Background
Hypertension (HPT) is a major risk factor for CVD and related diseases as well as for diseases leading to a marked increase in cardiovascular risk(Reference Mancia, De Backer and Dominiczak1).
The WHO reported that high blood pressure (BP) has been listed as the first cause of death worldwide(1) with an age-standardised prevalence of raised BP ranging from 15·2 to 31·7 %, as reported by the European Society of Cardiology in 2014(Reference Timmis, Townsend and Gale2). In particular, it has been reported that BP levels show a continuous linear relationship with the risk of stroke and myocardial infarction(Reference Timmis, Townsend and Gale2); the INTERHEART study(Reference Yusuf, Hawken and Ounpuu3) estimated that 22 % of myocardial infarctions in Europe are related to HPT, which almost doubles the risk compared with individuals with no history of HPT.
The relationship of BP with caffeine and caffeine metabolites is a major interest, given the widespread caffeine intake from food and beverage sources and the public health burden of high BP. Caffeine is the most widely consumed active pharmacological substance in the world and it is found also in common non-essential grocery items such as coffee, tea, cocoa, chocolate and soft drinks(Reference Barone and Roberts4).
Caffeine exerts several effects on the autonomic nervous system and blood vessels. The mechanisms by which caffeine exposure affects heart rate and BP levels might include increased catecholamine levels, which might subsequently lead to vasoconstriction(Reference Smith, Brice and Nash5, Reference Robertson, Frölich and Carr6). Possible mechanisms for the acute cardiovascular effects of caffeine include antagonistic effects on adenosine receptors (particularly, A1 and A2A receptors), activation of the sympathetic nervous system, stimulation of the adrenal cortex (release of corticosteroids), renal effects (diuresis, natriuresis and activation of the renin–angiotensin–aldosterone system) and inhibition of phosphodiesterase(7).
However, the role of caffeine in the regulation of BP levels is controversial. According to the guidelines for the management of arterial HPT of the European Society of Hypertension(Reference Wierzejska8) and of the European Society of Cardiology(Reference Wierzejska8), a firm recommendation or discouragement of coffee consumption cannot be issued due to insufficient quality of most studies(Reference Wierzejska8).
Recently, the European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies reported its Scientific Opinion on the safety of caffeine(7). The effect of caffeine consumption was observed at single doses of caffeine ranging from 80 to 300 mg, inducing a mean increase in systolic BP of about 3–8 mmHg and in diastolic BP of about 4–6 mmHg, with high inter-individual variability. In addition, the available data suggested that BP generally increases 30 min after caffeine consumption, reaches a peak after 60–90 min and returns to baseline after about 2 to 4 h, which is consistent with the pharmacokinetics of caffeine(7).
However, as well documented(7), results from prospective cohort studies on the relationship between habitual caffeine intake and long-term changes in BP and on HPT risk are conflicting and difficult to interpret because the data on clinical effects are mixed: equivocal, null and positive.
In the last 10 years, few systematic reviews and meta-analyses(Reference Zhang, Hu and Caballero9–Reference Xie, Cui and Zhu14) have been conducted with the purpose of clarification of the role of caffeine consumption on BP and HPT risk/incidence.
Zhang et al. (Reference Zhang, Hu and Caballero9) described an inverse J-shaped relationship between coffee consumption and the incidence of HPT, showing that more than three cups/d habitual coffee consumption, compared with less than one cup/d consumption, was not associated with an increased risk of HPT while a slightly elevated risk appeared to be associated with light to moderate use (i.e. 1–3 cups/d)(Reference Zhang, Hu and Caballero9).
Shah et al. (Reference Shah, Chu and Lacey10) assessed the BP and heart rate effects of energy drinks in healthy individuals and described a significant dose–effect increase, where the systolic BP elevation was under 4 mmHg when caffeine consumed was <200 mg and more than 6 mmHg when caffeine consumption was ≥200 mg(Reference Shah, Chu and Lacey10).
On the contrary, Steffen et al. (Reference Steffen, Kuhlea and Hensruda11) reported that no strong recommendation or discouragement of coffee consumption related to BP and/or HPT risk could be suggested, revealing that coffee consumption was not associated with any significant change in BP.
