Introduction
Depression is a common mental illness with a complex aetiology and is one of the leading causes of disability worldwide, affecting around 10–20% of the general population in their lifetime (Lim et al., Reference Lim, Tam, Lu, Ho, Zhang and Ho2018). There is now increasing evidence suggesting an association between depression and inflammation (Goldsmith et al., Reference Goldsmith, Rapaport and Miller2016). For instance, ‘sickness behaviour’ commonly seen following an acute infection, shares many characteristics with depression, such as fatigue, sleep disturbance and decreased motivation (Dantzer et al., Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008); early-life infection and autoimmune diseases are associated with a higher risk of depression in adulthood (Benros et al., Reference Benros, Waltoft, Nordentoft, Østergaard, Eaton, Krogh and Mortensen2013); people with chronic immune-mediated inflammatory diseases such as rheumatoid arthritis exhibit a higher prevalence of depression (Dickens et al., Reference Dickens, McGowan, Clark-Carter and Creed2002). Depression is also associated with other conditions linked with elevated inflammatory markers, such as cardiovascular disease (CVD) (Ridker, Reference Ridker2003).
C-reactive protein (CRP) is a marker of acute phase response which has been used most extensively as a measure of low-grade inflammation in psychiatric (von Känel et al., Reference von Känel, Hepp, Kraemer, Traber, Keel, Mica and Schnyder2007; Fernandes et al., Reference Fernandes, Steiner, Bernstein, Dodd, Pasco, Dean, Nardin, Goncalves and Berk2016) and physical conditions (Visser et al., Reference Visser, Bouter, McQuillan, Wener and Harris1999; Danesh et al., Reference Danesh, Whincup, Walker, Lennon, Thomson, Appleby, Gallimore and Pepys2000). CRP is associated with cardiovascular risk, including myocardial infarction, stroke, sudden cardiovascular death and peripheral vascular disease (Ridker, Reference Ridker2003). Meta-analyses of cross-sectional studies confirm that mean concentrations of circulating CRP and inflammatory cytokines such as interleukin 6 (IL-6) are higher in patients with acute depression compared with controls (Howren et al., Reference Howren, Lamkin and Suls2009; Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctôt2010; Haapakoski et al., Reference Haapakoski, Mathieu, Ebmeier, Alenius and Kivimäki2015; Goldsmith et al., Reference Goldsmith, Rapaport and Miller2016). Population-based longitudinal studies show that higher levels of CRP and IL-6 at baseline are associated with an increased risk of depression in subsequent follow-ups (Gimeno et al., Reference Gimeno, Kivimäki, Brunner, Elovainio, De Vogli, Steptoe, Kumari, Lowe, Rumley and Marmot2009; Wium-Andersen et al., Reference Wium-Andersen, Ørsted, Nielsen and Nordestgaard2013; Khandaker et al., Reference Khandaker, Pearson, Zammit, Lewis and Jones2014; Zalli et al., Reference Zalli, Jovanova, Hoogendijk, Tiemeier and Carvalho2016), suggesting that inflammation could be a cause rather than simply a consequence of the illness.
The association between inflammation and depression is clinically relevant. Poor response to antidepressants is associated with the activation of inflammatory immune responses (Lanquillon et al., Reference Lanquillon, Krieg, Bening-Abu-Shach and Vedder2000; Benedetti et al., Reference Benedetti, Lucca, Brambilla, Colombo and Smeraldi2002; Carvalho et al., Reference Carvalho, Torre, Papadopoulos, Poon, Juruena, Markopoulou, Cleare and Pariante2013; Chamberlain et al., Reference Chamberlain, Cavanagh, de Boer, Mondelli, Jones, Drevets, Cowen, Harrison, Pointon and Pariante2018). It has been reported the mean CRP levels are higher in treatment-resistant compared with treatment-responsive patients with depression (Maes et al., Reference Maes, Bosmans, De Jongh, Kenis, Vandoolaeghe and Neels1997; Sluzewska et al., Reference Sluzewska, Sobieska and Rybakowski1997). Anti-inflammatory treatment has antidepressant effects (Müller et al., Reference Müller, Schwarz, Dehning, Douhe, Cerovecki, Goldstein-Müller, Spellmann, Hetzel, Maino and Kleindienst2006; Köhler et al., Reference Köhler, Benros, Nordentoft, Farkouh, Iyengar, Mors and Krogh2014; Kappelmann et al., Reference Kappelmann, Lewis, Dantzer, Jones and Khandaker2018). Randomised controlled trials (RCTs) indicate that anti-inflammatory drugs are likely to be beneficial particularly for depressed patients who show evidence of inflammation (Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013; Kappelmann et al., Reference Kappelmann, Lewis, Dantzer, Jones and Khandaker2018). Currently, a number of ongoing RCTs of anti-inflammatory treatments are recruiting specifically depressed patients with elevated CRP levels (e.g. ⩾3 mg/L): NCT02473289; ISRCTN16942542 (Khandaker et al., Reference Khandaker, Oltean, Kaser, Dibben, Ramana, Jadon, Dantzer, Coles, Lewis and Jones2018). Therefore, a better understanding of the prevalence of low-grade inflammation in depression, and of factors associated with inflammation could inform future research and clinical practice.
