In the present-day climate, concern surrounding radiological and nuclear incidents has increased. Despite being low probability events, they are very high risk. Reference Arora, Chawla and Marwah1 Highlighted by recent events such as the Fukushima nuclear disaster, there is an increased urgency in the disaster community to develop measures to limit or prevent the impact of such incidents on the public. Reference Borrie and Tim2 Preparedness remains the key to reducing morbidity and mortality. While safety and security measures are in place to prevent or mitigate such events, accidents secondary to failure or intentional attacks remain a threat. When such a radiological or nuclear event occurs, emergency medical personnel must be trained to care for patients injured or contaminated by radioactive material. Reference Bullock, Haddow and Coppola3
The medical aftermath of such events includes acute radiation syndrome (ARS) along with multiple trauma and thermal or radiation burns. Among survivors of the initial event, continued exposure to radiation poses a prolonged threat of cutaneous radiation injury (CRI). Reference DiCarlo, Bandremer and Hollingsworth4 Events that cause ARS or CRI are both unpredictable and potentially devastating with the ability to quickly generate large numbers of patients requiring treatment. However, there are currently no medical countermeasures specifically approved by the US Food and Drug Administration for the management of ARS or CRI due to the ethical inability to perform human research trials. Reference Szumacher, Wighton and Franssen5 Current literature proposes radiation dermatitis (RD) as a research model for the study of ARS/ CRI, despite a difference in exposure conditions due to identical biological pathophysiology. Reference DiCarlo, Bandremer and Hollingsworth4
RD occurs in 95% of patients receiving radiation therapy (RT) for cancer treatment, affecting 800 million patients annually. Reference Singh, Alavi, Wong and Akita6 Tumor sites most related to RD include the breast, brain, head and neck, soft tissue, perineum, and anal canal. Increased incidence in these cancer patient populations is due to a higher total prescribed radiation dose for treatment. Reference Bensadoun, Humbert and Krutman7 Acute RD occurs within 90 days of treatment initiation, occurring gradually. Physical manifestations of RD include erythema, edema, pigment changes, hair loss, dry desquamation, and moist desquamation. Reference Bray, Simmons, Wolfson and Nouri8 Skin changes may also present with patient-experienced symptoms, which are often underreported, including pain, sensitivity, numbness, itching, and burning, which may also be associated with fatigue, sleep disturbances, changes in body image, and emotional distress. Reference Schnur, Love and Scheckner9 Despite the prevalence and impact of RD on patient quality of life, documentation of RD onset and progression is inconsistent or lacking.
Based on current literature, it is believed that research on RD is impeded by the absence of an unambiguous scoring scale to evaluate skin reactions. Reference Schnur, Love and Scheckner9 While several well-accepted grading scales exist for assessing RD, with the most common the Radiation Therapy Oncology Group (RTOG) and Common Toxicity Criteria for Adverse Events (CTCAE), they are observer-rated methods vulnerable to error. Studies show that providers tend to underreport and underestimate the incidence and severity of treatment-emergent adverse events, including RD. In a cohort study of over 10 000 patients with breast cancer, more than half of the patients experienced at least 1 significant symptom that was under-recognized by physicians utilizing the CTCAE assessment. Reference Jagsi, Griffith and Vicini10 Interobserver agreement in skin reaction assessments is also variable, ranging from 65% to 97%. Reference Delaney, Fisher, Hook and Barton11
To better quantify the characteristics of RD, including but not limited to appearance and timing of symptoms, use of scoring systems, and guidelines for treatment, we developed the Radiation Induced Skin Reactions (RISREAC) cohort by examining the clinical documentation and management of RD in breast cancer patients who received RT. Radiation-induced skin reactions continue to be a problem during cancer treatment, as well as during unpredictable radiological or nuclear events. Documentation of RD in the electronic medical record could be an untapped resource of standard care information and understanding longitudinal progression necessary to improve severity assessments and management of RD and CRI in the clinic and in the field. This is the first study to attempt the establishment of a retrospective historical control cohort for radiation dermatitis using clinical documentation.
