Introduction
California is the largest producer of processing tomato, accounting for more than 90% of U.S. production (Winans et al. Reference Winans, Brodt and Kendall2020). However, the profitability of the tomato industry in California is seriously threatened due to the presence of the parasitic weed branched broomrape. This parasitic weed was first reported on tomato in California in 1928 (Stout and Wagnon Reference Stout and Wagnon1953, quoted in Musselman Reference Musselman1980) and became the target of an eradication effort about four decades ago (Osipitan et al. Reference Osipitan, Hanson, Goldwasser, Fatino and Mesgaran2021). Branched broomrape is currently classified as an “A” pest in California. An “A” pest is a pest of known economic importance subject to state-enforced action involving “eradication, quarantine regulation, containment, rejection, or other holding action” (CDFA 2020). Detection of branched broomrape in a commercial tomato field leads to quarantine and crop destruction without harvest. Egyptian broomrape has also been reported in several tomato fields in California (Miyao Reference Miyao2017) but is currently assumed to be less of a threat than branched broomrape.
Broomrapes (Orobanche and Phelipanche spp.) have been known as the most destructive parasitic plants globally, and controlling them is a challenge. Branched broomrape is a holoparasitic weed that attaches to the host root and absorbs water, minerals, and carbohydrates from its host. It can severely damage the host by reducing the aerial biomass and leaf chlorophyll content (Mauromicale et al. Reference Mauromicale, Monaco and Longo2008) with yield losses of up to 80% (Eizenberg and Goldwasser Reference Eizenberg and Goldwasser2018). To germinate, seeds of branched broomrape need to be close to the host roots because the host root exudation triggers germination. Because seeds can maintain their viability in the seed bank for several decades (Joel Reference Joel, Joel, Gressel and Musselman2013), eradication is difficult. Branched broomrape has many hosts besides tomato, including cabbage (Brassica oleracea), canola (B. napus), carrot (Daucus carota L.), celery (Apium graveolens L.), pepper (Capsicum fruitisence), potato (Solanum tuberosum L.), sunflower (Helianthus annuus L.), and lettuce (Lactuca sativa; Osipitan et al. Reference Osipitan, Hanson, Goldwasser, Fatino and Mesgaran2021). The broad range of hosts can further complicate control and eradication plans. Moreover, branched broomrape has been noted to extend its host range to affect new species (Le Corre et al. Reference Le Corre, Reibel and Gibot-Leclerc2014); for example, the seeds of a new race of sunflower broomrape (Orobanche cumana Wallr.) has expanded its host range to Solanaceae crops by parasitizing tomato and tobacco (Nicotiana tabacu L.; Dor et al. Reference Dor, Plakhine, Joel, Larose, Westwood, Smirnov, Ziadna and Hershenhorn2020).
Branched broomrape produces hundreds of thousands of tiny seeds (0.2 to 0.4 mm), which can be transported easily by humans, water, wind, and animals (Eizenberg et al. Reference Eizenberg, Aly and Cohen2012; Ginman et al. Reference Ginman, Prider, Matthews, Virtue and Watling2015). Farm machinery (e.g., harvesters) is one of the most important ways of dispersal of broomrape seeds in tomato fields (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009; Rubiales and Fernández-Aparicio Reference Rubiales and Fernández-Aparicio2012). Because cropping systems in California are highly mechanized, dispersal of broomrape seeds via farm machinery is particularly concerning. Movement of farm equipment between plots of the same farm or between farms is very common, which can facilitate both the short- and long-distance dispersal of broomrape seeds. To effectively contain branched broomrape, farming implements used in an infested field must be cleaned and sanitized before entering other fields or farms. The first step in equipment sanitation is removing all plant and soil residues. The next step involves using effective disinfecting chemicals to disinfect broomrape seeds left on the equipment (Osipitan et al. Reference Osipitan, Hanson, Goldwasser, Fatino and Mesgaran2021).