Inflammation is unlikely to be relevant for all patients with depression (Khandaker et al., Reference Khandaker, Dantzer and Jones2017). While it is established that mean concentrations of peripheral inflammatory markers are higher in depressed patients compared with controls (Howren et al., Reference Howren, Lamkin and Suls2009; Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctôt2010; Haapakoski et al., Reference Haapakoski, Mathieu, Ebmeier, Alenius and Kivimäki2015; Goldsmith et al., Reference Goldsmith, Rapaport and Miller2016), it is unclear what proportion of depressed patients show evidence of low-grade inflammation. Many studies have reported on the prevalence of inflammation in depressed patients using various CRP level thresholds to define inflammation, e.g. >3 or >1 mg/L. These studies have been conducted in different settings and populations, e.g. inpatient, outpatient, population-based (Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013; Wium-Andersen et al., Reference Wium-Andersen, Ørsted, Nielsen and Nordestgaard2013; Shin et al., Reference Shin, Jung, Kim, Kim and Lim2016). The reported prevalence of inflammation varies widely among these studies; for example, for low-grade inflammation (CRP >3 mg/L) it has been reported to vary between 0% and 60% in existing studies (Ma et al., Reference Ma, Chiriboga, Pagoto, Rosal, Li, Merriam, Hébert, Whited and Ockene2011; Hannestad et al., Reference Hannestad, DellaGioia, Gallezot, Lim, Nabulsi, Esterlis, Pittman, Lee, O'Connor and Pelletier2013). However, as far as we are aware, a systematic review and meta-analysis of the prevalence of low-grade inflammation in patients with depression is currently lacking. While it is likely that the prevalence of low-grade inflammation is higher in patients with depression compared with controls, to our knowledge, no systematic review and meta-analysis has examined the odds ratio for inflammation in depressed patients compared with matched controls.
We conducted a systematic review of existing studies to: (1) quantify the prevalence of low-grade inflammation in patients with depression using meta-analysis; (2) calculate the odds ratio for low-grade inflammation in depressed patients compared with matched healthy controls using meta-analysis; (3) identify sociodemographic and other factors associated with inflammation prevalence in patients with depression using meta-regression analysis. We defined low-grade inflammation as serum CRP levels >3 mg/L. This cut-off has been chosen based on the American Heart Association and Center for Disease Control and Prevention recommendations, which defined CRP levels of >3 mg/L as high (Pearson et al., Reference Pearson, Mensah, Alexander, Anderson, Cannon III, Criqui, Fadl, Fortmann, Hong and Myers2003). In addition, we carried out additional analyses using CRP levels >1 mg/L to define ‘elevated CRP’, and >10 mg/L to define ‘very high CRP’ indicative of current infection. We also carried out a number of sensitivity analyses; for example, meta-analyses using >1 and >3 mg/L thresholds for CRP were repeated using only studies that excluded patients with suspected infection (defined as CRP >10 mg/L); and after excluding poor quality studies.
