Sialic acids (Sia) are a diverse family of sugars having neuraminic acid, a nine-carbon sugar acid, as the parent compound. They are typically found attached to the non-reducing terminus of cell surface glycans in all vertebrates( Reference Schauer 1 – Reference Cohen and Varki 5 ). N-Acetylneuraminic acid (Neu5Ac) and its hydroxylated form N-glycolylneuraminic acid (Neu5Gc) are the two major forms of Sia in mammals( Reference Troy 3 ). A less common Sia is ketodeoxynonulosonic acid (KDN), which is frequently found in lower vertebrates( Reference Inoue and Kitajima 6 ). However, human erythrocytes and ovarian cancer cells express unusually high levels of free KDN compared with the mostly conjugated forms of Neu5Ac and Neu5Gc( Reference Inoue, Lin and Chang 7 ). More than fifty naturally occurring derivatives of Sia have been discovered, resulting often from the modification of the parent Sia with phosphate, sulphate, lactyl and multiple acetyl groups( Reference Troy 3 , Reference Wang 8 ). The negative charge of the polySia moiety on neural cell adhesion molecules and its extended chain length or degree of polymerisation( Reference Nakata and Troy 9 ) are of critical importance for the mediation of many specific biological functions, including cell recognition, cell-to-cell adhesion, receptor-mediated cell signalling and immune function modulation( Reference Inoue, Lin and Chang 7 – Reference Drake, Nathan and Stock 10 ).
Sia are expressed in the tissues and body fluids of all vertebrates including cerebrospinal fluid, saliva, gastric juice, serum, urine, tears, milk( Reference Wang 8 , Reference Wang, Miller and McNeil 11 ) and fish eggs( Reference Inoue and Kitajima 6 ) and in the neurovirulent capsule of neuroinvasive bacteria causing meningitides( Reference Troy 3 ). Sia levels are highest in the central nervous system, primarily in neural cell membranes, which contain levels that are approximately twenty times higher than those present in other cell membranes( Reference Wang 8 , Reference Wang, Miller and McNeil 11 ). Dietary Sia supplementation increases Sia concentrations in the brain and improves learning and memory( Reference Wang and Brand-Miller 12 – Reference Wang, Yu and Karim 15 ). The results of earlier studies show that Sia is essential for the neural structure and function of newborns( Reference Wang 8 , Reference Wainwright, Lomanowska and McCutcheon 14 , Reference Wang, Yu and Karim 15 ). The pre-school period (e.g. 3–5 years) is an important period in life, as rapid postnatal brain development takes place during this time, particularly neural plasticity( Reference Rosales, Reznick and Zeisel 16 ). Anatomical MRI studies have shown that total cerebral volume peaks at the age of 14·5 years in boys and 11·5 years in girls and that by 6 years of age 95 % of the brain volume is achieved( Reference Lenroot and Giedd 17 ). During this neural developmental period, children's spoken vocabulary also increases significantly. They gain greater motor coordination and are able to engage in tasks including working memory, attention and inhibitory control( Reference Sakai 18 ). These special aspects and functions of brain development are reflected in a higher need for many nutrients such as iodine, Fe, Zn, divalent cations, gangliosides, glycoproteins and sphingolipids( Reference Rosales, Reznick and Zeisel 16 ). It is postulated that Sia, a key precursor of brain gangliosides and glycoproteins, in particular, for the synthesis of polysialylated neural cell adhesion molecules, may facilitate neural development and cognitive function in pre-school children.
Humans cannot synthesise Neu5Gc because of a human-specific mutation in the CMP-Neu5Ac hydroxylase (CMAH) gene( Reference Varki 19 – Reference Padler-Karavani, Yu and Cao 22 ). However, Neu5Gc can be metabolically incorporated into human tissues from animal-derived foods, primarily red meat and milk products. This incorporation occurs even in the presence of an anti-Neu5Gc ‘xeno-autoantibody’ response( Reference Taylor, Gregg and Padler-Karavani 21 , Reference Padler-Karavani, Yu and Cao 22 ). Varki( Reference Varki 23 ) has shown that the dietary uptake of Neu5Gc correlates with human inflammatory diseases including atherosclerosis and cancer. Human anti-Neu5Gc antibodies first appear in children during infancy and their level correlates with weaning and exposure to dietary Neu5Gc in cows' milk-based infant formula( Reference Varki 20 , Reference Taylor, Gregg and Padler-Karavani 21 ). The long-term impact of the uptake of the non-human Neu5Gc on health and disease remains to be determined( Reference Varki, Schauer, Varki, Cummings, Esko, Freeze, Stanley, Bertozzi, Hart and Etzler 2 , Reference Varki 19 – Reference Wang 24 ). To our knowledge, there are no published studies on the uptake, metabolism and catabolism of exogenous dietary KDN in human subjects.