Quaternary ammonium compounds are surface-active chemicals that are widely used as sanitation solutions in the food processing industry and in disinfectants, fabric softeners, and cosmetics (Martínez-Carballo et al. Reference Martínez-Carballo, Sitka, González-Barreiro, Kreuzinger, Fürhacker, Scharf and Gans2007). Initial research with some ammonium compounds as potential equipment disinfectants suggests that didecyl dimethyl ammonium chloride, alkyl dimethyl benzyl ammonium chloride, dioctyl dimethyl ammonium chloride, octyl decyl dimethyl ammonium chloride, and ammonium bromide can effectively prevent germination of Egyptian broomrape seeds (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009). However, data on the effectiveness of sanitation methods for both branched broomrape and Egyptian broomrape is scarce. Thus the objective of this study was to test the ability of various quaternary ammonium compounds to prevent the germination of branched and Egyptian broomrape seeds with the goal of finding a material to disinfect farm machinery.
Materials and Methods
Collection of Broomrape Seeds
Soil samples were collected from preidentified branched broomrape–infested fields near Woodland, CA (38.7574°N, 121.7677°W) in 2019. The soil samples were transferred to the Contained Research Facility of the University of California, Davis. In this facility, 80 plastic pots (2.4 L) were filled with the collected soil, moistened, and placed in a growth chamber. The growth chamber was set at a 28/20 C (day/night) temperature regime for 2 wk to precondition the branched broomrape seeds that might occur in the collected soil (Murdoch and Kebreab Reference Murdoch, Kebreab, Joel, Gressel and Musselman2013). Following this conditioning period, two beefsteak tomato seedlings (10 to 15 cm tall) were transplanted to each pot to stimulate the germination of the presumed broomrape seeds. About 100 d after planting, the emerged branched broomrape plants were mature and produced seeds. Capsules were collected from plants, crushed, and sieved to obtain clean seeds. The collected seeds (∼0.2 mm in size) were stored in darkness at a temperature of 4 C in the Contained Research Facility until use.
Evaluation of Quaternary Ammonium Compounds
We evaluated five quaternary ammonium compounds for their effectiveness in preventing the germination of branched broomrape seeds. These chemicals included ammonium bromide (AB), didecyl dimethyl ammonium bromide (DDAB), ammonium chloride (AC), alkyl dimethyl benzyl ammonium chloride (ADAC), and didecyl dimethyl ammonium chloride (DDAC). The compounds were supplied in technical form (≥90% concentration) by Sigma-Aldrich (St. Louis, MO). For AB, AC, DDAB, and DDAC we tested seven concentrations: 0% (distilled water), 0.01%, 0.05%, 0.125%, 0.2%, 0.5%, and 1% (wt/vol) of distilled water. For ADAC, the concentrations were 0%, 0.2%, 0.5%, 1%, 2.5%, 5%, and 10% (wt/vol) of distilled water.
Each solution (500 µl) was added to a 5-ml Eppendorf tube equipped with a paper filter, and about 100 seeds were placed in the Eppendorf tubes for 10 min and then centrifuged at 1,500 rpm for 2 min using Z206-A® compact centrifuge (Benchmark Scientific, Beachwood, OH). The centrifuge was used to drain the solutions from the seeds. Afterward, distilled water was added to the tubes to wash the seeds, and the water was drained by centrifuging for 2 min. Seeds were then extracted from the tubes, placed on filter papers (Whatman®; Global Life Sciences Solutions, Marlborough, MA) in 5-cm-diameter Petri dishes, moistened with distilled water, and kept at 25 C in darkness for 10 d within an incubator (Isotemp Incubator; Fisher Scientific) as the preconditioning period.
Following the preconditioning period, 1 ml of 10−5 M of a strigolactone analog (GR24) was added to the Petri dishes. GR24 is widely used to induce germination in seeds of broomrapes in the absence of host (Fernández-Aparicio et al. Reference Fernández-Aparicio, Yoneyama and Rubiales2011; Ibdah et al. Reference Ibdah, Dubey, Eizenberg, Dabour, Abu-Nassar, Gal-On and Aly2014). Seeds were incubated (Isotemp Incubator; Fisher Scientific) at 25 C and kept in the dark for 14 d where germinated, and ungerminated seeds were counted 7 and 14 d after incubation using a stereomicroscope (Nikon SMZ 1500). Seeds were considered germinated when a protruded radicle was visible. The experiment was conducted with three replicates per concentration of each compound in April 2020 and was repeated in July 2020.