Methods
Search strategy and study selection
This systematic review has been performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The search protocol was prospectively published on PROSPERO (see: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42018106640). The PubMed database was searched for published studies from its inception to 5 of July 2018 using the following keywords: ‘(CRP OR “C-reactive protein” OR “hs-CRP” OR hsCRP) OR (C-Reactive Protein[mesh] AND depressi*)’. No language restriction was applied; we only selected studies based on human participants. The electronic search was complemented by hand-searching of meta-analyses and review articles. Abstracts were screened, and full texts of relevant studies were retrieved. Two authors applied the inclusion/exclusion criteria independently and selected the final studies for this review (LB and EFO).
Selection criteria
We included studies that: (1) examined CRP levels in people with depression; (2) assessed depression using clinical criteria (DSM or ICD) or a validated tool (e.g. Hamilton Depression Rating Scale), and reported it as a categorical variable (yes/no); (3) reported CRP levels allowing the calculation of the proportion of ‘inflamed’ patients using cut-offs of either 3, 1 or 10 mg/L. One study used CRP cut-offs of 0.99 and 3.13 mg/L, which was included (Penninx et al., Reference Penninx, Kritchevsky, Yaffe, Newman, Simonsick, Rubin, Ferrucci, Harris and Pahor2003), as these values are very close to the thresholds above. Exclusion criteria were (1) studies reporting measures of inflammation other than CRP, e.g. interleukins or genetic markers; (2) in vitro or animal studies; (3) non-original data, e.g. reviews; (4) studies exclusively based on patients with a medical condition, e.g. cancer.
Recorded variables
The main outcome measure was the proportion of subjects showing elevated CRP in patients and, where reported, in non-depressed controls. We also extracted the following data: author; year of publication; sampling criteria; diagnostic criteria for depression; age of participants; treatment status (antidepressant-free, treatment resistant); ethnicity; matching criteria for patients and controls (if present); study setting and sample source (e.g. community or inpatient); presence of comorbidities. If there were multiple publications from the same data set, we used the study with the largest sample.
Data synthesis
We performed meta-analyses of the prevalence of inflammation in depressed patients using three different CRP cut-offs to define inflammation: >3 (primary), >1 and >10 mg/L. The pooled prevalence of inflammation was calculated using quantitative random-effect meta-analysis, expressed as percentage and 95% CI. The use of random-effect meta-analysis, as opposed to fixed effect, is appropriate when there is heterogeneity between studies. Pooling of studies was performed using the inverse variance method, so that studies with bigger samples were given greater weight. The Clopper–Pearson method was used to compute confidence interval for individual studies, and the logit transformation was used for the transformations of proportions, with a continuity correction of 0.5 in studies with zero cell frequencies. Heterogeneity between studies was measured using the I 2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity. Heterogeneity was tested using Cochrane's Q-Test (Higgins and Thompson, Reference Higgins and Thompson2002). Publication bias was assessed for each group of studies by visual inspection of funnel plots, and tested with an Egger's regression test for funnel plot asymmetry (mixed-effects meta-regression model). P values <0.05, two tailed, were considered statistically significant. We used meta-regression analyses to evaluate the association of inflammation prevalence with age, sex, body mass index (BMI), sample source, proportion of antidepressant-free patients and ethnicity. Seventeen studies reported CRP levels in matched non-depressed controls; these were used to calculate the meta-analytic odds ratio for inflammation in patients with depression v. healthy controls using random-effects estimates for meta-analyses with binary outcome data; pooling of studies was performed using the inverse variance method and with a continuity correction of 0.5 in studies with zero cell frequencies. Study quality was assessed using the Newcastle–Ottawa Scale (Stang, Reference Stang2010). Analyses were repeated with poor quality studies removed. Meta-analyses were carried out using the meta package [version 4.9 (Schwarzer, Reference Schwarzer2007)] in R 3.4 (R Core Team, 2017), and plotted using packages meta and Cairo v1.5 (Urbanek and Horner, Reference Urbanek and Horner2015). Additional information on the methods can be found in the Supplementary Materials.