Methods
Study Design and Patient Population
The RISREAC historical control cohort includes 245 breast cancer patients who received radiation therapy from January 2017 through December 2021 at the University of Rochester Medical Center (URMC). Eligibility criteria for the cohort were: adult females ages ≥ 22 years, with a diagnosis of breast cancer and prescribed conventional fractionation RT (ie, 1.8–2.0 Gy in 25–40 fractions, with or without boost) or short-course fractionation RT (ie, 2.0–3.0 Gy in 15–20 fractions, with or without boost). The total prescribed radiation dose (whole breast +/- boost) ranged from 34.0 Gy to 66.0 Gy in 15 to 40 fractions. We excluded any patients who were deceased at the time of chart review. This study was reviewed and exempted by the University of Rochester Research Subjects Review Board (RSRB, STUDY00004868).
Retrospective Review of Skin Reactions
The retrospective chart review was performed in EPIC electronic medical record (EMR) and involved the collection of demographic information, radiation treatment information, and radiation-skin reactions. Weekly clinician chart notes during RT were reviewed for “documentation of skin reactions” for each patient starting the first week of RT (ie, Week 1 of RT) and ended with the first follow-up visit after completion of RT (ie, Post-RT). The first Post-RT visit may not have been performed by the treating radiation oncologist. The chart notes were screened for the following descriptors and documentation related to RD: erythema, desquamation (dry or moist), flaking, itching, ulceration, weeping, scabbing/patchy crusting, grade (if documented), location of skin reaction (if documented), and treatments provided for skin reaction (eg, topical corticosteroid, silver sulfadiazine). Nurse clinic notes were also reviewed to ensure capture of any skin treatments provided during RT. All data were entered into a data collection sheet in REDCap.
Descriptor-Based Severity Score
Although clinicians did not always report “grade” of RD, the providers always described the skin reactions in a consistent manner. We generated descriptor-based severity and erythema scores, using this clinical documentation. We referenced the RTOG scale and Radiation Induced Skin Reaction Assessment Scale (RISRAS)—erythema scale to assign descriptor-based severity scores based on erythema and desquamation descriptors. Erythema descriptors were scored using a 5-point scale: 0 = No erythema; 1 = Faint erythema/pink; 2 = Hyperpigmentation/erythema present and noted; 3 = Definite redness/bright red/confluent red; and 4 = Deep red/deep purple. Unlike RTOG or RISRAS, hyperpigmentation was included in descriptor-based scoring because hyperpigmentation is a melanocytic response to radiation in darkly pigmented skin that is often noticeable before erythema. 12,Reference Healy13 Desquamation descriptors were scored: 2 = Dry desquamation and 3 = Moist desquamation. Dry desquamation is characterized as peeling of dry, scaly skin, which may or may not produce symptoms in patients. Moist desquamation, however, is typically painful and results from sloughing of the epidermis, as a result of a weakened epithelial barrier. Reference Kole, Kole and Moran14
The descriptor-based severity score was the highest score between erythema descriptors and desquamation descriptors and ranged from 0 to 4, similar to the RTOG scale. The descriptor-based erythema score was solely based on erythema descriptors and ranged from 0 to 4, similar to the RISRAS-erythema scale.
Statistical Analyses
This retrospective study is primarily descriptive. All statistical tests (Analysis of Variance [ANOVA] and Pearson’s correlation) were performed at 0.05 level of significance in JMP 16 Pro.