Urine is the most important excretory product containing Sia and an important marker for the clinical diagnosis of several inborn errors of Sia metabolism, including Salla disease, infant Sia storage disease and sialidosis( Reference Valianpour, Abeling and Duran 25 – Reference Seppala, Renlund and Bernardini 28 ). Abnormal concentrations of urinary Sia have also been reported in adults and children with renal diseases( Reference van Aswegen, van der Merwe and du Plessis 29 ), diabetes( Reference Shivananda Nayak, Duncan and Lalloo 30 ) and cancer( Reference Labdenne and Heikinheimo 31 ). Given their potential importance, it is surprising that there is little information on the concentrations of urinary Sia such as Neu5Ac, Neu5Gc and KDN and the possible relationship of these concentrations with dietary Sia intake in pre-school children. The present study provides new information on the metabolic fate of dietary Sia present in red meat and other dietary sources and its possible correlation with urinary Sia levels in pre-school children. The present study thus provides the basis for follow-up studies to determine the lifelong effects of Sia intake, principally from dietary red meat in early life, on health and disease in adulthood( Reference Varki 32 ).
Subjects and methods
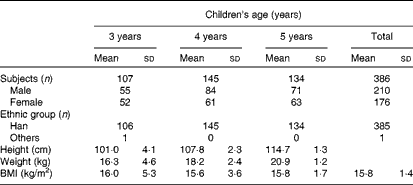
A total of 386 healthy children were recruited from a large kindergarten in Xiamen, China, in three groups: 3–4-year-olds (n 107), 4–5-year-olds (n 145) and 5–6-year-olds (n 134). All the children were provided breakfast, lunch and afternoon dessert from 07.30 to 17.30 hours. Fresh drinking-water was provided throughout the day. Children ate dinner at home. Total nutrient intake levels, principally dietary Sia intake levels, were recorded and analysed. Body weight and height were also recorded. Children were excluded from the study if they were taking any medication. The characteristics of children on the day of urine sample collection are given in Table 1. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Human Ethics Committee of Xiamen University, with written informed consent from the parents of all subjects.
Table 1 General characteristics of the study participants (Mean values and standard deviations)
Method of urine sample collection
Spot urine samples were collected daily from each child at three different time periods (07.00, 11.30 and 16.30 hours). Morning urine samples were collected by the parents. Urine sample collection at noon and late afternoon was carried out by trained researchers. Urine samples were collected from 183 3–5-year-old children at all the three time periods at the kindergarten. Major reasons for children missing urine samples included ‘they do not have urine and have just now passed urine in the sample collection period. Each sample was collected in a sterile container and stored at − 20°C and then at − 80°C before analysis.
Food sample collection
A total of twenty-one different types of fresh raw foods and products including meat, poultry, eggs, seafood, milk and milk products were purchased from three to five different local supermarkets (Table 2) and stored at − 20°C until use.
Table 2 Concentration and distribution of different forms of sialic acids (Sia) in conventional foods of China (μg/g wet tissue)

Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; KDN, ketodeoxynonulosonic acid; ND, not detected; Tr, trace amounts.
