INTRODUCTION
Acute gastrointestinal illness (AGI) is an important cause of morbidity and mortality worldwide. In developed countries AGI is generally of low severity, but affects a high number of people [Reference Scallan1, Reference Scallan2]. These diseases may therefore still represent a considerable disease burden and cost to society through medical expenses and lost days of work. Only a minority of these infections are diagnosed and incidence has to be estimated through scientific studies. Measuring the disease burden within the population is of importance for the understanding of disease dynamics, for estimation of the foodborne disease burden and, ultimately, for preventive strategies. Within the last 10–15 years cross-sectional telephone surveys to quantify AGI have been conducted in a number of countries [Reference Cantwell3–Reference Gauci13], and a common understanding for the methodology has begun to develop internationally [Reference Flint14, Reference Majowicz15].
A study of the incidence of gastrointestinal illness has not previously been performed in Denmark, but was undertaken here. The objective of the present study was to estimate the burden of self-reported AGI in Denmark.
METHODS
Study design and data collection
The study was initiated through a European collaborative burden-of-illness project which was performed as part of the Med-Vet-Net network of excellence [Reference Belcher and Newell16]. The data collection was performed during the 1-year period from 1 January to 31 December 2009. Interviews were performed using a structured questionnaire stored as a Microsoft Access entry form. The questionnaire and data entry system was tested in a pilot period preceding the study period. Interviews were performed in-house by trained interviewers, consisting of a group of nine students working in afternoons and weekends. A sample size of 1500 interviews was calculated to detect a period prevalence of 10% in a 4-week recall period with 2% allowable error and 95% confidence (calculation done as part of the Med-Vet-Net collaboration). A target sample size of 1800, corresponding to 150 interviews per month, was chosen.
The Danish population consisted of 5·52 million people on 1 July 2009 [17]. Complete information on age, gender and addresses is available for all Danish residents through the Danish Civil Registration System (CPR, Central Person Registry, a complete population database) [Reference Pedersen18]. Using this system, the proportion of Danes alive within the two genders and within eight age groups (0–9, 10–19, …, ⩾70 years) was calculated. Based on this the number of interviews needed within each of these 16 age and gender groups was calculated, summing up to 1800 interviews in total. A total of 10 000 Danish residents were then randomly selected from the Civil Registration System with proportional representation according to the five major Danish administrative geographical areas (‘regions’); these potential study participants were distributed according to age and gender groups. This process of selecting potential study participants was repeated halfway through the study period. Name and address as listed in the Civil Registration System (for children, the parents' addresses were also looked up) were used to find the participant's household phone number and/or mobile number in the web-based national phonebooks. If no phone number could be located, the person was excluded.
Seasonal distribution in the occurrence of gastroenteritis was taken into account, by performing equal numbers of interviews each calendar month. Landline phone numbers were tried first. If there was no response or if a landline number was not available, mobile phone numbers were tried. If persons could not be reached after three attempts on different days, further attempts to contact this individual were not made. For children aged ⩽15 years, a parent or guardian was asked to answer the questions. For individuals aged 16–17 years verbal consent was sought from a parent or guardian before interviewing the adolescent. Interviews were not performed if the study subject was unable to speak Danish.
The questionnaire collected information on occurrence of episodes of gastroenteritis within different time periods. The participants were first asked if they had had either diarrhoea or vomiting on the day before the interview. Upon negative answers, the same questions were repeated for the period of the past week and, upon a further negative response, the preceding 4-week period. Diarrhoea was defined as ⩾3 loose stools on the same day. Each participant could only be counted as a case once. Participants that reported symptoms were asked about further symptoms to diarrhoea and vomiting and in addition asked about the date of onset and the duration of symptoms. Furthermore, they were asked about background illness and medication, including chronic gastrointestinal illness, previous abdominal surgery, pregnancy and other potential competing causes of gastrointestinal symptoms such as usage of drugs and alcohol. In addition, information was collected on demographic characteristics, medical care, days lost from work and on different exposures such as perceived drinking-water quality (unusual taste/odour/colouring/clarity or reduced pressure) in the 2 weeks prior to interview/disease onset and broad categories of food products consumed (chicken, beef, pork, fish, vegetables) in the 7 days prior to interview/disease onset. The core of the questionnaire was developed within the project framework of the Med-Vet-Net work package 23 on prioritization of foodborne infections [Reference Mangen19] and based on the questionnaire used in the English IID2 study [Reference O'Brien20]. Clearance for the study was obtained by the Research Ethics Committee in Denmark.