Results
The literature search yielded 1545 results, out of which 37 studies met the inclusion criteria for meta-analysis (Legros et al., Reference Legros, Mendlewicz and Wybran1985; Penninx et al., Reference Penninx, Kritchevsky, Yaffe, Newman, Simonsick, Rubin, Ferrucci, Harris and Pahor2003; Ladwig et al., Reference Ladwig, Marten-Mittag, Löwel, Döring and Koenig2005; Liukkonen et al., Reference Liukkonen, Silvennoinen-Kassinen, Jokelainen, Räsänen, Leinonen, Meyer-Rochow and Timonen2006; O'brien et al., Reference O'brien, Scott and Dinan2006; Almeida et al., Reference Almeida, Flicker, Norman, Hankey, Vasikaran, van Bockxmeer and Jamrozik2007; Kling et al., Reference Kling, Alesci, Csako, Costello, Luckenbaugh, Bonne, Duncko, Drevets, Manji and Charney2007; Danese et al., Reference Danese, Moffitt, Pariante, Ambler, Poulton and Caspi2008; Nilsson et al., Reference Nilsson, Gustafson and Hultberg2008; Cizza et al., Reference Cizza, Eskandari, Coyle, Krishnamurthy, Wright, Mistry and Csako2009; Harley et al., Reference Harley, Luty, Carter, Mulder and Joyce2010; Ma et al., Reference Ma, Chiriboga, Pagoto, Rosal, Li, Merriam, Hébert, Whited and Ockene2011; Naghashpour et al., Reference Naghashpour, Amani, Nematpour and Haghighizadeh2011; Hannestad et al., Reference Hannestad, DellaGioia, Gallezot, Lim, Nabulsi, Esterlis, Pittman, Lee, O'Connor and Pelletier2013; Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013; Shanahan et al., Reference Shanahan, Copeland, Worthman, Angold and Costello2013; Park et al., Reference Park, Joo, McIntyre and Kim2014; Uher et al., Reference Uher, Tansey, Dew, Maier, Mors, Hauser, Dernovsek, Henigsberg, Souery and Farmer2014; Wium-Andersen et al., Reference Wium-Andersen, Ørsted and Nordestgaard2014; Courtet et al., Reference Courtet, Jaussent, Genty, Dupuy, Guillaume, Ducasse and Olie2015; Wysokiński et al., Reference Wysokiński, Margulska, Strzelecki and Kłoszewska2015; Cepeda et al., Reference Cepeda, Stang and Makadia2016; Haroon et al., Reference Haroon, Fleischer, Felger, Chen, Woolwine, Patel, Hu and Miller2016; Rapaport et al., Reference Rapaport, Nierenberg, Schettler, Kinkead, Cardoos, Walker and Mischoulon2016; Shin et al., Reference Shin, Jung, Kim, Kim and Lim2016; Ekinci and Ekinci, Reference Ekinci and Ekinci2017; Euteneuer et al., Reference Euteneuer, Dannehl, Del Rey, Engler, Schedlowski and Rief2017; Gallagher et al., Reference Gallagher, Kiss, Lanctot and Herrmann2017; Horsdal et al., Reference Horsdal, Köhler-Forsberg, Benros and Gasse2017; Jha et al., Reference Jha, Minhajuddin, Gadad, Greer, Grannemann, Soyombo, Mayes, Rush and Trivedi2017; Cáceda et al., Reference Cáceda, Griffin and Delgado2018; Chamberlain et al., Reference Chamberlain, Cavanagh, de Boer, Mondelli, Jones, Drevets, Cowen, Harrison, Pointon and Pariante2018; Felger et al., Reference Felger, Haroon, Patel, Goldsmith, Wommack, Woolwine, Le, Feinberg, Tansey and Miller2018; Osimo et al., Reference Osimo, Cardinal, Jones and Khandaker2018b; Porcu et al., Reference Porcu, Urbano, Verri, Barbosa, Baracat, Vargas, Machado, Pescim and Nunes2018; Shibata et al., Reference Shibata, Ohara, Yoshida, Hata, Mukai, Kawano, Kanba, Kitazono and Ninomiya2018; Wei et al., Reference Wei, Du, Wu, Fu and Xia2018). Please see Supplementary Fig. S1 for the PRISMA diagram of study selection, and Table 1 for details of the included studies.