LC–MS/MS for quantitative analysis of N-acetylneuraminic acid, N-glycolylneuraminic acid and ketodeoxynonulosonic acid levels in the urine and foods
The major forms of free Sia (Neu5Ac, Neu5Gc and KDN) were analysed using a highly sensitive LC–MS/MS method (Agilent1100-Agilent6430; Agilent Technology)( Reference van der Ham, Prinsen and Huijmans 33 ). Urine samples were filtered through a 0·22 μm membrane filter (Millipore Corporation), diluted and mixed with 0·5 μmol/l internal standard. Subsequently, 1 g of food samples was homogenised in an Eppendorf tube with Tris-buffered saline using a high-speed disperser (T10 basic ULTRA-TURRAX, IKA) at 4°C( Reference Inoue, Lin and Chang 7 ). Then, the homogenate was centrifuged at 3000 g for 30 min, and the supernatant was filtered through a 0·22 μm membrane filter before analysis for free Sia. Because only free Sia can be measured using this method, optimal conditions for the release of conjugated Sia were established by prior hydrolysis of urine samples and precipitation of food samples under a variety of acidic conditions. The maximum release of conjugated Sia from food samples was achieved using an equal volume of 0·1 mol/l trifluoroacetic acid for 60 min at 80°C and from urine samples using 0·15 m-H2SO4 at 80°C for 90 min. All foods samples were filtered after hydrolysis using a 0·22 μm Millipore membrane. To 100 μl of diluted samples, 25 μl of 0·5 μmol/l [13C3]Sia were added as an internal standard before analysis using LC–MS/MS. Negatively single charged ions for Neu5Ac (m/z 308·0), Neu5Gc (m/z 324·0), KDN (m/z 267·0) and internal standard (m/z 311·1) were selected as parent ions and m/z 87·1, m/z 116·1, m/z 87·0 and m/z 90·1 were selected as daughter ions, respectively. The ratio of Sia:[13C3]Sia (peak area) was used to quantify the concentrations of Sia.
Quantitative analysis of urinary creatinine levels
The Nanjing Jiancheng Bioengineering Institute creatinine (Cr) kit was used with a semi-automatic biochemical analyser (Microlab300, Vital Scientific) to quantitatively determine urinary Cr levels. All samples were analysed in duplicate, and the final concentrations of total, free and conjugated Sia are expressed as mmol Sia/mol Cr.
Levels of sialic acid intake from dietary food sources
Sia is predominantly conjugated to mammalian glycoconjugates in red meat and dairy products( Reference Varki, Schauer, Varki, Cummings, Esko, Freeze, Stanley, Bertozzi, Hart and Etzler 2 – Reference Cohen and Varki 5 , Reference Tangvoranuntakul, Gagneux and Diaz 34 , Reference Chen, Wang and Han 35 ). All food intake levels of children at the kindergarten were recorded on the day of urine sample collection. Food intake levels were analysed exactly as described in our previous published method( Reference Chen, Wang and Han 35 ). Briefly, a trained researcher used the ‘China Food Composition’ to calculate individual nutrient intake levels of the 1506 foods and thirty-one nutrients( 36 ). The intake levels of selected nutrients were analysed using software for ‘Intake Distribution Estimation’, based on Chinese Food Nutrition Composition( 36 – 38 ). Sia intake levels for each participant are expressed as μg Sia/kg body weight.
Statistical analysis
Data were analysed by a two-factor repeated-measures ANOVA model with three time points using the Greenhouse–Geisser adjustment for asphericity. To determine the different time trends for the three age groups, an interaction between the dependent variable and grouping factor was analysed. The overall comparison of different levels of Sia between the three age groups was made using the repeated-measures ANOVA model, and comparisons at individual time points were made using t tests. Values were considered significant at P <0·05.
Pearson's correlation was used for analysing the correlation between urinary Sia concentrations and dietary Sia intake. Significant correlations were obtained if P <0·01. The F test was used for the comparison of the slopes of the regression lines of urinary Sia concentrations and dietary Sia intake. All analyses were carried out using SPSS for Windows 19.0 (SPSS, Inc.).