Results
Patient Characteristics
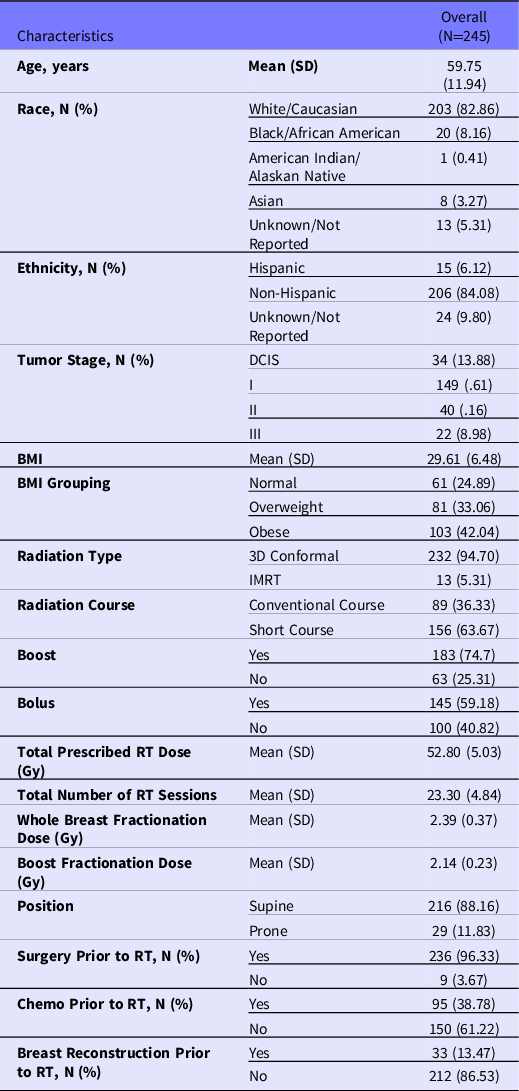
The baseline characteristics of the historical cohort (N = 245) are provided in Table 1. The majority of patients were white (83%), obese (42%), with a mean age of 60 years, and the most common tumor stage at the start of RT was non-invasive ductal carcinoma in situ (DCIS) (14%) followed closely by stage 3 breast cancer (10%).
Table 1. Patient characteristics
Documentation of Radiation Dermatitis
Radiation dermatitis grade was documented for 169 (68.9%) patients (Table 2). Although the grading scale was not specified, clinicians referred to RTOG or the CTCAE scale, which are the most commonly used scales using similar grading. The trajectory of RD severity showed the worst documented severity at the end of RT (Figure 1), with the majority of patients having Grade 1 or Grade 2 RD (see Table 2). The mean severity at the end of RT was 1.57 [1.46, 1.68]. Post-RT assessments were completed on 240/245 (98%) patients with a mean of 110 days between the end of RT and the first post-RT clinic note discussing RT treatment. Five patients did not have any skin evaluation documented in chart notes after completion of RT. Of the 240 patients, 39 (16.3%) patients had a documented RD grade by the clinician with Grade 1 being the most commonly reported (see Table 2).
Table 2. Documented radiation dermatitis grade at End RT and Post-RT
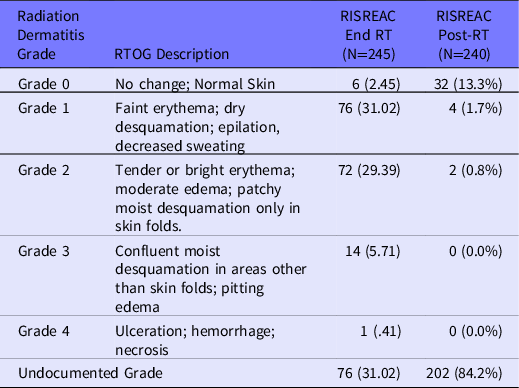
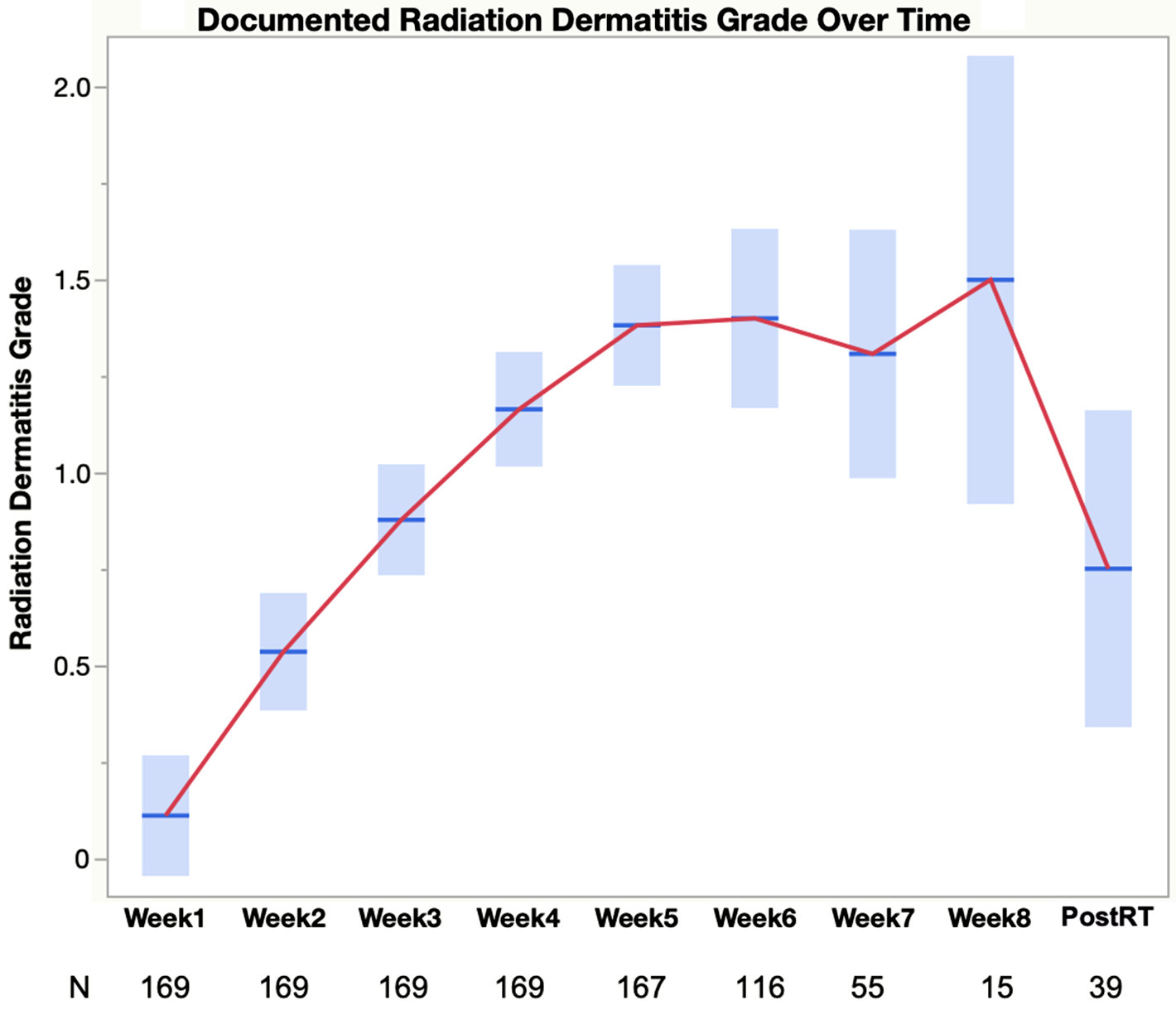
Figure 1. Trajectory of radiation dermatitis based on clinical documentation of grade. The graphical plot shows mean documented radiation dermatitis grade and 95% confidence interval for each week during radiation therapy for the 169 patients with clinical documentation of radiation dermatitis grade. Due to varying prescribed radiation therapy regimens, the number of patients at each week varies across some weeks. Therefore, N is included at each week in the plot.