Table 1. Characteristics of studies included in the meta-analysis
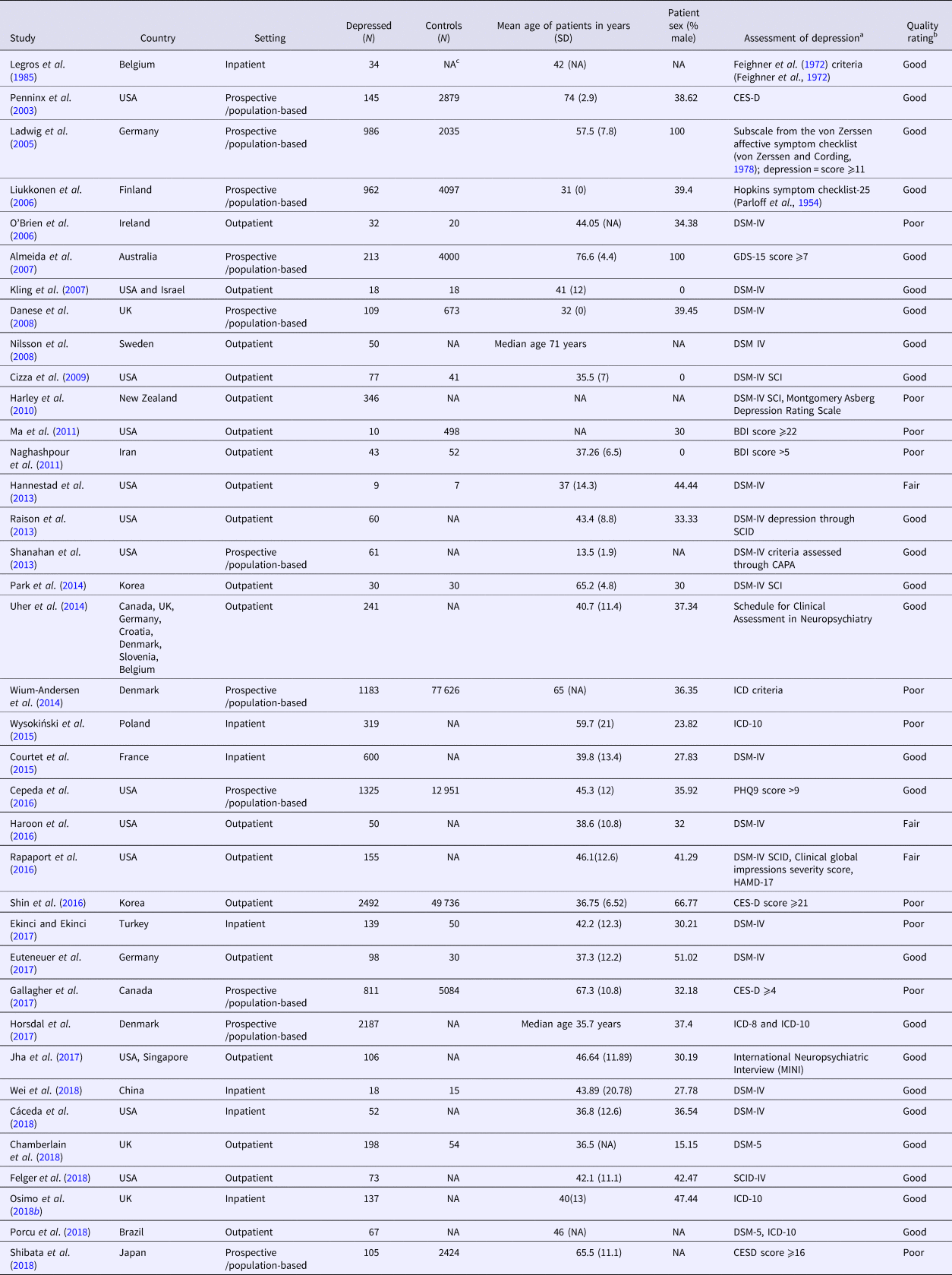
a CES-D, The Center for Epidemiologic Studies Depression Scale; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th. Edition; GDS, Geriatric Depression Scale; BDI, Beck's Depression Inventory; SCID, Structured Clinical Interview for DSM; CAPA, The Child and Adolescent Psychiatric Assessment; ICD, World Health Organisation International Classification of Diseases; PHQ9, Patient Health Questionnaire-9; HAMD-17, Hamilton Depression Rating Scale (HDRS).
b Studies evaluated using the Newcastle–Ottawa Scale (see Supplementary methods and Table S1), then converted to Agency for Healthcare Research and Quality – AHRQ – standards (good, fair and poor) using these thresholds:
• Good quality: ⩾75% in Selection domain AND ⩾50% in Comparability domain AND ⩾50% in Outcome domain.