Results
Urinary sialic acid (N-acetylneuraminic acid, N-glycolylneuraminic acid and ketodeoxynonulosonic acid) levels in pre-school children
Except in the urine sample of one 4-year-old girl, no Neu5Gc was detected in the 855 urine samples collected from 386 healthy children aged 3–5 years. The concentrations of free and conjugated Neu5Ac and KDN in the urine samples of pre-school children at the three different collection time periods are given in Table 3. The concentrations of total urinary Sia were highest in the morning and decreased during the day in 4- and 5-year-olds, but not in 3-year-olds (Table 3). The slight decline in urinary Sia levels, including total, free and conjugated Neu5Ac and free KDN levels, between the age groups (i.e. interaction) was statistically significant (P= 0·008, 0·002, 0·029 and 0·004, respectively, using a two-way repeated-measures ANOVA with the Greenhouse–Geisser adjustment for asphericity), when all data were analysed or in the 183 children who had complete datasets for all the time points (Fig. 1(a)–(e)). The levels of conjugated KDN in the urine samples of 3- and 4-year-old children increased during the day (P< 0·05; Fig. 1(e)). Interestingly, the difference in the levels of all the different forms of urinary Sia including free and conjugated Neu5Ac and total and conjugated KDN in the morning urine samples between the different age groups was statistically significant (P< 0·006, using the general linear model (ANOVA) with Bonferroni's adjustment for multiple comparisons; Table 3). In the morning urine samples of 5-year-old children, the levels of total Sia, Neu5Ac and KDN were 34–81, 25–87 and 48 %, approximately 76 % greater than those in the samples of 3- and 4-year-old children (P< 0·05; Table 3). In the noon urine samples of 3-year-old children, the levels of free Neu5Ac and KDN were 36 and 33 % lower than those in the samples of 4-year-old children (P< 0·05), but not in those of 5-year-old children (P>0·05; Table 3). Furthermore, in the afternoon urine samples of 5-year-old children, the levels of total urinary KDN were 36 %, approximately 37 % lower than those in the samples of 3- and 4-year-old children, irrespective of all the data being included in the analysis (as shown in Table 3) or the 183 children with complete datasets for all time periods being analysed separately (P< 0·05; Fig. 1).
Table 3 Concentration and distribution of spot urinary sialic acids (Sia) (N-acetylneuraminic acid (Neu5Ac) and ketodeoxynonulosonic acid (KDN))* in children aged 3–5 years at three different time periods (Mean values with their standard errors)

Cr, creatinine.
* No Neu5Gc was detected in the urine samples of 3- to 5-year-old children any time point throughout the course of the study, except in the noon urine sample of one 4-year-old child.

Fig. 1 Time trend of concentrations of total and each form of sialic acids (Sia) in the three age groups (![]() , 3 years (n 49);
, 3 years (n 49);![]() , 4 years (n 73);
, 4 years (n 73);![]() , 5 years (n 61)) during the day. Only those children whose data were complete for all the three time periods were included in the analyses. The difference between the three groups (i.e. interaction) was significant (P= 0·008, 0·002, 0·029, 0·004 and 0·325, using a two-way repeated-measures ANOVA with the Greenhouse–Geisser adjustment for asphericity) in relation to the levels of (a) total Sia, (b) free N-acetylneuraminic acid, (c) conjugated N-acetylneuraminic acid, (d) free ketodeoxynonulosonic acid (KDN) and (e) conjugated KDN, respectively. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different between the three groups: * P< 0·05, ** P< 0·01 (one-way ANOVA). Cr, creatinine.
, 5 years (n 61)) during the day. Only those children whose data were complete for all the three time periods were included in the analyses. The difference between the three groups (i.e. interaction) was significant (P= 0·008, 0·002, 0·029, 0·004 and 0·325, using a two-way repeated-measures ANOVA with the Greenhouse–Geisser adjustment for asphericity) in relation to the levels of (a) total Sia, (b) free N-acetylneuraminic acid, (c) conjugated N-acetylneuraminic acid, (d) free ketodeoxynonulosonic acid (KDN) and (e) conjugated KDN, respectively. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different between the three groups: * P< 0·05, ** P< 0·01 (one-way ANOVA). Cr, creatinine.
Variation and distribution of N-acetylneuraminic acid, N-glycolylneuraminic acid and ketodeoxynonulosonic acid in the urine samples of 3–5-year-old children
The present results also showed that there was a large variation in urinary Sia concentrations between children in the same age group and at the same period during the day. For example, the lowest and highest levels of total urinary Sia in the morning, noon and late afternoon varied between 7-, 54- and 21-fold; 18-, 68- and 49-fold; and 32-, 22- and 12-fold, respectively, for 3-, 4- and 5-year-olds. The level of free Neu5Ac showed the most significant variation among the levels of Sia in all the age groups. In particular, 5-year-old children showed the greatest variation in the levels of all the detected forms of urinary Sia at the same time point or at the three different time periods (P< 0·05–0·01). However, only 3- and 4-year-old children showed the greatest variation in urinary Sia levels within the same time point, but not during the different time periods of a day.