D’Elia et al.(Reference D’Elia, La Fata and Galletti12), exploring the relationship between habitual coffee consumption and the risk of HPT in the general population, reported a non-linear inverse dose–response relationship. In fact, consuming one or two cups of coffee/d was not significantly associated with HPT risk while a significant protective effect of coffee consumption was observed when 3–7 cups/d were consumed compared with no coffee consumption(Reference D’Elia, La Fata and Galletti12).
Mesas et al. (Reference Mesas, Leon-Muñoz and Rodriguez-Artalejo13) reported conflicting results on the influence of caffeine intake on BP distinguishing between acute and long-term effects. Intake of 200 to 300 mg of caffeine/d led to a significant BP increase, observed in the first 60 min post-ingestion up to 180 min, while drinking coffee for 2 weeks (range of caffeine intake/d: 79–300 mg) did not appear to increase BP(Reference Mesas, Leon-Muñoz and Rodriguez-Artalejo13).
Finally, the most recent meta-analysis of cohort studies, conducted by Xie et al. (Reference Xie, Cui and Zhu14), provided quantitative evidence that coffee consumption was inversely associated with the risk of HPT in a dose–response manner. The authors described a reduction of about 2 % per one cup/d increment of coffee consumption(Reference Xie, Cui and Zhu14).
Given the conflicting results described above, in the present narrative review, we provided a critical analysis of the existing literature. From a methodological point of view, summarising various methodological inaccuracies/aspects and confounding factors makes it difficult to compare studies in order to obtain a single consensus on the effects of caffeine intake on BP and HPT risk.
With this purpose, we considered cohort studies and randomised controlled trials (RCT) of the last 10 years (Tables 1 and 2), excluding the systematic reviews and meta-analyses that we mentioned above(Reference Zhang, Hu and Caballero9–Reference Xie, Cui and Zhu14).
Characteristics of the study population
Previous studies(Reference Mancia, De Backer and Dominiczak1, Reference Timmis, Townsend and Gale2, Reference Rizzo, Hispard and Dolbeault15) have reported that the BP response to intake of caffeine is influenced by multiple factors, such as age, sex, genetics(Reference Mancia, De Backer and Dominiczak1, Reference Timmis, Townsend and Gale2, Reference Nehlig16) and lifestyle factors (cigarette smoking, ethanol consumption, weight, Na and K intake)(Reference Mancia, De Backer and Dominiczak1, Reference Rizzo, Hispard and Dolbeault15), that affect either directly BP response or caffeine metabolism (such as genetic polymorphism).
Age is the factor that most influences BP response to caffeine intake; greater increases in systolic and diastolic BP have been reported in response to caffeine with increasing age(Reference Farag, Whitsett and McKey17). In the present review, few cohort studies(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18–Reference Navarro, Martinez-Gonzalez and Gea28) and only one RCT(Reference Farag, Whitsett and McKey17) considered age as a possible confounding factor (Table 1).
Table 1. Confounding variables affecting blood pressure response or caffeine metabolism
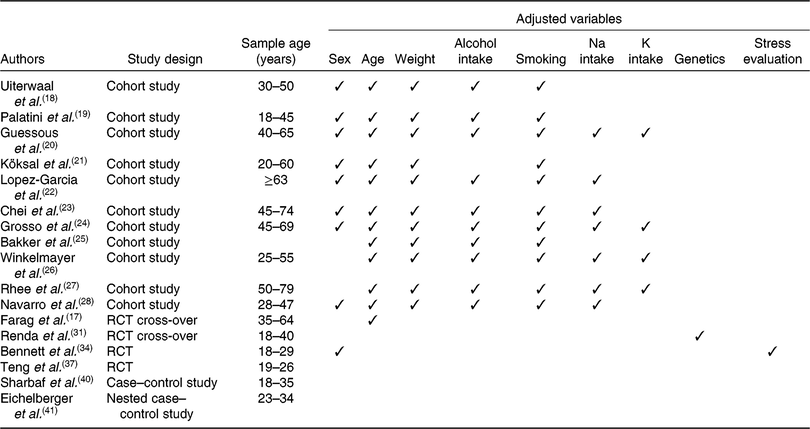
RCT, randomised controlled trial.
