig nor the pig-farm density in the CD was associated with Salmonella status at any level.
Farm was proportionally the most variable level, and consequently expected to contain variables that will influence the outcome to the greatest extent. This is in accordance with the findings of Carstensen & Christensen [Reference Carstensen and Christensen11], who found a large between-farm variability in pig Salmonella serological status over time. Funk et al. [Reference Funk, Davies and Nichols12] argued that it is the level of the time cohort, not the farm nor the company level, that most influences the shedding of pigs. Unlike our study, the study of Funk et al. [Reference Funk, Davies and Nichols12] included only multi-site production units, and that might have minimized the importance of farm for Salmonella status. Furthermore, Beloeil et al. [Reference Beloeil13] found a significant batch effect for seroconversion on a single farm.
Most variables associated with Salmonella positivity at any level were typical farm-level variables. This study had a cross-sectional design and, therefore, no inference about timing could be made. Hence, the question remains whether farm effect was indeed the effect of farm, cohort, or both; where the farm effect can be defined as processes related to facilities and procedures that change either rarely or slowly (time invariant), and the cohort effect is defined as all processes specific to the pigs that entered the facility as a group (time variant).
Pen effect was statistically significant in our study, although estimates of variance and proportion of variance at the pen level differed between the PQL-based model, and the other two methods (ML and MCMC) that gave similar results. Underestimation bias of PQL-based methods, particularly for random effects, has been discussed elsewhere [Reference Dohoo22]. Data used in this study were probably prone to this type of bias, because mean number of pigs per pen was 2·2. Thus, variance estimates and proportion of variance based on the ML and MCMC models are probably more accurate. Sampling more pigs per pen in future similar studies could decrease the extent of such potential bias. Clustering of Salmonella infection or exposure by pen was identified in some previous studies [Reference Beloeil13, Reference Davies14], but not in others [Reference Nollet10]. Our results suggest that farm-level variables are central in determining Salmonella shedding in pigs, but factors operating at the pen level are also influential. Our sampling strategy might have influenced estimates of pen-level variability. Sampling five pens in herds with small number of pens, but existing variation in age among pens, and under an assumption of age-related shedding might have increased variability at the pen level. Only by narrowing the initial inclusion criteria could this problem have been avoided, but this would limit a generalization of the findings.
Cross-sectional studies based on multiple farms and considering within-farm variables as potential risk factors are rare [Reference Funk, Davies and Gebreyes15]. In our study, we attempted to analyse within-farm variables (room, pen, and pig), and to explore the nature of their association with Salmonella status of the appropriate level.
Random-coefficient models, as used in this study, will allow the coefficients as well as the intercepts to vary among farms [Reference Fitzmaurice, Laird and Ware24]. Hence, the slopes of the random-coefficient models may differ by farm. By fitting both types of model, we intended to determine not only the possible association between the Salmonella positivity and a covariate on the ‘average’ farm as represented by the fixed effect from the model, but also the type of farm-specific random variation around that fixed effect as represented by the estimates of covariance and visualized by the farm-specific associations. Of all pen- and pig-level variables evaluated, only mean pen weight and pig weight tended to be univariably associated with the Salmonella status of pigs, with the absence of random variation among farms. As the individual pig weight and mean pen weight increased, the likelihood of shedding decreased, and this association did not vary among the study farms. From a biological perspective, this may be either because of the correlation between weight and age, with the latter being associated with Salmonella shedding [Reference Beloeil13, Reference Kranker25]; or because a lower weight may be associated with a lower disease status and lower disease status could be associated with prolonged shedding [Reference Wills26]. Whether weight was more reflective of age or health status in this study may also be farm dependent. From a causal perspective, both age and health might be components of the association between Salmonella shedding and weight, and we have no means of separating them. From a sampling perspective, however, our results imply that the sensitivity of the sampling scheme for Salmonella at the pig level, based on bacteriological culture, is lower if we sample heavier pigs, regardless of the farm type. Thus, pigs of lower weight should be sampled for sensitivity of on-farm culture-based Salmonella monitoring programmes to be maximized. Because of the pig-level inclusion criteria applied in the study, this statement technically applies only to the relatively narrow window of pigs close to market weight. Although it would seem plausible from previous studies, it remains to be seen whether this recommendation could be extrapolated back to younger age categories (i.e. early grower period immediately after shipping from the nursery phase).
From the study design perspective, it also suggests that the results of on-farm cross-sectional studies based on culture may be influenced by the point in time that the pigs were sampled on these farms; which, under practical conditions, may vary. Prevalence estimates based on shedding could be underestimated, both at the pig- and herd-level. In addition, possible differences in the shape of Salmonella shedding curves among different farm types, coupled with sampling of pigs of different weight could bias existing associations in unpredictable ways. For example, a management procedure that facilitates Salmonella shedding early in the production phase could appear as unimportant if pigs were sampled close to market, when shedding stopped or was reduced, and the herd test was unable to detect the existing level of shedding. Thus, repeated studies of this nature are needed, and agreement among them would be beneficial before considering a management procedure as a risk or a sparing factor.