Descriptor-Based Skin Severity Scores
Although clinician-documented RD could not be found on all patients during the retrospective chart review, a clinical description of the radiation-induced skin changes was found on all patients during RT and 98% of patients post-RT. Due to the consistency of descriptions in the chart notes, we utilized a descriptor-based scoring to generate an RD severity score and erythema score for the end of RT on all patients (Table 3). The overall mean descriptor-based skin severity score was 2.22 [2.10, 2.34], and the mean erythema score was 2.16 [2.04, 2.28]. For patients with clinician documented RD grade (n = 169), the descriptor-based severity scores moderately correlated (r = 0.532, P < 0.0001) with and were higher than documented RD grade (Figure 2). The descriptor-based scores were significantly higher than the documented RD grade for 2 main reasons: (1) the inclusion of hyperpigmentation in scoring and (2) the presence of “Definite redness/bright red/confluent red” and/or moist desquamation resulted in a score of 3. Clinicians documented “hyperpigmentation” but reported RD grade as “Grade 0/Normal.” Among the 87 patients who had a descriptor-based score of 3, 46 (52.9%) had erythema of 3 without dry or moist desquamation, 10 (11.5%) had erythema of 3 with moist desquamation, and 9 (10.3%) had erythema of 3 with both dry and moist desquamation, 3 (3.4%) had erythema of 2 with moist desquamation, and 1 (1.1%) had erythema of 1 with moist desquamation.