• Fair quality: 50% in Selection domain AND ⩾50% in Comparability domain AND ⩾50% in Outcome domain.
• Poor quality: ⩽50% in Selection domain OR 0% in Comparability domain OR ⩽50% in Outcome domain.
c Not available.
Prevalence of low-grade inflammation (CRP >3 mg/L) in depressed patients
Results based on all available studies
Thirty studies comprising 11 813 patients with depression were used for this analysis. The meta-analytic pooled prevalence of low-grade inflammation in depressed patients was 27% (95% CI 21–34%); see Fig. 1. There was evidence of heterogeneity among studies (I 2 = 97.7%; 95% CI 97.3–98.1%; Cochrane's Q = 1264; p = <0.01). Further analyses after grouping studies by setting showed that the prevalence of inflammation in inpatient samples (N = 1265) was 30% (95% CI 21–42%; I 2 = 91.9%; Cochrane's Q = 62); in outpatient samples (N = 3528) it was 29% (95% CI 19–43%; I 2 = 95.9%; Cochrane's Q = 338); and in population-based samples (N = 7020) it was 24% (95% CI 17–34%; I 2 = 98.3%; Cochrane's Q = 483).
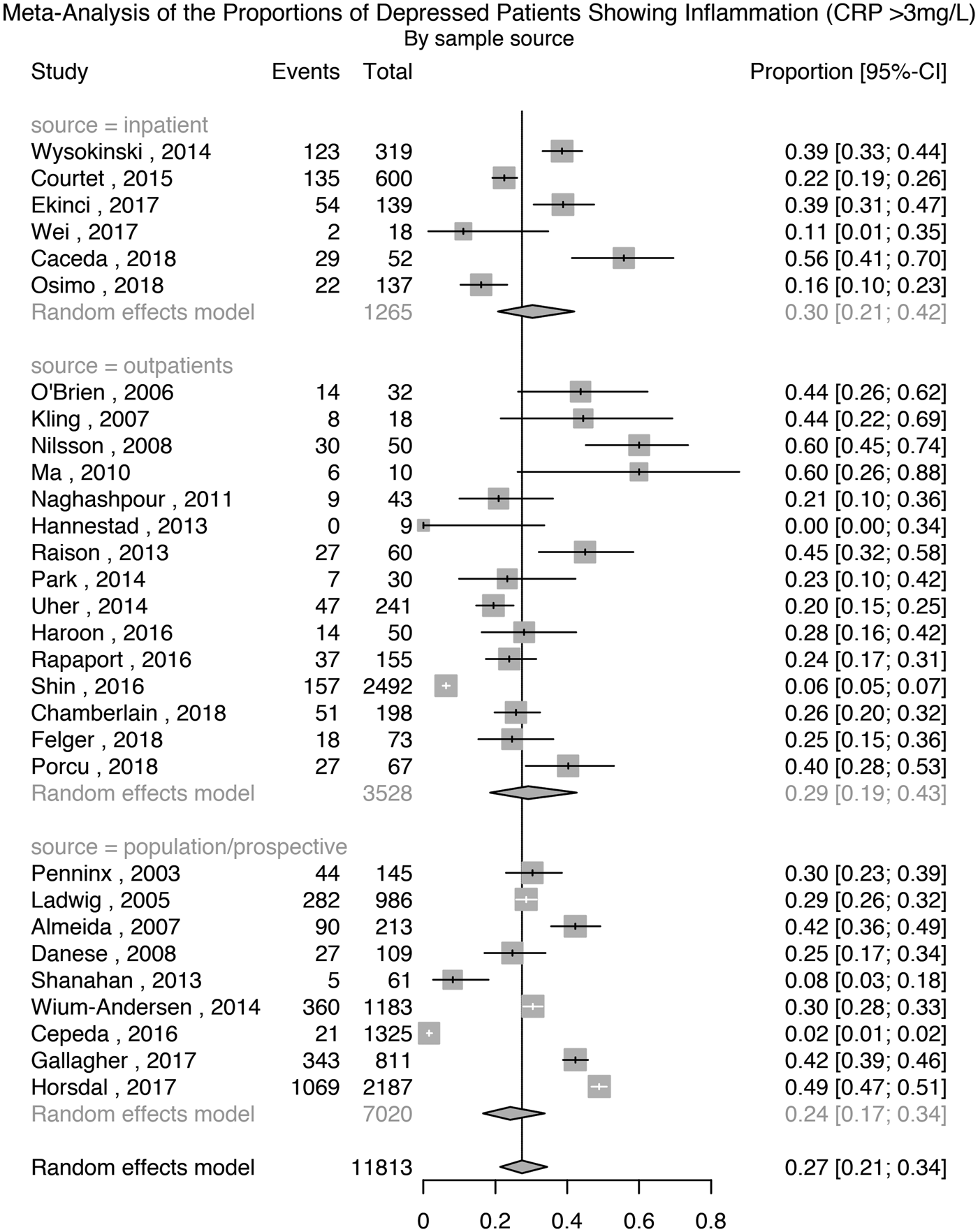
Fig. 1. Prevalence of low-grade inflammation (CRP >3 mg/L) in depressed patients.
Analyses excluding poor quality studies or cases with past depression
A sensitivity analysis excluding six poor quality studies, comprising 8778 patients, showed that the prevalence of inflammation was 27% (95% CI 22–33%); Supplementary Table S1 and Fig. S2. There was evidence of heterogeneity (I 2 = 96.4%; 95% CI 95.5–97.1%; Cochrane's Q = 644; p = <0.01). A sensitivity analysis excluding two studies where depression was not active in all patients, comprising 11 763 patients, showed that the prevalence of inflammation was 26% (95% CI 20–34%); Supplementary Fig. S3. There was evidence of heterogeneity (I 2 = 97.9%; 95% CI 97.4–98.2%; Cochrane's Q = 1261; p = <0.01).
Analysis after excluding cases of suspected infection (CRP >10 mg/L)