No other variables at either the pig or pen level were associated with pig or pen Salmonella status. The only covariate that showed significant random variation and no significant fixed effect association at the same time was cleanliness score. We believe this was because of the discrete nature of the measurement. Once the slopes were allowed to vary, the model was able to account for the fact that pens or pigs of different Salmonella status had the same cleanliness score. Consequently the random variation was significant and the fit of the model improved. Hence, the significant random variation was more of an artefact of the measurement than of biological significance.
Higher pig density was not associated with a higher prevalence of Salmonella in our study but was identified in a study by Funk et al. [Reference Funk, Davies and Gebreyes15]. Density in that study was measured at the cohort level, within two 3-site all-in/all-out systems, and was determined primarily by the number of pigs marketed prior to the sampling occasion. In contrast, we measured the pig density at the pen level within different management systems representative of those currently present in the Ontario swine industry.
The absence of any associations between Salmonella status and pen-level covariates in our study was unexpected, because up to 25% of the variation resided at the pen-level. It was therefore expected that at least some variables identified as putative risk factors by other authors would be associated with the Salmonella status of pigs or pens, or show a significant random variation among farms. Thus, the second objective of our study was fulfilled only in part. It is possible that some component causes of Salmonella infection at the pen level are formed when the pens are populated with pigs, such as the residual contamination of pens [Reference Beloeil13], and/or the introduction of shedders from a prior management phase. Other component causes may accumulate over time or show other types of time-varying effects, such as environmental temperature, housing conditions [Reference Beloeil13], and density [Reference Carstensen and Christensen11]. These types of measurements were not possible in our study. A longitudinal study with specific inclusion criteria is better suited to study the causal association between pen-level covariates and Salmonella shedding. However, from a practical sampling standpoint our results suggest that if the investigator is faced with a decision to sample pigs or pens for bacteriological diagnosis of subclinical salmonellosis, he or she cannot rely on cleanliness, number of pigs in a pen, or pig density to increase the herd sensitivity (i.e. the probability of culturing Salmonella in an infected herd).
Farm-level variables had the greatest influence on the Salmonella status of farms, pens and pigs. This was concordant with the results of our empty model for Salmonella shedding that identified farm as the most variable level. Risk factors identified during univariable and multivariable analyses could be classified into four general categories: feed management as measured by feed type; medication usage, specifically antimicrobials; hygiene practices such as washing and disinfection; and the spatial separation of age groups as represented by having only finishers on site.
The type of feed has frequently been identified in epidemiological studies as an important contributor to Salmonella infection in pigs. Liquid feed, compared to other types of feed, has been reported as a protective factor in some studies [Reference van der Wolf7, Reference van der Wolf27], and pelleted feed, compared to non-pelleted, has been identified as a risk factor in other studies [Reference Lo Fo Wong8, Reference Davies14]. In our study, the use of either liquid or mash feed in the finisher ration was a protective factor compared to the use of pelleted feed. The beneficial effect of liquid feeding [Reference van der Wolf7] and coarsely ground non-pelleted feed [Reference Mikkelsen28, Reference Hedemann29] on the Salmonella status of pigs have been described earlier. In this study, we did not record the particle size of the feed during our visit, but it is probable that the feed was coarsely ground on at least some farms that used high-moisture corn as part of their finisher ration. Moreover, feed must be finely ground prior to pelleting.
An increase in herd size was associated with a linear increase in the log odds of farm Salmonella positivity. This finding is in contrast with the results of other authors [Reference van der Wolf7], who found that smaller herds were more likely to be positive, possibly due to higher hygiene standards in large herds. Data in our study, examined by descriptive means, did not show a consistent pattern that would suggest such an association. This may be because barns housing a small number of pigs consisted both of traditional small farms, and barns that were part of multi-site finishing operations, with the latter probably having higher hygiene standards. However, our results are in partial agreement with the results reported by Carstensen & Christensen [Reference Carstensen and Christensen11]. Herd size in their study was defined as the annual number of pigs sent to market, and in our study herd size was defined as the number of pigs in the barn. The definition of herd size may have important implications concerning the interpretation of the association between occurrence of disease and herd size [Reference Gardner, Willeberg and Mousing30]. The apparent association between Salmonella status and herd size in our study may be due either to a higher likelihood of introducing or maintaining infection in larger herds, or to some other unmeasured factor.
In our study, completely closed barn was associated with a lower likelihood of Salmonella positivity for the farm. Closed barns may minimize the introduction of Salmonella by minimizing contact with the outside environment and other animal species [Reference Lo Fo Wong31].