Table 3. Descriptors used for descriptor-based severity and erythema at end of RT
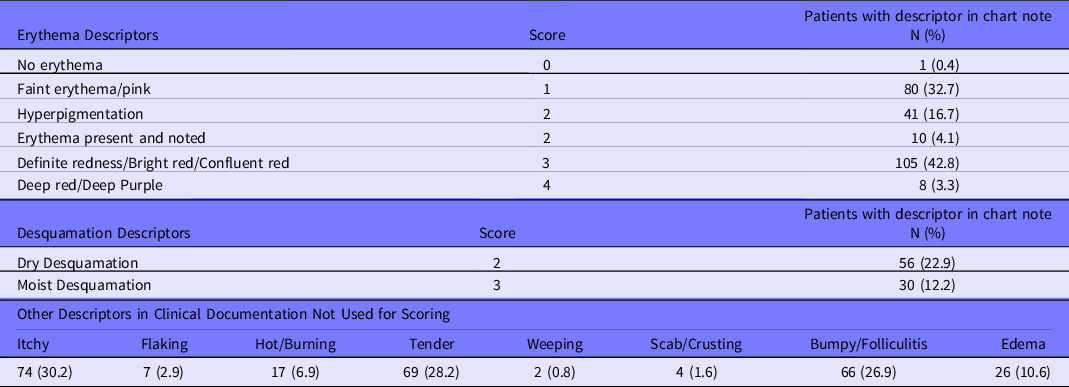

Figure 2. Mean descriptor-based severity in patients with grade in clinical documentation. The boxplots compare descriptor-based severity and clinician-documented severity for radiation dermatitis for the 169 patients with clinical documentation of radiation dermatitis grade. Descriptor-based severity scores were higher than documented severity (P < 0.0001).
Skin Care Treatments
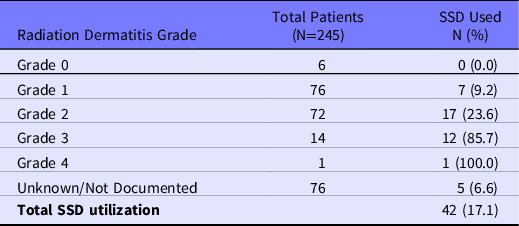
Most patients (225/245, 91.8%) received some type of skin care treatment during their prescribed course of RT, with 66.7% (150/225) receiving 2 or more skin care modalities. The most commonly provided topical treatments were RadiaPlex, Hydrocortisone, and Aquaphor (Figure 3). Skin treatments were most frequently provided during Week 1 (71.1%), Week 2 (7.6%), and Week 3 (8.9%) of RT. Although silver sulfadiazine (ie, SSD) is often considered a standard treatment comparator in clinical trials, our RISREAC cohort shows SSD was used in only 17.1% of patients and primarily for higher grade skin reactions (Table 4).
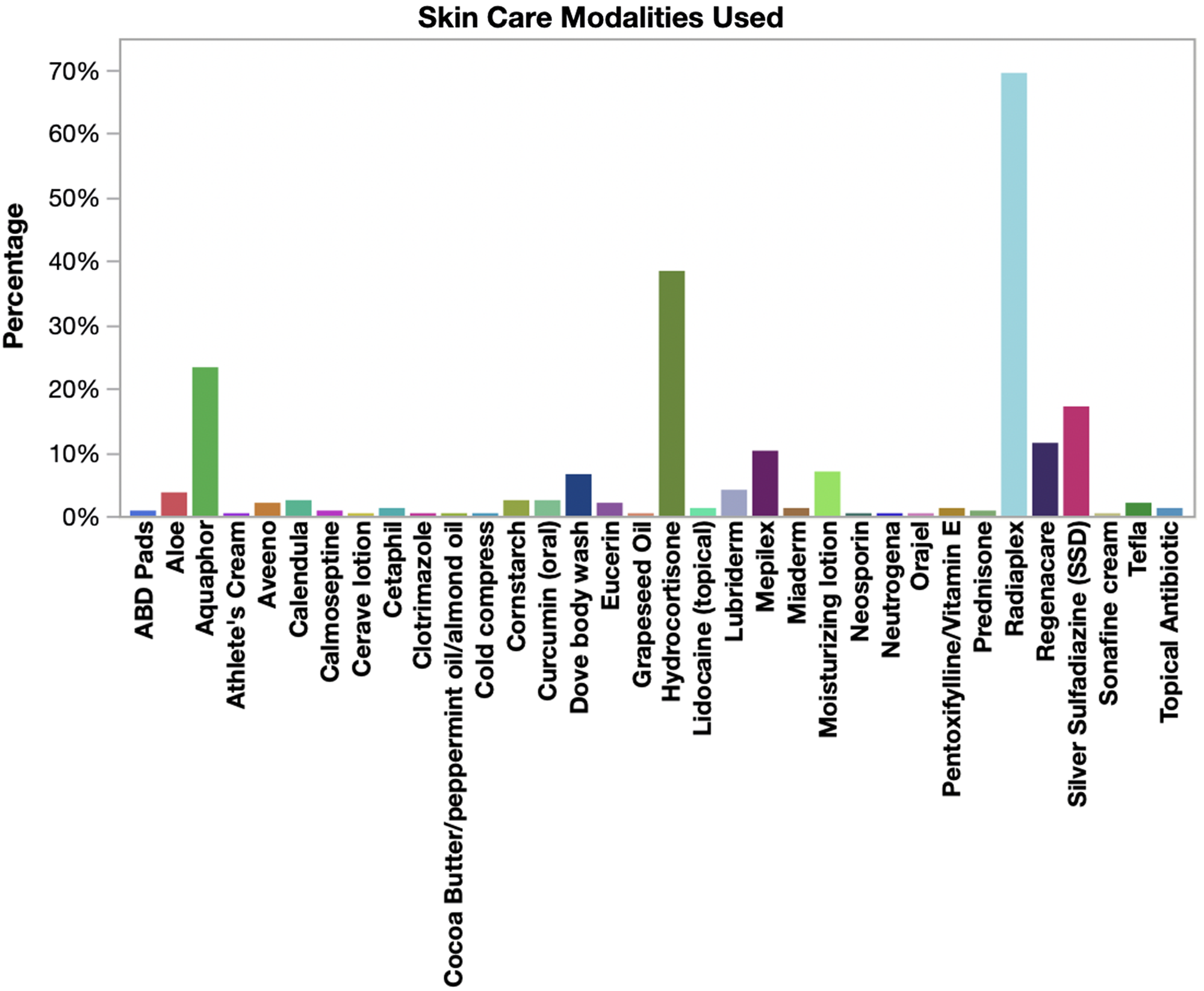
Figure 3. Skin care modalities utilized during radiation therapy. The bar chart shows the percentage of patients (N = 245) who used the various skin modalities reported in clinical documentation during radiation therapy. Most patients used more than 1 skin treatment.
Table 4. Silver Sulfadiazine (SSD) utilization
Limitations