Effects of Exposure Duration
A second study was conducted to determine the impact of the exposure duration of the quaternary compounds on the germination of branched broomrape and Egyptian broomrape seeds. The exposure times tested were 10 minutes, 1 hour, and 24 hours including centrifuge time. In this study, three compounds, ADAC, DDAB, and DDAC were tested at seven concentrations of 0 (distilled water), 0.01, 0.05, 0.125, 0.2, 0.5, and 1% (wt/vol) of distilled water. Treatment application, seed conditioning, and germination test were as described above. All treatments were replicated three times in September 2020, and the experiment was repeated in October 2020.
Data analysis
Seed germination data (as a surrogate for seed viability) were subjected to dose-response analysis using the drc package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015) in R software (R Core Team 2020) following the guideline provided by Keshtkar et al. (Reference Keshtkar, Kudsk and Mesgaran2021). For both studies, data from the two experimental runs were pooled because there was no difference between the full model (with the experimental run as a covariate) and the reduced model (without the experimental run), as detailed below. A three-parametric log-logistic function (Equation 1; Streibig et al. Reference Streibig, Rudemo, Jensen, Streibig and Kudsk1993) best described the seed germination of branched broomrape in relation to the concentration of ADAC, DDAB, DDAC, and AB:
where Y is broomrape total seed germination (percentage), e is the effective dose needed for a 50% response (ED50), u is the upper limit, and b is the relative slope around the inflection point (e). In the exposure duration experiment, the three-parametric log-logistic function (Equation 1) also showed the best fit for branched broomrape seed germination. However, for AC data, a four-parametric log-logistic function (Equation 2; (Streibig et al. Reference Streibig, Rudemo, Jensen, Streibig and Kudsk1993) was found to provide better fits:
with the addition parameter l indicating the lower limit of the curve. Note that in the above four-parameter model, parameter e shows the response halfway between the upper, u, and lower, l, limits and should not be used as the ED50 (Keshtkar et al. Reference Keshtkar, Kudsk and Mesgaran2021). Germination data from all chemical compounds, except AC, were fitted to Equation 1 simultaneously. To test whether the parameters of the model differ between the chemical compounds, we compared the size of error between a full model and various reduced models (lacking 1 or 2 parameters). An F-test was conducted to compare the error across these two types of models using the “anova()” function of the mass package in R. For example, to test the null hypothesis that parameter e (ED50) does not differ between ADAC, DDAB, DDAC, and AB compounds, we first fitted a (reduced) model that assumed a single e parameter across the dose-response curves of these four chemistries. A full model was then fitted to the same data using four individual e parameters for each chemistry. The latter more complex model (i.e., full) is justified, and hence the four curves differ in parameter e, if its size of model error is significantly smaller than that of the reduced model as indicated by the F-test. A similar test was performed for all other parameters of the models to test whether they differ across chemical compounds or exposure duration treatments.
Results and Discussion
Screening of Ammonium Compounds
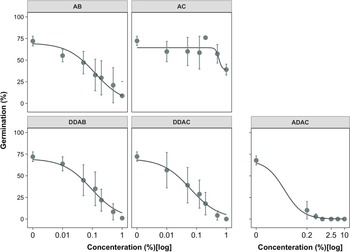
All five quaternary ammonium compounds, except AC, were effective in preventing the germination of broomrape seeds (potentially making them nonviable), as shown by our dose-response analysis (Figure 1). Comparing various reduced models vs. the full model showed that both the upper limit, u, and slope, b, parameters can be fixed across curves of ADAC, DDAB, DDAC, and AB without significantly reducing the goodness of fit (Table 1);that is, these parameters did not vary between the tested compounds. As represented by parameter u, total seed germination in control was ∼70% (SE = 3.3). The effective dose for 50% reduction in germination (parameter e) varied significantly across the above four chemistries and ranged from 0.014 (SE = 0.016) in ADAC to 0.124 (SE = 0.033 (wt/vol) in AB (Table 1). A four-parameter logistic model with a lower limit l > 0 was used for AC, because branched broomrape germination never approached zero over the tested doses. For AC, the ED50 was not estimable because the lower limit of the model (∼39%) was greater than half the maximum response (i.e., 64.4% × 0.5 = 32.2%).