Genetics also play a role in caffeine-related BP modulation(Reference Timmis, Townsend and Gale2, Reference Rizzo, Hispard and Dolbeault15). In humans, caffeine is rapidly and completely absorbed after oral intake and the main route of metabolism is via N-3 demethylation to paraxanthine, catalysed by cytochrome P450 (CYP) 1A2 in the liver(7, Reference Nehlig16); the activity of CYP1A2 accounts for about 95 % of caffeine clearance(7). Genetic polymorphism of the CYP1A2 gene has been reported to be a source of variability in the metabolism of caffeine(7, Reference Nehlig16).
Moreover, previous genetic studies, conducted on three CYP1A2 alleles (rs762551, rs1133323 and rs1378942)(Reference Levy, Ehret and Rice29, Reference Newton, Gateva and Tobin30), reported that CYP1A2 variants are influenced by cigarette smoking, which increases caffeine metabolism, with caffeine BP response higher in smokers than in non-smokers. Additionally, it has been previously reported that long-term ethanol consumption masks the induction of CYP1A2 activity(Reference Rizzo, Hispard and Dolbeault15). Therefore, these findings suggest that the interaction between lifestyle factors and genetic assessment may explain a key role in the variability of the cardiovascular effects of caffeine intake.
In the present literature review, only one study(Reference Renda, Zimarino and Antonucci31) investigated the CYP1A2 polymorphism while other authors considered alcohol intake(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18–Reference Guessous, Pruijm and Ponte20, Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22–Reference Navarro, Martinez-Gonzalez and Gea28) and smoking habits(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18–Reference Navarro, Martinez-Gonzalez and Gea28) as possible confounding factors that directly affect BP response, not considering the genetic aspects (Table 1).
The sex-related effect of caffeine consumption on BP should also be considered(7). In fact, it has been well recognised that even if BP is generally lower in premenopausal women than in men, postmenopausal women have a higher prevalence of HPT than do men of a similar age(Reference Rhee, Qin and Hedlin27). Moreover, it has been previously reported(7, Reference Nehlig16) that CYP1A2 activity is higher in men than in women and lower in women on oral contraceptives. In the present review, only eight studies(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18–Reference Grosso, Stepaniak and Polak24, Reference Navarro, Martinez-Gonzalez and Gea28) considered sex as a possible confounding factor (Table 1).
Excess body weight, decreased K intake and increased Na consumption with diet are well-recognised risk factors for BP elevation and HPT onset(Reference Mancia, De Backer and Dominiczak1). In the present review, eleven studies(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18–Reference Navarro, Martinez-Gonzalez and Gea28) adjusted their results for weight, seven(Reference Guessous, Pruijm and Ponte20, Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22–Reference Grosso, Stepaniak and Polak24, Reference Winkelmayer, Stampfer and Willett26–Reference Navarro, Martinez-Gonzalez and Gea28) considered dietary Na intake and only four(Reference Guessous, Pruijm and Ponte20, Reference Grosso, Stepaniak and Polak24, Reference Winkelmayer, Stampfer and Willett26, Reference Rhee, Qin and Hedlin27) dietary K intake (Table 1).
Finally, it has been previously demonstrated that caffeine and stress appear to affect BP more in those with a positive family history of HPT compared with those with no non-contributory history(Reference Al’Absi, Bongard and Buchanan32, Reference Al’Absi, Lovallo and McKey33); studies reported in the present review did not consider this aspect, excluding Bennett et al. (Reference Bennett, Rodrigues and Klein34) (Table 1), overshadowing the impact on BP of stress and caffeine combined.