The limitations of this study include a single-institution study, limited sample diversity, and a maximum dose range for patients receiving radiation therapy. The generalizability of this study is limited due to it is a single institution representation. A collaborative approach across multiple institutions to document RD would increase understanding of CRI progression and the ability to develop comprehensive countermeasures. Additionally, there may be variations in the documentation and description of radiation-induced skin reactions across institutions, which could only be evaluated in a future multi-institutional study. Although our cohort reflects the patient population receiving care at URMC in radiation oncology (83% white), additional diversity in sampling would further improve the generalizability and inclusivity of the study results. This retrospective cohort could be expanded to include other cancer patient populations at risk for RD (ie, head and neck cancer) and inclusion of all 6 Fitzpatrick skin types to improve its representation across all patients. The limited maximum total prescribed radiation dose range allowed us to focus on the documentation of radiation-induced skin changes without the inclusion of severe skin reactions. This cohort should expand the study cohort to include breast cancer patients with greater than 66 Gy total prescribed doses, as well as other cancer patient populations at high risk of radiation-induced skin reactions (ie, head and neck, lung, colorectal, soft tissue cancer, etc). This study demonstrated the ability to establish a historical control cohort using retrospective data in the EMR and improve the generalizability of the cohort through the expansion and inclusion of additional patients.