Case definition
We employed the case definition proposed by the International Collaboration on Enteric Disease ‘Burden of Illness’ Studies [Reference Majowicz15], which states that a case of AGI is defined as ‘an individual with ⩾3 loose stools, or any vomiting, in 24 hours, but excluding those (a) with cancer of the bowel, irritable bowel syndrome, Crohn's disease, ulcerative colitis, cystic fibrosis, celiac disease, or another chronic illness with symptoms of diarrhoea or vomiting, or (b) who report their symptoms were due to drugs, alcohol, or pregnancy’.
Data analysis
The primary outcome measure of incidence rate was the proportion of cases calculated as an annual rate. The 95% confidence interval (CI) for the incidences was estimated for the Poisson distribution as:
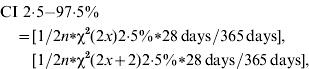
where n=number of persons included in the study, and x=number of cases.
Symptomatic persons that did not meet the case definition, due to underlying illness or other conditions, were not included in the denominator as they were not, by definition, eligible to be cases and therefore not part of the at-risk study population. For the calculation of the 4- and 1-week incidence rates, only cases with first onset of symptoms within the 4- or 1-week periods were included (to measure incidence rather than prevalence); however, cases that could not state the date of onset were all kept in the case group. Slightly more interviews than pre-determined were performed for some gender and age groups, and therefore the overall incidence measure was presented as a standardized incidence rate taking this into account. According to the proposed standardization criteria for burden-of-illness surveys, the main reported incidence rate measures were based on the 4-week period responses. The degree of urbanization of the study participants was examined by assigning an urbanization code to each participant's residential address, as previously described [Reference Ethelberg21]. Briefly, this system classifies the study subjects into five population density levels, ranging from very rural to inner-city areas, according to the total number of persons living in a 1 km2 area surrounding the address.
Data were analysed using the software packages Stata 10 (StataCorp, USA) and SAS v. 9.13 (SAS Institute, USA).
RESULTS
Table 1 shows the number of attempted and successful interviews by age and gender group. Contact was attempted with 3372 persons, 2421 of which answered the telephone call resulting in 1949 interviews being performed. This gives a participation rate of 80·5% for persons that answered the phone, Unsuccessful attempts to include persons in the study were more often a result of difficulties in reaching them (67%) than refusal to participate (31%). Of the 1949 persons with completed interviews, 1853 were included in the study.
Table 1. Study population, number of interviews completed and attempted by age and gender groups, Denmark 2009
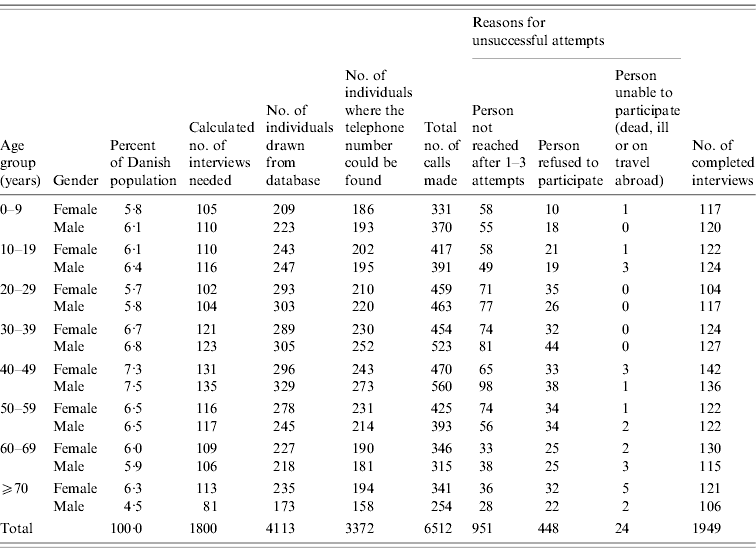
Whether participants were reached by a landline or by a mobile phone number could be assessed for 1385 participants after the data collection had been completed (this information was not generally recorded as part of the study). Of these, 54% had been contacted by a landline phone and 46% by a mobile phone. There was a clear difference by age, as 20–29 and 30–39 years age groups were more often interviewed by mobile phone (90% and 69%, respectively), whereas all other age groups were more often reached by landline numbers.