Blood pressure assessment
As well described, BP is characterised by large spontaneous variations both during the day and between days, months and seasons; the diagnosis of HPT should be based on multiple BP measurements, taken on separate occasions over a period of time(Reference Mancia, De Backer and Dominiczak1). BP can be measured by a doctor or nurse in the office or clinic (office or clinic BP), by the patient him/herself or by a relative of him/her at home, or automatically over 24 h monitoring (ambulatory BP monitoring)(Reference Mancia, De Backer and Dominiczak1). There are different procedures to measure BP, such as mercury sphygmomanometer or other non-invasive devices (auscultatory or oscillometric semiautomatic devices)(Reference Mancia, De Backer and Dominiczak1); however, since BP measurements are sensitive, standardised measures are needed(Reference Tolonen, Koponen and Naska35).
Some authors cited in Table 2 registered BP by using automatic techniques(Reference Palatini, Fania and Mos19–Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22, Reference Bakker, Steegers and Raat25); this could introduce some bias because these are not ‘gold standard’ methods(Reference Perloff, Grim and Flack36) and their accuracy should be checked periodically by comparing with mercury sphygmomanometric values(Reference Mancia, De Backer and Dominiczak1). Moreover, some studies considered ambulatory BP monitoring(Reference Palatini, Fania and Mos19, Reference Guessous, Pruijm and Ponte20, Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22), others isolated BP measurements(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18, Reference Grosso, Stepaniak and Polak24, Reference Bakker, Steegers and Raat25, Reference Rhee, Qin and Hedlin27, Reference Teng, Lim and Chua37) and others repeated BP measurements(Reference Farag, Whitsett and McKey17, Reference Köksal, Yardımcı and Kocaadam21, Reference Renda, Zimarino and Antonucci31, Reference Bennett, Rodrigues and Klein34).
Table 2. Methodological aspects influencing the interpretation of results
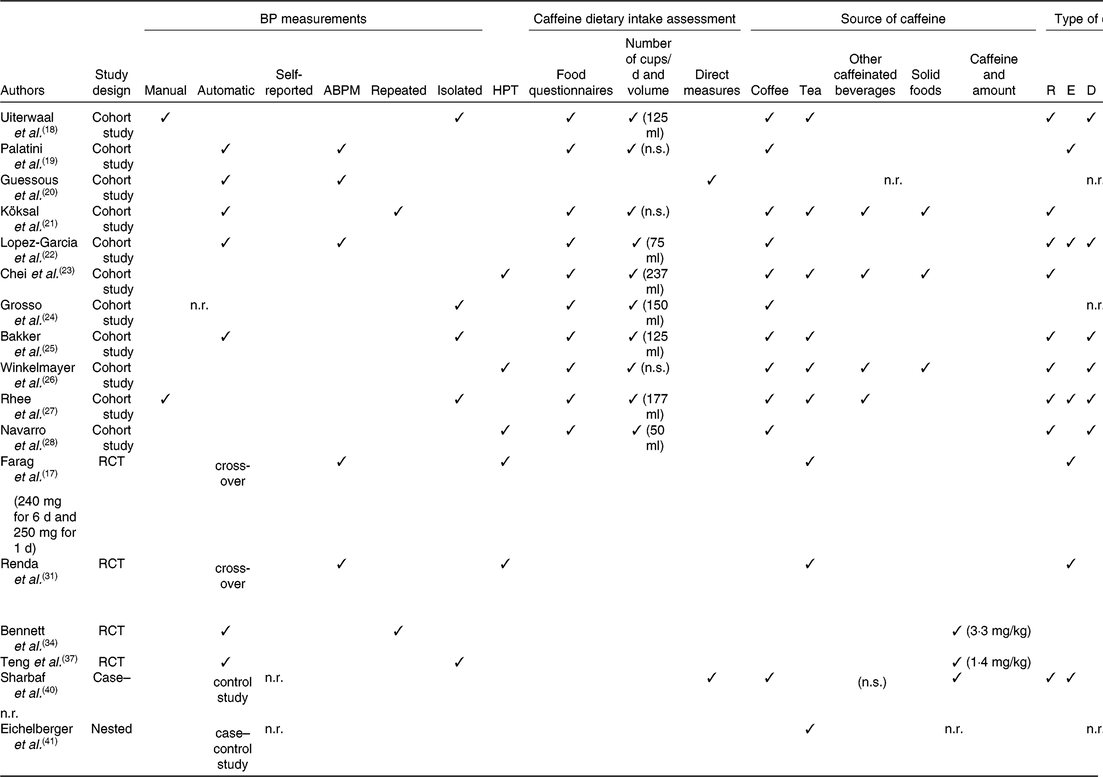
BP, blood pressure; ABPM, ambulatory blood pressure monitoring; HPT, hypertension; R, regular; E, espresso; D, decaffeinated; n.s., not specified; n.r., not reported; RCT, randomised controlled trial.