Figure 1. Dose response curves of branched broomrape seed germination in response to doses of five different ammonium products. A three-parameter logistic model (Equation 1) was used for AB, ADAC, DDAC, and DDAB, whereas AC data were fitted to a four-parameter model (Equation 2). Lines are fitted values, and solid circles indicate observed germination averaged across two experimental runs with three replicates each (i.e., n = 6). Error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 1. Abbreviations: AB, ammonium bromide; AC, ammonium chloride; DDAC, didecyl dimethyl ammonium chloride; ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide.
Table 1. Estimated parameter values for the three-parameter (Equation 1) and four-parameter (Equation 2) log-logistic models were used to describe the branched broomrape seed germination responses to the increasing doses of different ammonium compounds. e,f

a These parameters were fixed across ammonium compounds because of the nonsignificant P-value for the comparison of full vs. reduced models.
b This parameter (lower limit) only applies to the four-parameter log-logistic model.
c Values in parenthesis are standard errors.
d P-values indicate significant differences between ammonium compounds for a given parameter in the three-parameter log-logistic model only.
e Abbreviations: AB, ammonium bromide; AC, ammonium chloride; DDAC, didecyl dimethyl ammonium chloride; ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide; RMSE, root mean square error.
f In these models, b represents the steepness of the inflection point, u is the upper limit, l indicates the lower limit, and e is the dose that produces a germination response halfway between u and l.
Germination responses of branched broomrape seeds to DDAB and DDAC, and to a lesser extent to AB, were similar: the decline occurred slowly, and complete prevention was achieved at the maximum concentration (i.e., 1%). However, the declining response to ADAC was more abrupt than that of other compounds (Figure 1), as shown by its very low ED50 value (Table 1). AC had a poor sanitation effect on branched broomrape seeds, and even at the highest concentration rate more than half the seeds were able to germinate (Figure 1). Two ammonium compounds, AB and AC, were eliminated for the exposure duration experiment.
Exposure Duration
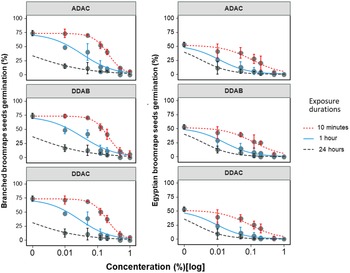
A three-parameter log-logistic model was used to characterize the seed germination responses of both branched and Egyptian broomrape to different sanitation doses across three exposure durations of 10 min, 1 h, and 24 h (Figure 2 and Table 2). All three chemical compounds (ADAC, DDAB, and DDAC) displayed a significant effect on seed germination of both broomrape species seeds, and complete prevention was achieved with the three tested exposure durations when these chemicals were applied at the maximum rate (i.e., 1% wt/vol). However, ED50 values for all disinfectants decreased with increased exposure duration in both broomrape species. For ADAC, for example, a concentration of 0.216% (wt/vol) was required to provide a 50% reduction in branched broomrape seed germination when seeds were exposed to this chemical for 10 min. In contrast, only 0.0006% (wt/vol) was enough to give the same level of reduction in seed germination when exposure duration increased to 24 h. The same trend was observed with the two other products, DDAB and DDAC, across both broomrape species. A comparison of ED50 values between the two broomrape species indicated that these ammonium compounds could halt germination in Egyptian broomrape seeds at lower concentrations compared to branched broomrape seeds, suggesting greater sensitivity of Egyptian broomrape seeds to ammonium compounds (Table 2).

Figure 2. Dose response curves of branched broomrape and Egyptian broomrape seed germination in response to doses of three different ammonium products under three exposure durations of 10 min, 1 h, and 24 h. A three-parameter logistic model (Equation 1) was fitted to germination data. Lines are fitted values, and solid circles indicate observed germination averaged across two experimental runs with three replicates each (i.e., n = 6). Error bars indicate 95% confidence intervals. Model parameter estimates are shown in Table 2. Abbreviations: DDAC, didecyl dimethyl ammonium chloride; ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide.