Nine studies reported the prevalence of low-grade inflammation after excluding participants with suspected infection, defined as CRP >10 mg/L. A separate meta-analysis based on these studies, comprising 6948 patients, showed that the prevalence of inflammation was 16% (95% CI 8–32%); Supplementary Fig. S4. There was evidence of heterogeneity (I 2 = 98.8%; 95% CI 98.5–99.1%; Cochrane's Q = 675; p = <0.01).
Association between prevalence of low-grade inflammation (CRP >3 mg/L) and characteristics of depressed patients
Meta-regression was used on 19 studies comprising 7858 patients to test the association between the prevalence of inflammation and the proportion of patients who were antidepressant-free at the time of study. There was no association between these factors (estimate: −0.007; z = −1.03; p = 0.30). Similarly, sex, age, non-White ethnicity, BMI and sample source (inpatient, outpatient or population-based) were not associated with the prevalence of inflammation (see Supplementary Results).
Assessment of publication bias
A funnel plot of the 30 studies assessing the prevalence of low-grade inflammation (CRP >3 mg/L) in depression visually appeared symmetrical (Supplementary Fig. S5). Egger's regression test for funnel plot asymmetry was non-significant (t = −1.3; df = 28; p = 0.21), suggesting there was no evidence of publication bias.
Odds ratio for low-grade inflammation (>3 mg/L) in depressed patients
Seventeen studies reported the prevalence of inflammation in 7761 depressed patients and 155 728 matched non-depressed controls (see Supplementary Table S2 for matching details). The meta-analytic OR for inflammation in depressed patients compared with matched controls was 1.46 (95% CI 1.22–1.75; p < 0.0001); see Fig. 2. There was evidence of heterogeneity (I 2 = 71.9%; 95% CI 54.3–82.7%; Cochrane's Q = 57; p = <0.01).