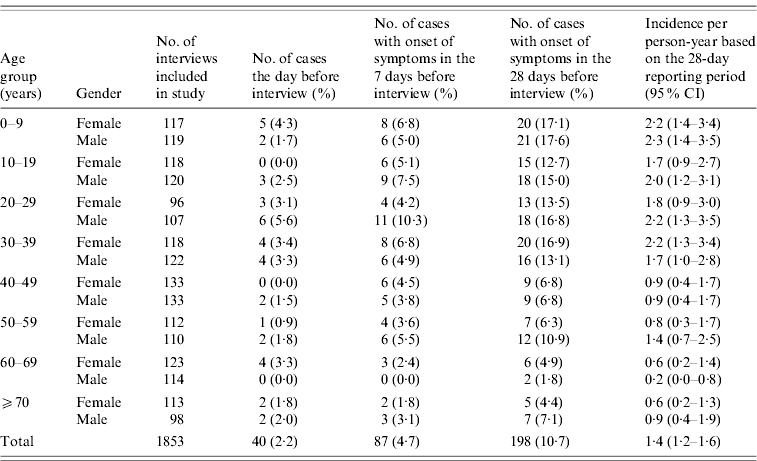
When asked about illness within a 4-week period, a total of 248 (13·4%) persons reported suffering from diarrhoea, vomiting or both symptoms within the 4-week period. Of these 206 (11·1%) met the case definition and 198 (10·7%) of these had onset of symptoms within the 4-week period (Table 2). Adjusting for the fact that the number of interviewees in each gender age group was slightly different from the calculated number, the 10·7% of cases corresponds to an overall standardized incidence rate of 1·4 (95% CI 1·2–1·6) episodes of AGI per person-year.
Table 2. Cases of acute gastrointestinal illness by age group and gender, Denmark 2009
Gender, age, symptoms, seasonality, urbanization and potential risk factors
The overall gender difference was small; 95 (48%) cases were female [relative risk (RR) 1·0, 95% CI 0·91–1·2]. However, there was a clear effect of age; the adjusted incidence rate was generally higher in the younger age groups being 2·3 (95% CI 1·6–3·1) per person-year in the 0–9 years group, 1·9 (95% CI 1·5–2·3) per person-year in the 10–39 years group, but 0·81 (95% CI 0·6–1·0) per person-year in those aged ⩾40 years. When dividing the younger age group into two groups consisting of those aged <5 years and those aged 5–9 years, the corresponding incidence rate was 2·75 (95% CI 1·7–4·1) for the former and 1·85 (95% CI 1·1–2·9) for the latter.
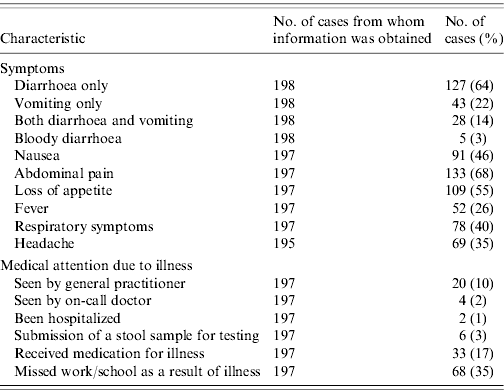
Among the 198 cases, 127 (64%) reported suffering from diarrhoea, 43 (22%) suffering from vomiting and 28 (14%) cases reported both symptoms. Abdominal cramps, loss of appetite or nausea were frequently reported (Table 3), whereas bloody diarrhoea was only reported by 3% of cases. Respiratory symptoms, not generally associated with intestinal disease, were reported by 40% of cases. The proportion of cases reporting concomitant respiratory symptoms was higher in the younger age groups, being 50% in both the children and teenage groups. The majority of cases had not been in contact with the medical system; 24 (12%) cases were seen by a physician as a result of the illness and only two (1%) cases were hospitalized. Six (3%) cases reported having had a stool sample submitted for microbiological analyses. However, after completion of the data collection we only succeeded in finding two stool sample analysis results in laboratory databases; both were negative for pathogenic bacteria. Among the 146 cases who reported diarrhoea within the 4-week period and who did not report being ill at the time of interview, the average duration of illness was 3·0 days (range 1–30). The similar figure for vomiting, calculated in 66 cases was 1·8 days (range 1–14). Among all cases, 68 (35%) reported having missed work or school as a result of their illness.