No Neu5Gc was detected in the urine samples of 3–5-year-old children during the course of the present study. The single exception was the noon urine sample of a 4-year-old girl who appeared to have total Sia intake levels similar to those of the other children. The proportional composition of urinary Sia in the three age groups was conjugated Neu5Ac (70·0 (sd 10·6) %), free Neu5Ac (21·2 (sd 8·5) %), conjugated KDN (4·5 (sd 4·0) %) and free KDN (3·7 (sd 1·6) %, Fig. 2). In 3-year-old children, the proportions of free Neu5Ac in the morning urine samples, conjugated Neu5Ac in the noon urine samples and conjugated KDN in the late-afternoon urine samples were significantly higher than those at the other two time periods (P< 0·05; Fig. 2(a), (d) and (g)). In 4-year-old children, only the proportion of conjugated KDN was higher in the afternoon urine samples than in the morning and noon urine samples (P< 0·05; Fig. 2(b), (e) and (h)). In 5-year-old children, however, the proportion of free urinary Neu5Ac was significantly high, while that of conjugated Neu5Ac was lower than those at the other two time periods (P< 0·05; Fig. 2(c), (f) and (i)). Thus, for children in the same age group, the proportions of urinary Neu5Ac and KDN varied during the three daily urine sample collection periods. Children of different ages also had slightly different proportions of each urinary Sia at different time points (Fig. 2).

Fig. 2 Average proportion of free N-acetylneuraminic acid (Neu5Ac) (■), conjugated Neu5Ac (□), free ketodeoxynonulosonic acid (KDN) (![]() ) and conjugated KDN (
) and conjugated KDN (![]() ) in the spot urine samples of pre-school children throughout the day (07.00, 11.30 and 16.30 hours). The numbers of children from whom morning, noon and afternoon urine samples were collected in the 3-year age group were (a) n 76, (d) n 76 and (g) n 79; in the 4-year age group (b) n 108, (e) n 107 and (h) n 112; and in the 5-year age group (c) n 103, (f) n 85 and (i) n 110.
) in the spot urine samples of pre-school children throughout the day (07.00, 11.30 and 16.30 hours). The numbers of children from whom morning, noon and afternoon urine samples were collected in the 3-year age group were (a) n 76, (d) n 76 and (g) n 79; in the 4-year age group (b) n 108, (e) n 107 and (h) n 112; and in the 5-year age group (c) n 103, (f) n 85 and (i) n 110.
Total sialic acid and its distribution in food products
The concentration of total Sia expressed as μg Sia/g wet tissue in Chinese foods was highest in Crucian eggs, followed by hen egg yolk, hen egg-white, milk products, red meat, poultry and seafood (Table 2). In milk products, the concentration of total Sia was highest in cheese. However, in red meat, lamb contained the highest levels of total Sia, followed by beef and pork. The concentrations of Sia in poultry were higher in ducks than in chickens. Only trace levels of Sia were detected in shrimp, clams and abalones. The difference in Sia levels between the different food types was statistically significant (P< 0·05), as shown in Table 2.
Neu5Ac is the predominant form of Sia in most conventional foods, except crab, in which KDN is the major form. However, in ducks, chickens, hen eggs and fish, only Neu5Ac, and not Neu5Gc or KDN, was detected. All milk products, however, were found to contain all the three forms of Sia as follows: 71–83 % Neu5Ac; 2–5 % Neu5Gc; 12–27 % KDN. The proportions of Neu5Gc in lamb, beef and pork were 22·5, 39·5 and 8·9 %, respectively, which were higher than those in all the other categories of foods studied (Table 2).
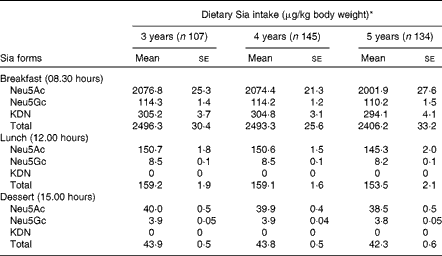
Levels of daily sialic acid intake in pre-school children
The most common form of dietary Sia in humans is derived from animal and dairy products( Reference Wang 8 ). The mean levels of Sia intake from different food sources are given in Table 4. These values were derived from the Sia levels in conventional foods of China (Table 2). The absolute level of total Sia intake during the day was higher in 5-year-old children than in 3- and 4-year-old children, although the differences were not statistically significant (P>0·05). However, total Sia intake levels in 3-year-old children were marginally higher than those in 4- and 5-year-old children (P= 0·051) during the day, when Sia intake levels were calculated based on mg Sia/kg body weight per d (Table 4). Sia intake levels were also higher in all the three age groups of children at breakfast than at lunch, probably because they consumed larger amounts of milk or milk products at breakfast.