Therefore, the lack of standardisation to obtain BP measurements has led to values that cannot be compared with each other.
Finally, as far as HPT is concerned (Table 2), some studies(Reference Chei, Loh and Soh23, Reference Winkelmayer, Stampfer and Willett26, Reference Navarro, Martinez-Gonzalez and Gea28) analysed only the risk of HPT by means of a self-reported medical diagnosis or through the prescribed HPT medication reported by the patient during a semi-structured interview(Reference Chei, Loh and Soh23, Reference Winkelmayer, Stampfer and Willett26, Reference Navarro, Martinez-Gonzalez and Gea28).
Caffeine source and daily caffeine intake
The terms caffeine and coffee are often conflated in both the biomedical literature and public perception; however, the terms are not synonymous, and the biological effects of coffee cannot be reduced to the isolated effects of the caffeine that it contains(Reference O’Keefe, Bhatti and Patil38).
The main sources of caffeine in the diet include coffee, tea, caffeinated soft drinks (including ‘energy drinks’ containing also guarana, taurine and ginseng that may also cause haemodynamic changes) and chocolate(Reference Mancia, De Backer and Dominiczak1).
However, a high variability in caffeine levels for different foods and beverages has been noticed within the same product and for the same country(7). For instance, caffeine concentrations in coffee beverages depend on the manufacturing process, on the type of coffee beans used and on the type of preparation(7); as reported by Fox et al. (Reference Fox, Wu and Yiran39) who evaluated twenty-eight varieties of coffee with caffeine concentrations ranging from 10·6 and 19·9 mg/g.
Other food items that might present significant variability in caffeine levels are cocoa-based beverages, depending on the amount and type of cocoa that differ according to the brand(Reference Mancia, De Backer and Dominiczak1).
Cohort(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18, Reference Palatini, Fania and Mos19, Reference Köksal, Yardımcı and Kocaadam21–Reference Navarro, Martinez-Gonzalez and Gea28) and case–control studies(Reference Sharbaf, Dehghanpour and Shariat40), reported in Table 2, evaluated the effects of caffeine on BP and HPT risk; in all these studies coffee was always considered as a source of caffeine, but other sources of caffeine, such as tea(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18, Reference Köksal, Yardımcı and Kocaadam21, Reference Chei, Loh and Soh23, Reference Bakker, Steegers and Raat25–Reference Rhee, Qin and Hedlin27, Reference Sharbaf, Dehghanpour and Shariat40), energy/soft drinks(Reference Köksal, Yardımcı and Kocaadam21, Reference Chei, Loh and Soh23, Reference Winkelmayer, Stampfer and Willett26, Reference Rhee, Qin and Hedlin27, Reference Sharbaf, Dehghanpour and Shariat40) and solid foods(Reference Köksal, Yardımcı and Kocaadam21, Reference Chei, Loh and Soh23, Reference Winkelmayer, Stampfer and Willett26) were also included, increasing the variability of results. Therefore, to better elucidate the variability and effect of caffeine on BP and HPT risk, we should consider the average concentration of caffeine rather than specific foods or beverages, as already suggested by the EFSA panel(7).