Table 3. Self-reported symptoms and use of medical system by cases with acute gastrointestinal illness over a 4-week period, Denmark 2009
The distribution of cases according to month of the year is shown by symptom in Figure 1. Illness was more prominent in the winter months and less prominent during the spring months. The distribution of study participants according to the rural–urban classification scheme was not markedly different from that of the Danish population, although there were relatively fewer participants in the two extreme categories, i.e. the least and most populated levels. The distribution of cases was not different from that of non-cases, except for the most rural level where there were fewer cases (3·4% vs. 6·7%) although this difference was not significant (P=0·06, χ2 test).
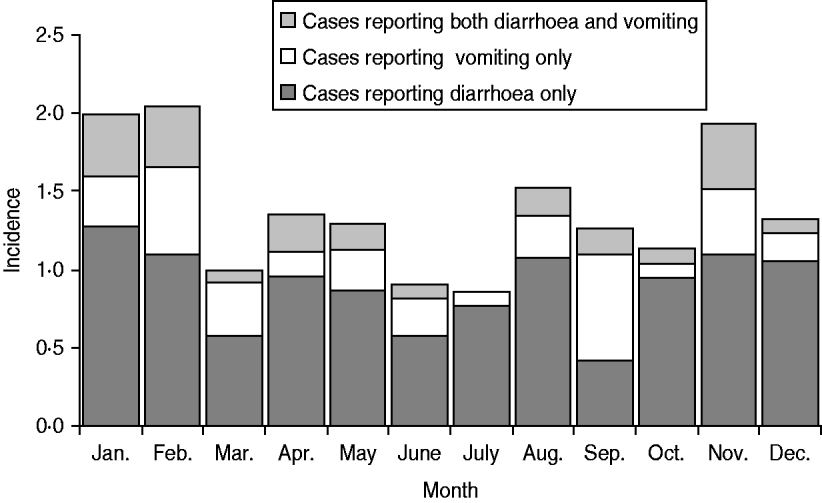
Fig. 1. Incidence per person-year of acute gastrointestinal illness by major symptom groups, Denmark 2009.
In an analysis of the questions concerning the perceived quality of household tap water, an elevated risk was associated with noting a changed taste to the water (RR 2·8, 95% CI 1·3–5·9), although this rested on five cases only. Consumption of pork (RR 0·7, 95% CI 0·5–0·9), fish (RR 0·6, 95% CI 0·5–0·8) and vegetables (RR 0·5, 95% CI 0·3–0·9) were inversely associated with illness (data not shown).
The effect of using different recall periods
In addition to the 4-week period, the study subjects were asked about illness the day before the interview and the 7-day period preceding the interview. The number of cases produced by these two alternative recall periods is presented for each age and gender group in Table 2. Observing first the number of persons who reported having suffered gastrointestinal illness the day before the interview, a total of 40 (2·2%) persons fulfilled the case definition. Of these 35 (88%) reported diarrhoea and 11 (28%) reported vomiting. Thus this indicates that the daily point prevalence, i.e. the proportion of the Danish population suffering acute intestinal illness at any given day, can be estimated at 2·2% (corresponding to 120 000 persons).