Table 4 Dietary sialic acid (Sia) intake levels during the day in 3- to 5-year-old kindergarten children (Mean values with their standard errors)
Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; KDN, ketodeoxynonulosonic acid.
* There was no significant difference in dietary Sia intake levels between children in the three age groups.
Relationship between sialic acid intake levels and urinary sialic acid levels
There was only a marginal correlation between total Sia intake levels at breakfast or lunch and urinary conjugated KDN levels in the afternoon (P= 0·064). The intake level of total Sia or total Neu5Ac at breakfast was also only marginally correlated with the percentage of free urinary Neu5Ac (P= 0·033). A marginally positive correlation existed between the intake level of total Sia or total KDN and the proportion of conjugated urinary KDN in the afternoon (P= 0·024). In summary, we did not find a statistically significant correlation between the levels of dietary Sia intake and the levels of urinary Sia in 3–5-year-old children (P>0·01; Fig. 3).

Fig. 3 Lack of statistically significant correlation between total sialic acid (Sia) intake levels and excretion levels of Sia in the urine. (a) Marginal correlation between total Sia intake levels and conjugated ketodeoxynonulosonic acid (KDN) levels in the afternoon urine samples (n 386, P= 0·064, Pearson's correlation) (y= 0·02x–0·56; R 2 0·011). (b) Modest correlation between total Sia intake levels at breakfast and urinary percentage of free N-acetylneuraminic acid (Neu5Ac) at noon (n 386, P= 0·033, Pearson's correlation) (y= 0·00x+0·14; R 2 0·016). (c) Slight correlation between total KDN intake levels in the morning or total Sia intake levels at lunch and urinary percentage of conjugated KDN in the afternoon urine samples (n 386, P= 0·024, Pearson's correlation) (y= 0·00x–0·02; R 2 0·016). Cr, creatinine; BW, body weight.
Discussion
The important finding of the present study is that the spot urine of 3–5-year-old children contains significant levels of Sia, predominantly as Neu5Ac and KDN, a finding that has been reported for the first time. No Neu5Gc was detected using a highly sensitive LC–MS/MS method( Reference Valianpour, Abeling and Duran 25 , Reference van der Ham, Prinsen and Huijmans 33 ). Previous studies have reported that urinary Sia levels were highest during the first month of life, decreased significantly during the first 2 years and gradually declined over the next several years( Reference Fang-Kircher 26 , Reference van der Ham, Prinsen and Huijmans 33 ). These earlier studies have reported a urinary level of total Sia of 63·7 mmol Sia/mol Cr in 3–5-year-old children. In the present study, the values obtained for 3- and 4-year-old children (40–47 and 52–53 mmol Sia/mol Cr, respectively) were slightly lower than those reported previously for children in the Netherlands, but similar to those reported for 5-year-old children (40–79 mmol Sia/mol Cr). Unfortunately, the Netherlands study did not report Sia intake levels for its group of children( Reference van der Ham, Prinsen and Huijmans 33 ). In the present study, in 3–5-year-old children, approximately 70·8 % of the urinary Sia was conjugated Neu5Ac, while approximately 21·3 % was free Neu5Ac. The conjugated and free forms of KDN accounted for more than 5 % of the total urinary Sia (Fig. 2), reflecting that human urine is a relatively rich source of sialylated (Neu5Ac) glycoconjugates. In the morning urine samples, the average proportion of total free Sia (Neu5Ac and KDN) was approximately 25·1 %. This level was lower than that reported previously, which was approximately 40–47 %( Reference Fang-Kircher 26 ). This difference is probably related to the use of the thiobarbituric acid method used in the earlier study( Reference Fang-Kircher 26 ) for the analysis of spot urine or 24 h urine samples for the same age group of children. The thiobarbituric acid method is considerably less sensitive for the quantification of urinary Sia levels than the LC–MS/MS method used in the present study. Thus, our new findings show that the proportion of free and conjugated Neu5Ac and KDN in the urine remained relatively constant throughout the day for 3–5-year-old children and the absolute level of total Sia or total Neu5Ac declined slightly in 5-year-old children during the day (Fig. 2).