Discussion
This study retrospectively evaluated 245 patients with breast cancer undergoing RT for the clinical documentation of radiation-induced skin reactions for a better understanding of RD progression, severity, grading, and treatment. Individuals receiving RT for cancer or other medical disorders can provide insight into radiation effects on the human body and valuable information for medical countermeasures to unpredictable radiological events. Although CRI events usually involve a single high dose radiation exposure, unlike the fractionated exposure during RT, the clinical presentation of the skin reactions is similar. Reference DiCarlo, Bandremer and Hollingsworth4 Therefore, information from RISREAC cohort could potentially serve as a control cohort in radiation dermatitis trials and aid in countermeasures development for CRI in the field.
The peak RD assigned to our RISREAC cohort was at the end-RT point. Comparatively, a prospective study by Drost et al. consisting of 148 patients with breast cancer received an average of 50 Gy total prescribed radiation dose over 25 fractions and showed peak RD 2 weeks post-RT. Reference Drost, Li and Vesprini15 The observed difference in RD grade between the 2 cohorts is most likely due to the difference in RD follow-up. While RD grade was documented weekly during RT for patients in our RISREAC cohort, documentation became inconsistent following the end-RT point. Further, there are currently no definite guidelines for when or how often patients should be evaluated for RD during RT. For example, the first skin documentation post-RT in the RISREAC cohort occurred after an average of 110 days or 15 weeks with 84% of patients without a documented RD grade post-RT. Additionally, a randomized clinical trial of RT in head-and-neck cancer patients, similarly, found considerable variation in the time points used to report toxicity. Reference Denis, Garaud and Bardet16 Our findings, nonetheless, correlate with current research regarding RD severity and underscore the importance of documentation of radiation-induced skin changes after completion of RT.
Despite the irregularity in RD grade documentation, providers demonstrated awareness of RD indications. A chart review of the cohort revealed universal documentation of symptom descriptors by providers to describe RD skin injury. Descriptors were reported for erythema/ pigmentation, dry desquamation, and moist desquamation (see Table 3). These descriptors were then used to assign a severity score and erythema score to assign an RD grade to patients. Comparatively, the descriptor-based severity scores for the RISREAC cohort were significantly higher (mean = 2.22) compared to those of the 169 RISREAC patients with clinician documented RD grades (mean = 1.57). Although there are many possibilities for this observed difference in RD severity, the difference is most likely due to the lack of accounting for hyperpigmentation in clinical reporting of RD grade (ie, RTOG/CTCAE score). Reference Hymes, Strom and Fife17–Reference Milam, Rangel and Pomeranz19 Clinicians reported Grade 0/Normal even if they documented hyperpigmentation. The under-recognition of hyperpigmentation as a symptom of RD impacts RD management in skin of color. These findings are supported by Jagsi et al., Reference Jagsi, Griffith and Vicini10 reporting that in a study of 5510 patients underreporting of at least 1 symptom of RD was associated with being of black or non-white ethnicity in 53% of cases. Reference Jagsi, Griffith and Vicini10 The significance of these findings is magnified in the context of current understanding that black patients experience worse RD severity. Reference Wright, Takita and Reis20 Our descriptor-based scoring accounts for the documented radiation-induced skin changes in a standardized manner. Future research can explore the utilization of computational algorithms to improve our descriptor-based scoring by the inclusion of additional symptoms and descriptors, such as pruritus/itch, folliculitis, and edema. Overall, our results further support the need for objective and accurate RD severity scoring for all skin types.
Machine learning (ML) is a valuable instrument that may prove beneficial to standardizing RD management. ML is already implemented in dermatology to assist in disease classification. VisualDx DermExpert is a commonly used smartphone-based ML app that assists clinicians in a primary care setting to identify skin lesion morphology and build a differential diagnosis for review. Reference Dulmage, Tegtmeyer and Zhang21 Although ML is currently not utilized for radiation skin injury classification, the dose-dependent clinical presentation of radiation skin injury makes it an ideal candidate. A study by Ranjan et al. Reference Ranjan, Partl and Erhart22 supports this position. Reference Ranjan, Partl and Erhart22 The study finds that AI-based algorithms can act as a pre-screening and decision support tool to provide an efficient assessment of erythema grading in radiation-skin injury. The employed algorithm in the Ranjan et al. Reference Ranjan, Partl and Erhart22 study obtained an accuracy of 73%, 66%, and 82% for estimating the severity grade of each class, respectively. The CTCAE grades were predicted by trained algorithms through visual inspection of radiation skin injury images.