Table 2. Estimated parameter values for the three-parameter (Equation 1) log-logistic models used to describe the branched broomrape and Egyptian broomrape seed germination responses to the increasing doses of ammonium compounds over various exposure durations. d,e

a If there is a single value for the parameter, it means that that parameter is fixed across ammonium compounds and exposure durations because of the nonsignificant P-value for the comparison of full vs. reduced models.
b Values in parenthesis are standard errors.
c P-values indicate significant differences between ammonium compounds and exposure durations for a given parameter in the three-parameter log-logistic model (Equation 1).
d Abbreviations: DDAC, didecyl dimethyl ammonium chloride; ADAC, alkyl dimethyl benzyl ammonium chloride; DDAB, didecyl dimethyl ammonium bromide; RMSE, root mean square error.
e In the model (Equation 1), b represents the steepness of the inflection point, u is the upper limit (i.e., maximum seed germination when the dose of the ammonium compound is zero), and e is the dose that produces a germination response half the u value.
Sanitation of farm equipment before entering other farms can help reduce the introduction of broomrape seeds to non-infested fields. Quaternary ammonium compounds are widely used as disinfectants and have been found to be effective for broomrape seed eradication. As part of a national eradication program in southern Australia, a quaternary ammonium compound containing didecyl dimethyl ammonium chloride (DDAC; commercial name NiproQuat) was found to significantly render the branched broomrape seeds nonviable on farm machinery (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009).
ADBC (alkyl dimethyl benzyl ammonium chloride), known commercially as Zoharquat 50, is a bactericidal, fungicidal, and algicidal agent that has provided promising results for sanitation of tomato harvesters contaminated with Egyptian broomrape seeds (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009). At 1% (wt/vol) ADBC caused a 20% reduction in the germination of Egyptian broomrape seeds, but when the concentration increased to 10% (wt/vol), this product ultimately prevented the germination of all Egyptian broomrape seeds collected from the tomato harvester (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009).
DDAB, with the commercial name Bromosept 50, is a disinfectant agent with effectiveness against bacterial, viral, and fungal pathogens. The sanitation effect of DDAB against Egyptian broomrape seeds was tested in the laboratory and a commercial disinfecting facility (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009). This research concluded that soaking Egyptian broomrape seeds in DDAB at 0.1 % (wt/vol) for 5 min completely prevented the germination of seeds in a petri dish. DDAB sprayed at a higher rate of 1% (wt/vol) completely prevented the germination of Egyptian broomrape seeds attached to the sides of a commercial tomato harvester (Hershenhorn et al. Reference Hershenhorn, Eizenberg, Dor, Kapulnik and Goldwasser2009).
Several studies have noted that sanitation and prevention are integral parts of the noxious and quarantine plant eradication program (Panetta and Scanlan Reference Panetta and Scanlan1995; Quinn et al. Reference Quinn, Barney, McCubbins and Endres2013). Human activities and farm machinery are among the most effective agent of weed seed dispersal (Liebman et al. Reference Liebman, Mohler and Staver2001). The spatial distribution pattern of Egyptian broomrape in a tomato field showed a strong link between some specific farm activities and the level of infestation: the container collection site and the washing site of combines had the highest level of Egyptian broomrape contamination (Eizenberg et al. Reference Eizenberg, Aly and Cohen2012). Containers and farm equipment can collect broomrape seeds and transport them to another location, hence they should be disinfected and sanitized before exiting an infested field. Our study showed that most commercially available ammonium compounds are effective on broomrape seeds and can be used to sanitize farm equipment.
Removing seeds from equipment before leaving an infested field should constitute a key component of any broomrape eradication program. Further research should be conducted to evaluate the efficacy of other sanitizer classes (e.g., peracetic acid, acid-anionic sanitizers, fatty acid sanitizers, biguanide, and peroxides), which are used in the food industry and have been found to be more effective than quaternary ammonium compounds in sterilizing surfaces (Bernardi et al. Reference Bernardi, Garcia and Copetti2019). Furthermore, a shorter exposure time needs to be evaluated for equipment sanitation in further studies.
Acknowledgments
This research was funded by the California Department of Food and Agriculture through Specialty Crop Block Grant Program 19-0001-043-SF. The authors declare no conflicts of interest.