When asked about illness in the 7-day period preceding interview, 98 (5·3%) individuals met the case definition. Excluding cases that reported onset of symptoms before the beginning of the 7-day period, there were 87 (4·7%) cases. Of the 87 cases, 66 (76%) reported suffering from diarrhoea, 32 (37%) vomiting and 11 (13%) both symptoms. The 87 cases correspond to an adjusted incidence rate of 2·4 (95% CI 2·0–3·0) episodes of AGI per person-year.
DISCUSSION
This study was a cross-sectional telephone survey of randomly selected residents in Denmark. Similar burden-of-illness studies have been performed in a number of industrialized countries within the past 10–15 years [Reference Cantwell3–Reference Jones12]. In the USA, studies have repeatedly been performed within FoodNet, finding percentages of symptomatic persons within the past month or 4-week period ranging from 6% to 11% [Reference Cantwell3, Reference Herikstad5, Reference Imhoff7]. Comparison between different studies is complicated by the use of different case definitions and slightly different methodologies. This had led to an international recommendation of a common case definition; the one which was adopted in the present study. Thus re-analysis of datasets from studies performed in USA, Canada, Australia and Ireland using this case definition, led to annual incidence rates varying from 0·64 to 1·0 [Reference Majowicz15]. Cross-sectional burden-of-illness surveys appear to provide estimates of disease which are higher than those found in prospective population studies [Reference de Wit22, Reference Wheeler23]. They should therefore not stand alone, but be interpreted in the light of knowledge obtained from other types of studies. However, through the many cross-sectional studies performed in recent years and the international collaborative work performed in relation to these studies, a number of methodological issues have been resolved and comparability between study series have increased.
The only previous Danish data stems from a pilot study from 1992 where a question about gastrointestinal symptoms was included in a large survey performed by Statistics Denmark. A question regarding illness in household members over a 3-month period was put to 2000 households (N. Rosdahl, K. Schmidt, unpublished data). This resulted in an estimate of 1·4 for the annual rate of disease per person; although comparison with the present study is difficult because of the design, case definition and other circumstances relating to the 1992 pilot study.
The incidence rate of 1·4 found in the present study is at the high end of the spectrum of what has been found in other developed countries. It is possible of course that the incidence of self-reported disease is somewhat higher in Denmark than in some other countries. However, the fact that a relatively small proportion of cases (12%) sought medical contact or suffered bloody diarrhoea (3%) suggests that this study is primarily capturing the milder disease cases. Among the limitations of this study is the sample size, which was relatively small. It is possible that the use of a larger group of study participants might have increased the precision of the estimate as there is variation between incidence estimates between some age gender groups. Potential strengths of this study include the study design, which we believed helped to reach good representativeness of the underlying population in our sample. Study participants were not randomly chosen in the process of making the telephone call, but were selected from the Danish population register. This meant that the interviews targeted known, named persons and that a pre-determined quota of subjects in each age and gender group was contacted each month. By this approach it was possible to target a selection of the population which closely resembled the full population in terms of age and gender distribution. The use of both mobile and landline phone numbers also helped reach persons in all age groups and avoided the potentially serious bias introduced by only calling landline phones; something that may in particular restrict the representativeness of the group of young adults, as we estimated that 90% of participants in the 20–29 years age groups were interviewed by mobile phone. Finally, the participation rate was quite high in this study compared to comparable studies from other countries cited above.
A comment should also be made regarding the underlying study population. In this study, the study population constituted all Danish residents exclusive of those that (a) personally or as household members did not have a telephone number listed in the web-based national phone books, (b) were unable to speak Danish, or (c) were excluded owing to symptoms of a likely non-infectious aetiology in accordance with the case definition used. The first of these groups constituted 18% in our sample material, but we do not have particular reasons to believe that persons with unlisted numbers are different from the majority of the population with respect to AGI. This may not be true for the second group. However, it consisted of just one person. The third group constituted 96 persons in the present study, corresponding to 5% of all those interviewed. We note that in some other studies [Reference Majowicz15] it has been the practice to define these persons as non-ill (keeping them in the denominator) when calculating the incidence estimates. Doing so in the present study would reduce the overall incidence estimate from 1·4 to 1·3. As these persons, by case definition, are never at-risk of becoming cases, we would argue that they cannot be part of the study population. Nevertheless, it is particularly important in burden-of-illness surveys to carefully delineate this population group, as it may be quite large; in this study it was roughly half the size of the case group.