Total urinary Sia levels varied greatly in children of the three age groups. The most varied level was of free Neu5Ac. It is known that in humans free Sia is rapidly excreted into the urine and is poorly reabsorbed by the tubules of the kidney( Reference Seppala, Renlund and Bernardini 28 ). Thus, free Neu5Ac in the urine can be a marker for Sia excretion from the kidney and may, in principle, approximate the metabolic rate of Sia intake. Urinary Sia is an important marker for the diagnosis of Sia metabolic diseases. Of the 386 children in the present study, four had total free urinary Sia levels that were 8- to 13-fold higher than the mean levels of the other participants. These four children had a normal proportion of urinary free/total Sia and did not show any clinical symptoms consistent with a Sia metabolic disease. They also had similar levels of Sia intake compared with the other children. Follow-up studies should be carried out for these children to better understand the mechanism underlying the elevated levels of urinary Sia in pre-school children. While the precise molecular basis for this variation remains unknown, it may be related to genetic and age differences in the metabolic and catabolic rates of dietary Sia and excretory function of the kidney.
There are no published reports on the level of urinary KDN excretion in children. While the biosynthetic pathways of Neu5Ac and KDN are well established, and distinctly different( Reference Varki, Schauer, Varki, Cummings, Esko, Freeze, Stanley, Bertozzi, Hart and Etzler 2 – Reference Varki 4 , Reference Wang 8 , Reference Go, Sato and Yin 39 ), there is less detailed information on how exogenous KDN may be taken up by mammalian cells. The present study has shown that the morning urine of healthy 3–5-year-old children contains significant levels of KDN, ranging from 3·14 to 5·70 mmol KDN/mol Cr. Interestingly, the concentrations of free and bound KDN remained similar throughout the day in each age group, but 5-year-old children showed slightly higher levels than 3- or 4-year-old children (P< 0·05). To our knowledge, this is the first report on the concentration and distribution of urinary KDN in pre-school children.
Neu5Gc is widely expressed in all mammalian species except in humans( Reference Schauer 1 – Reference Varki 4 , Reference Wang 8 , Reference Varki 20 ). However, mice and humans are able to ingest and absorb dietary Neu5Gc and excrete it into the urine( Reference Varki, Schauer, Varki, Cummings, Esko, Freeze, Stanley, Bertozzi, Hart and Etzler 2 , Reference Varki 23 ). Low levels of dietary Neu5Gc can be incorporated into newly synthesised glycoproteins in human tissues( Reference Tangvoranuntakul, Gagneux and Diaz 34 ). We detected an elevated level of Neu5Gc in only one of the 856 spot urine samples. It is possible that the children in the present study had lower Neu5Gc intake levels ( < 5 mg) than the previously reported intake level of 150 mg( Reference Tangvoranuntakul, Gagneux and Diaz 34 ). We do not know whether dietary Neu5Gc may more readily be incorporated into rapidly growing cells and tissues in developing children compared with adults. Exogenous Sia exhibits a different metabolic and catabolic rate depending on the age, method of administration and form in which Sia is present, i.e. whether free or conjugated( Reference Wang 8 ). Free Sia that is orally administered is rapidly absorbed from the intestine and 60–90 % is excreted in the urine without chemical modification within 30 min in mice( Reference Wang 8 , Reference Nohle and Schauer 40 , Reference Wang, Downing and Petocz 41 ). In contrast, the absorption of oligosaccharide-bound Sia or Sia glycoproteins is delayed by several hours compared with that of free Sia( Reference Wang, Downing and Petocz 41 ).
We analysed Sia concentrations in conventional foods of China. The results showed that the highest concentration of Sia in the foods examined was in Crucian eggs, followed by eggs, milk products, red meat, poultry and seafood (Table 2). Most of the dietary Sia in the present study was conjugated. The concentration and distribution of Sia in conventional Chinese foods were slightly different from those in Australian( Reference Zeng and Wang 42 ) and American( Reference Wang 8 ) foods, as shown in Table 5. Total Sia concentration in Chinese pork was similar to that in Australian pork, but lower than that in American pork; however, the absolute amounts of Neu5Gc were 9·6 and 65·1 % lower than those in both Australian and American pork, respectively. Also, Chinese ducks contained a 3-fold higher level of total Sia than American ducks. Moreover, red meat is the primary source of dietary Neu5Gc in adults in America( Reference Tangvoranuntakul, Gagneux and Diaz 34 ). In China, however, particularly for children, milk and dairy products are the major sources of Neu5Gc (Table 3). However, we only analysed three samples of skeletal muscle per animal species. Thus, the number of animals and specific areas of red meat in each species should be increased in future studies. The present results suggest that Sia concentrations in red meat and dairy products are different in different countries, as the nutrition intake levels from animal foods and environment are different. The knowledge of Sia content in conventional foods may help us to better understand possible medical disorders involving the uptake of the ‘non-human’ Neu5Gc from diets.