Use of antimicrobials in the finisher ration (chlortetracycline and penicillin) was identified as a univariable risk factor at different levels, but it was not significant once feed type was added to the model. The above antimicrobials were used only in pelleted-based finisher rations. The parenteral administration of antimicrobials in clinical salmonellosis may have beneficial effects [Reference Schwartz and Straw32], but the effect of the per oral application on Salmonella shedding in non-clinical cases is controversial, reducing shedding in some studies [Reference Ebner and Mathew33], but not in the others [Reference Jacks34]. Two recent studies indicated that there is an increased risk of Salmonella-positivity for pigs fed feed containing antimicrobials as feed additives [Reference van der Wolf7, Reference Leontides, Grafanakis and Genigeorgis35] supporting univariable associations detected in this study. The use of most broad-spectrum antibiotics is associated with lower colonization resistance, which might lead to a lower infectious or colonization dose for pathogenic bacteria and longer shedding periods [Reference van den Bogaard and Stobberingh36]. However, findings in this study should be interpreted with caution due to the low number of herds that used the above-mentioned antimicrobials, complete confounding with the feed type, and variation in the quality of original records among herds, particularly with respect to the time-variant nature of this variable.
An unexpected finding in our study was that the likelihood of Salmonella positivity increased with higher hygiene scores as represented by measures such as pressure washing with cold water and disinfection. Such measures are often part of the recommended procedures for lowering Salmonella levels on a farm [Reference Fedorka-Cray, Gray, Wray, Wray and Wray4, Reference Lo Fo Wong31, Reference Schwartz and Straw32]. Cleaning and disinfection decrease residual contamination in pens, but do not completely eliminate Salmonella under field conditions [Reference Beloeil9, Reference Funk, Davies and Nichols12]. Residual contamination was identified as a risk factor for Salmonella status in a study by Beloeil et al. [Reference Beloeil9]. A similar association between seroprevalence and disinfection was reported in a study by van der Wolf et al. [Reference van der Wolf7], who hypothesized that herd personnel may assume that the disinfectant decreases residual contamination even when pressure washing is not sufficiently thorough. In order to clean a barn sufficiently to reduce Salmonella contamination producers need to produce a faeces-free environment and then disinfect. Cleaning to less than a faeces-free environment will inevitably lead to a build up of Salmonella once pigs are introduced to the pens.
In addition, since our study was based on bacteriological culture, it is possible that better hygiene practices, represented by higher hygiene scores, may have lowered the level of residual Salmonella contamination, but not below the minimum infectious dose for all animals. Alternatively, in a less clean environment, all pigs may become infected over a short time period and stop shedding by the end of finishing phase. In contrast, in a cleaner environment, spread of Salmonella in the barn may have been slower because the initial exposure was lower, resulting in a flatter shedding epidemic curve. Thus, when a cross-sectional study in finisher pigs close to market is employed, more pigs may appear to be shedders in facilities that try to reduce microbial contamination by following good hygiene practices. Finally, if pressure washing does not result in a faeces-free environment, this washing may then spread Salmonella in the barn so that more pens become contaminated.
Some limitations of this study include imperfect culture sensitivity, and selection procedure at the herd level. Imperfect sensitivity underestimated the prevalence of Salmonella positivity at different examined levels. We attempted to maximize the likelihood of detecting Salmonella-positive herds. First, we used 25 g faeces in two parallel enrichment procedures, and two selective plating media, which should optimize detection of Salmonella at the sample level. In a study by Funk et al. [Reference Funk, Davies and Nichols37], using a 25-g sample maximized the relative sensitivity (up to 78·3%), and these authors suggested that sensitivity increased as the number of selective enrichment media, incubation conditions, and plating media increased. Second, in our study, both pooled and individual pig samples were tested, which further increased the likelihood of detecting Salmonella-positive herds. Imperfect sensitivity might have led to misclassification of Salmonella status of herds, pens, and pigs. At the herd and the pig level this misclassification was probably non-differential. Under conditions of a binary predictor this could lead to diminished strength of association. In contrast, sensitivity at the pen level was probably higher in pens with more than two pig samples, or more than one pooled faecal sample tested. This occurred on eight farms with less than five pens included (due to limited number of pens), while number of samples (both pooled and pig) per herd was kept constant. Higher sensitivity could have led to possible non-differential misclassification. The most obvious influence of such misclassification would be overestimation of an association between pen positivity and number of pigs in a pen. However, due to a lack of statistical significance, the clinical impact of this misclassification was negligible.
In conclusion, farm was the most variable level in the variability of Salmonella shedding in finishing pigs. The importance of farm-level variables was confirmed in risk-factor analyses: all significant variables included in the final pig and pen model were farm-level variables. Liquid and mash feed and completely closed barns were identified as protective management procedures. In contrast, disinfection and washing with cold water were associated with a high risk of Salmonella shedding. Pen was the second most variable and statistically significant level for shedding, but no pen-level variables that were measured were associated with pen-level Salmonella status. Mean pen weight and pig weight tended to be associated with Salmonella shedding at the pig level, with no random variation around these associations. We made use of random-intercept and random-coefficient models to fully explore the nature of pen- and pig-level variables among farms.
ACKNOWLEDGEMENTS
We thank the Ontario Ministry of Agriculture, Food and Rural Affairs, Ontario Pork, and the Public Health Agency of Canada for funding. Expertise provided by the Laboratory Services Division, University of Guelph is greatly appreciated. We also thank the participating producers and veterinarians for their participation in this project. We are appreciative of the extensive effort of Bryan Bloomfield and Arturo Ruiz in the collection of samples and data.
DECLARATION OF INTEREST
None.