Finally, we should also take into consideration the direct administration of caffeine in RCT at different timings and concentrations. In humans, 99 % of caffeine is absorbed within 45 min after ingestion, reaching the plasma concentration peak in 30 min; however, caffeine metabolites become higher after 8–10 h from ingestion(Reference Nehlig16). Moreover, as previous reported, data suggested that BP generally increases 30 min after caffeine consumption, reaches a peak after 60–90 min and returns to baseline after about 2–4 h, which is consistent with the pharmacokinetics of caffeine(7). Among our selected RCT, only two studies(Reference Renda, Zimarino and Antonucci31, Reference Bennett, Rodrigues and Klein34) considered a period of abstinence from caffeine from 2 to 4 h. Furthermore, two studies(Reference Farag, Whitsett and McKey17, Reference Teng, Lim and Chua37) measured BP response within 1 h after caffeine administration and another two(Reference Renda, Zimarino and Antonucci31, Reference Bennett, Rodrigues and Klein34) within 2 h.
EFSA reported that the effect of caffeine consumption was observed at single doses of caffeine ranging from 80 to 300 mg(7). RCT showed heterogeneity also in the administered doses of caffeine; in fact, three studies(Reference Renda, Zimarino and Antonucci31, Reference Bennett, Rodrigues and Klein34, Reference Teng, Lim and Chua37) administered caffeine in mg/kg body weight, whereas one study(Reference Farag, Whitsett and McKey17) administered fixed doses.
Another source of bias is due to the fact that there are different indirect methods to assess daily caffeine consumption, making it difficult to relate caffeine intake to BP and HPT risk results among studies. In fact, most of the studies(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18, Reference Palatini, Fania and Mos19, Reference Köksal, Yardımcı and Kocaadam21–Reference Navarro, Martinez-Gonzalez and Gea28) estimated caffeine consumption by means of questionnaires or interviews; however, only seven studies(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18, Reference Köksal, Yardımcı and Kocaadam21, Reference Chei, Loh and Soh23, Reference Grosso, Stepaniak and Polak24, Reference Winkelmayer, Stampfer and Willett26–Reference Navarro, Martinez-Gonzalez and Gea28) used a validated FFQ, one study used FFQ together with a photographic food atlas(Reference Grosso, Stepaniak and Polak24), and another one(Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22) used a computerised diet history with photographs, to estimate portion sizes, new dishes and cooking methods and the degree of food processing(Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22). Other authors(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18, Reference Palatini, Fania and Mos19, Reference Köksal, Yardımcı and Kocaadam21, Reference Chei, Loh and Soh23–Reference Bakker, Steegers and Raat25, Reference Rhee, Qin and Hedlin27, Reference Navarro, Martinez-Gonzalez and Gea28) expressed daily caffeine consumption as coffee cups or tea cups with different volumes (Table 2).
Another issue that has been identified relies on the laboratory analytical methodology. It is well known that caffeine is metabolised by the liver CYP1A2 enzyme into paraxanthine (about 80 %), theobromine (about 12 %), and theophylline (about 4 %) and that the urinary excretion of caffeine and caffeine metabolites is a valid measure of caffeine intake(Reference Guessous, Pruijm and Ponte20). However, only one study conducted by Guessous et al. (Reference Guessous, Pruijm and Ponte20) analysed, for the first time, the associations between ABPM with urinary caffeine and caffeine metabolite excretion. Other authors(Reference Farag, Whitsett and McKey17, Reference Renda, Zimarino and Antonucci31, Reference Eichelberger, Baker and Woodham41) evaluated caffeine(Reference Farag, Whitsett and McKey17, Reference Renda, Zimarino and Antonucci31) and its metabolites (paraxanthine)(Reference Eichelberger, Baker and Woodham41) in plasma(Reference Renda, Zimarino and Antonucci31), serum(Reference Eichelberger, Baker and Woodham41) and salivary samples(Reference Farag, Whitsett and McKey17).
Type of coffee
The amount of caffeine in coffee (DM basis of green coffee beans) varies markedly between species and species(Reference Caprioli, Cortese and Sagratini42). For instance, amounts ranging from 65 to 120 mg of caffeine have been reported to be contained in a normal cup of coffee, whereas Arabica coffee normally contains less caffeine than the Robusta variety(Reference Caprioli, Cortese and Sagratini42).
Moreover, the content of bioactive compounds is influenced by the extraction mechanism(Reference Nehlig16).