The incidence of gastrointestinal disease was highest in children and there was clearly a decreasing gradient of disease with increasing age, the incidence being 2–3 times lower in the elderly compared to children. This finding is in accord with results from FoodNet and other studies [Reference Jones12] and it is plausible that both increased immunity acquired when getting older to important pathogens such as rotavirus and Campylobacter in addition to different patterns of behaviour is responsible for this. Another frequent finding in previous studies has been a slightly increased incidence in women compared to men [Reference Majowicz15]; however, we did not find this in the present study, where there was 1·1 male case for every female case.
In our study, respondents were first asked about illness the day before interview and subsequently about illness in the past 7 and 28 days. This approach was recently evaluated in a US study and found not to bias the results [Reference Cantwell3]. That same study also found that asking about disease in the 7-day period preceding the interview led to estimates of disease roughly twice as high as when asking about a 4-week period. The present study corroborates these results; we found annual incidence estimates of 1·4 and 2·4 for the 4-week and 7-day periods, respectively, and the percentage of cases going from 2·2 in 1 day to 5·3 in 7 days and 11·1 in 28 days. A number of factors, including inaccurate recall, may influence on these results, and it is difficult to say which are the more correct. However, these findings may suggest that common use of a 4-week recall period may underestimate the burden of illness and they underline how sensitive these studies are towards changes in design.
A relatively large group of cases also reported having respiratory symptoms. It has been noted as a general problem in acute intestinal illness telephone surveys that respiratory disease may sometimes result in gastrointestinal symptoms [Reference Hall24]. This means that the study may in part also measure symptoms caused by non-intestinal infections, a fact that should be borne in mind when evaluating the results. However, in the present study the definition of respiratory symptoms was somewhat vague; respondents were only asked if they had ‘symptoms of common cold’.
There was no striking pattern in the seasonality of cases, although there were more cases in the winter months. Similar results have been seen previously [Reference Sargeant, Majowicz and Snelgrove10]. In Denmark, some common infections show a marked seasonal distribution, but they are not necessarily in phase with one another. Thus the number of some bacterial diarrhoeal infections, most notably Salmonella infections, is higher in the summer months in Denmark, whereas norovirus outbreaks are often particularly prevalent in the winter months, and rotavirus infections peak in late winter or early spring. The number of stool samples referred for microbiological analysis has previously been found to be more or less unchanged throughout the year, although slightly lower in the second quarter of the year [Reference Ethelberg25]. This indicates that the seasonal differences in overall gastrointestinal illness in Denmark are minor.
This study adds to the picture of foodborne and gastrointestinal illness in Denmark. The data regarding care-seeking and the proportion of patients that have a stool sample submitted for analysis in particular will help fill a gap in the efforts to estimate the size of each layer in the surveillance pyramid for gastrointestinal infections in Denmark. Although the data obtained are not pathogen specific, the dataset, when seen in the context of national surveillance data, register studies [Reference Helms, Simonsen and Molbak26], model studies [Reference Korsgaard27] and even serological studies [Reference Simonsen28] can assist in the efforts to calculate cost and disease burden of gastrointestinal illness in Denmark. Due to the methodological challenges inherent in the cross-sectional telephone study design, the results or the present study are not conclusive; nevertheless, they suggest that a substantial amount of acute gastrointestinal disease occurs which leaves room for preventive measures, e.g. in terms of efforts to prevent outbreaks, improve food safety and improve hygiene, particular in day-care and similar institutions for children.
ACKNOWLEDGEMENTS
We thank Iben Onsberg Hansen, Bodil Helbech Hansen, Eva Tulin Kristensen, Henriette Juel Larsen, Mamuna Afzal, Nina Kamstrup-Larsen, Dominik Wessely, Louise Hansen and Kristine Bjørn Larsen who conducted the interviews. We also thank Kenn Schultz Nielsen who helped set up the MS Access database and interview form and Jacob Simonsen for help with accessing CPR data. The study was funded in part by Med-Vet-Net work package 23.
DECLARATION OF INTEREST
None.