We recommend that future studies assess the effectiveness of an automatic scoring system utilizing image inspection algorithms and symptom descriptors. Our results demonstrate the feasibility of descriptor-based scoring, as we observed clinicians more frequently document symptom descriptors rather than a grade for radiation-skin injury. However, despite the potential of ML in this field, there are challenges to implementation. A systemic review of current approaches of ML in dermatology notes, the development of an effective AI-based algorithm is highly dependent on the data available. Reference Jeong, Park, Henao and Kheterpal23 If there is a large supply of high-quality image data sets, the probability of the algorithm generating more accurate predictions increases. While public image data sets are available, lack of diversity in the data, quality control of images, and patient information present as barriers to generalizability. Reference Jeong, Park, Henao and Kheterpal23 Validation of AI-based algorithms for grading RD severity in the settings in which it would be implemented is needed. However, if proven effective, there is potential to eliminate the interobserver variability prone to a purely visual inspection.
Such implementation may allow for standardization of CRI diagnosis and quick identification of patients who require intensive local skin care, especially in remote areas where expertise may not be readily available. Though the demand for objective scoring of radiation-skin injury is high, supported by an increasing movement toward digitation, new methods must be built on internationally established standards.
In all, the RISREAC cohort provides important insight into the topical agents prescribed for RD. As illustrated in Figure 3, providers rely on a multitude of different skin care modalities. Without a standardized treatment algorithm to rely on and limited research on effective RD treatment modalities, clinicians must rely on their knowledge of treating skin diseases with similar presentation to RD to guide treatment. The most commonly provided topical treatments were RadiaPlex (ie, hyaluronate cream), Hydrocortisone, and Aquaphor (ie, petroleum-based ointment). Topical corticosteroids are the only proven effective treatment modality for RD. Reference Rosenthal, Israilevich and Moy24 Several randomized trials and 1 meta-analysis indicate that regular use of corticosteroids during RT successfully reduces the occurrence of severe RD. Reference Chan, Webster and Chung25–Reference Miller, Schwartz and Sloan29 Some studies have demonstrated benefits from hyaluronate or petroleum-based treatments; however, the evidence is limited and at times conflicting, thus requiring additional confirmatory studies regarding their efficacy. Reference Rosenthal, Israilevich and Moy24,Reference Liguori, Guillemin and Pesce30,Reference Pinnix, Perkins and Strom31 Silver sulfadiazine (ie, SDD) is another skin care modality for RD traditionally recommended for burn injuries for prevention of wound infection. Reference Hemati, Asnaashari and Sarvizadeh32 Despite some published studies reporting a decrease of RD severity with SSD, our RISREAC cohort shows that SDD use is nonetheless limited. Only 17% of the cohort was treated with SSD, and of these the majority had skin reactions of Grade 2 or higher. By sharing insight on the most prescribed agents, we aim to provide guidance for future research on uniform and efficient RD management.
Conclusions
The RISREAC cohort is the first historical control cohort established from clinical documentation of radiation-induced skin changes for the study of RD and CRI. Ultimately, we seek to grow the RISREAC cohort by increasing the diversity of patients and severity of radiation injury across a spectrum of patients with RD and/or CRI. Our results support the need for consensus on and standardization of radiation dermatitis treatment and severity scoring to improve RD and CRI management. Our study revealed that radiation dermatitis symptom descriptors were more reliably documented than RTOG or CTCAE grades and accounted for hyperpigmentation, unlike RTOG and CTCAE. Our findings, thus, reveal the potential for a more sensitive and objective severity scoring scale through the incorporation of symptom descriptors and ML. We aim to employ descriptor-based scoring with the incorporation of computational algorithms, such as potentially natural language processing and ML, to increase inclusion of descriptors and scoring accuracy. A new descriptor-based ML scoring tool would be useful in both the clinic and the field for medical countermeasures for CRI.
Author contributions
AG contributed to the data collection, writing, preparation, and revising of the manuscript. YX contributed to data collection, data analyses, results interpretation, and revising of the manuscript. PA contributed to the study design, results interpretation, and revising of the manuscript. JRW contributed to the study design, data collection, data analyses, results interpretation, writing, preparation, and revising of the manuscript.
Funding statement
This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under Contract No. HHSO100201800022C.