McCusker et al.(Reference McCusker, Goldberger and Cone43) previously evaluated the caffeine content of caffeinated and decaffeinated espresso coffee (EC) and coffee brew purchased ready-to-drink from coffee shops. The caffeine dose (expressed in mg) in caffeinated EC was lower than in caffeinated brewed coffees. However, EC volumes were smaller than regularly brewed coffee and, in terms of caffeine concentration, EC reported higher caffeine concentration than brewed coffees(Reference McCusker, Goldberger and Cone43).
Finally, another study by Fujioka et al. (Reference Fujioka and Shibamoto44) found that the caffeine content in regular coffees ranged from 10·9 to 16·5 mg/g while that of decaffeinated coffees was from 0·34 to 0·47 mg/g.
Studies considered in the present narrative review reported different types of coffee such as regular(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18, Reference Köksal, Yardımcı and Kocaadam21, Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22, Reference Chei, Loh and Soh23, Reference Bakker, Steegers and Raat25–Reference Navarro, Martinez-Gonzalez and Gea28), espresso(Reference Palatini, Fania and Mos19, Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22, Reference Rhee, Qin and Hedlin27), decaffeinated(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18, Reference Lopez-Garcia, Orozco-Arbeláez and Leon-Muñoz22, Reference Bakker, Steegers and Raat25–Reference Navarro, Martinez-Gonzalez and Gea28) and other(Reference Uiterwaal, Verschuren and Bueno-de-Mesquita18), even not specified(Reference Grosso, Stepaniak and Polak24, Reference Sharbaf, Dehghanpour and Shariat40).
Conclusion
Coffee consumption has long been a suspected cause of HPT, but the available evidence from the literature is sometimes equivocal, making it difficult to compare studies in order to obtain a single consensus on the effects of caffeine intake on the risk of BP and HPT, as also demonstrated by different systematic reviews and meta-analyses(Reference Zhang, Hu and Caballero9–Reference Xie, Cui and Zhu14) in the last 10 years.
Also worth mentioning in the context of the present review is that Grant et al. (Reference Grant, Magruder and Friedman45) also considered the protocols used to control for caffeine’s effects on cardiovascular parameters in the extant cardiovascular literature(Reference Grant, Magruder and Friedman45). Grant et al. (Reference Grant, Magruder and Friedman45) summarised the widely differing protocols used to identify correctly some primary means to control cardiovascular response after caffeine intakes, such as controlling variables that significantly alter the half-life of caffeine, timing of caffeine administration, and methods of administration. In conclusion, Grant et al. (Reference Grant, Magruder and Friedman45) stressed that creating a standard for ‘caffeine controls’ requires more attention and interest in examining the potentially confounding vascular effect of caffeine. As a result, improvements in methodological controls for caffeine can have significant health consequences. Reliable, methodologically correct cardiovascular research depends on the appropriate control of a myriad of factors that can systematically alter cardiovascular responses(Reference Grant, Magruder and Friedman45).
Similarly, in the present critical narrative review we highlighted how conflicting results could be due to various confounding/different factors such as: (i) variables affecting BP response (age, sex, genetics, smoking habits, alcohol consumption, Na and K intake); (ii) different caffeine content of food and beverages due to different items (coffee, tea, caffeinated beverages or solid foods) and extraction mechanism (espresso, regular or decaffeinated); and (iii) caffeine metabolism. Moreover, different methodological aspects in the evaluation of daily dietary caffeine intake (questionnaires or direct measurements of caffeine’s metabolites) and in the BP measurement, concerning instruments (manual or automatic) and timing (ambulatory BP monitoring, isolated or repeated), could add further bias in the interpretation of results.
Therefore, it is mandatory to take into consideration all methodological aspects and confounding factors to generate a standardised methodology in order to increase cross-study consistency and minimise confounding effects of different variables on the relationship between BP response and HPT risk/incidence after caffeine intake.
Acknowledgements
The authors are very grateful to Richard Patrick Tyrrell for linguistic consultation. R. D. G. and I. d. N. contributed equally to the conception and design of the review, conducted the literature review and drafted the manuscript; F. G. and A. M. conducted the literature review; H. C. drafted and revised the manuscript. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere.
The authors declare no potential conflicts of interest.