Table 5 Comparison of sialic acid (Sia) concentrations (μg/g wet tissue) and percentage of N-glycolylneuraminic acid (Neu5Gc) in red meat in three different countries

We also found that the mean intake levels of total Sia from 08.00 to 17.00 hours in 3-, 4- and 5-year-old kindergarten children were 2700, 2696 and 2602 μg/kg body weight, respectively (Table 4). Although 3-year-old children had relatively higher Sia intake levels at breakfast and lunch and on consuming afternoon dessert than 4- or 5-year-old children (Table 4), the differences between the age groups were not statistically significant (P>0·05). Importantly, there was no significant correlation between the levels of dietary Sia intake and the levels of urinary Sia in any age group at any time point. Thus, the rate of Sia excretion/metabolism depends on both the form of dietary Sia and its retention time in the gastrointestinal tract. Also, Sia utilisation between the three age groups may be different. For example, in 3-year-old children, the high intake levels of Sia did not correlate with the relatively high excretion of Sia in the urine compared with those in 5-year-old children (Table 3). We infer from these results that the more rapid brain and body development in younger children may require a higher level of dietary Sia for the synthesis of the key neural gangliosides and Sia glycoconjugates when compared with that in older children. However, the long-term impact of the uptake of Sia-rich (Neu5Gc) red meat in infants and pre-school children, and on later health and disease, remains to be determined. Neu5Gc can be metabolically incorporated into human cells and tissues after ingestion of a diet rich in red meat and/or milk products( Reference Varki 32 ). All humans have variable levels of circulating anti-Neu5Gc antibodies, thus raising potential concern about the effect of consuming a Neu5Gc-rich diet on inflammatory diseases( Reference Varki 23 , Reference Tangvoranuntakul, Gagneux and Diaz 34 ). Thus, our findings are of potential importance for future studies on the metabolism and utilisation of dietary Sia for neural development, disease prevention and diagnosis in pre-school children. The lack of correlation between dietary Sia intake levels and urinary Sia excretion levels in pre-school children has not been reported previously.
A potential limitation of the present study could be that we did not collect 24 h urine samples because of the difficulty of doing so for girls and 3-year-old children. However, it has been reported that morning spot urine samples correlate well with 24 h urine samples( Reference Fang-Kircher 26 ). Thus, our findings provide clinical evidence to better understand urinary Sia profiles in 3–5-year-old children and the lack of correlation between these profiles and dietary Sia intake levels. This lack of correlation may have clinical relevance because it may provide a means to identify pre-school-aged children with potential Sia metabolic disorders having elevated levels of urinary Sia, as four did in the present study. Accordingly, these new findings provide a strong rationale for monitoring urinary Sia levels in pre-school children.
Acknowledgements
The authors thank the mothers and children who participated in the present study and the kindergarten staff of Affiliated Kindergarten of Xiamen University. They also thank Dr Hongwei Li and Haijiao Chen for helping with urine sample collection. The present study was supported by a research grant from the School of Medicine, Xiamen University. It was also supported by a start-up research fund from the School of Medicine, Xiamen University. The School of Medicine, Xiamen University, had no role in the design, analysis or writing of this article.
The authors' contributions are as follows: Y. C. was involved in the data collection of urine samples, urinary Sia analysis, statistical analysis and manuscript drafting; L. P. was responsible for data collection for food Sia analysis; N. L. was involved in data collection for urinary Cr analysis; F. A. T. was responsible for the interpretation of the results and final writing and approval of the manuscript; B. W. was involved in the conceptualisation and design of the study, analysis and interpretation of the results, statistical analysis, final writing of the manuscript, and approval of the final manuscript.
None of the authors has any conflict of interest with respect to the study.