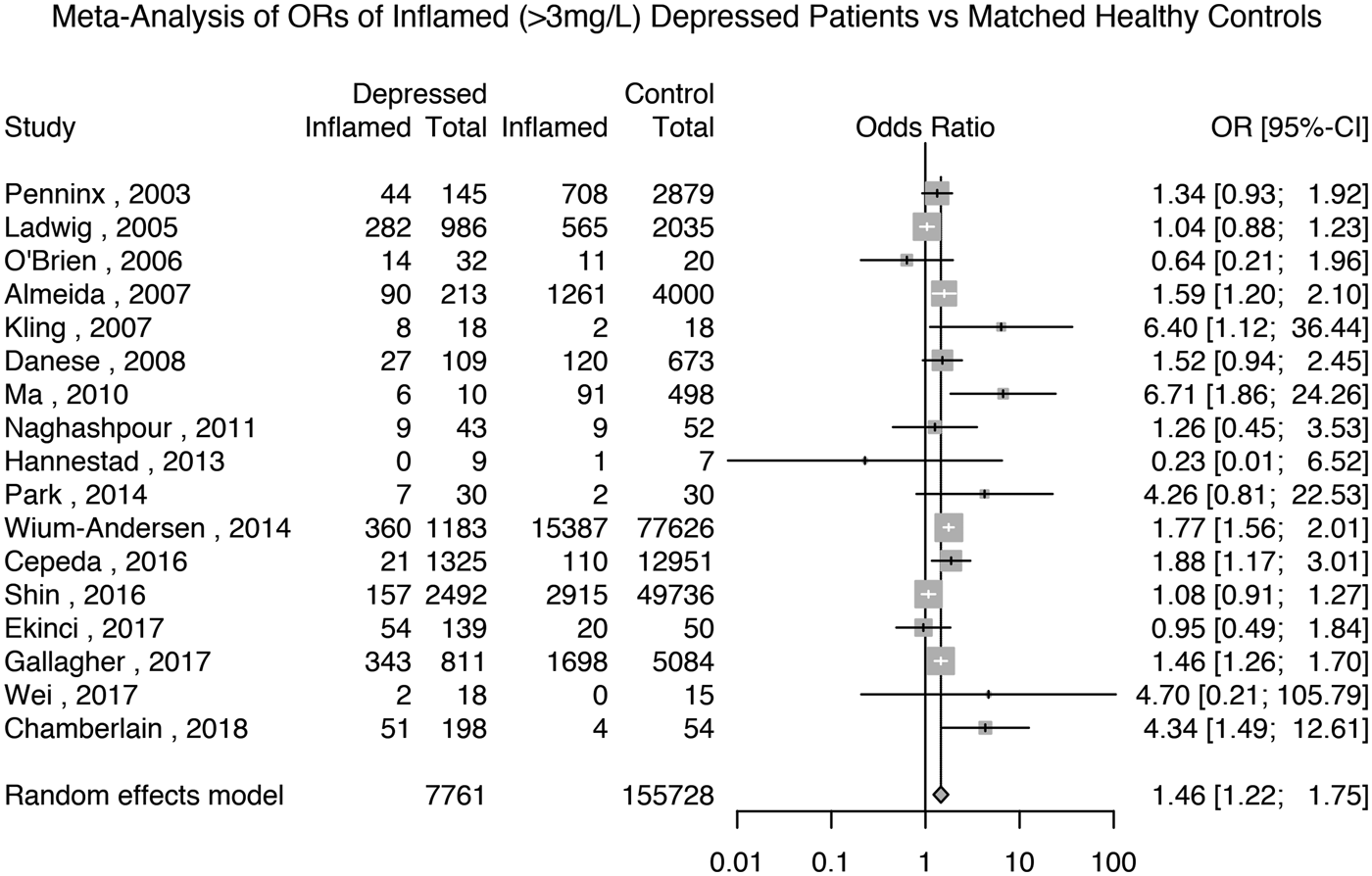
Fig. 2. Odds ratio for low-grade inflammation (CRP >3 mg/L) in depressed patients compared with matched controls.
Based on the same studies, we meta-analysed the prevalence of inflammation in depressed patients and matched non-depressed controls separately. The prevalence of inflammation in controls was 16% (95% CI 11–23%) and that in depressed patients was 24% (95% CI 17–34%); see Supplementary Figs S6 and S7.
We carried out sensitivity analyses based on five available studies of depressed patients and matched healthy controls that excluded subjects with very high levels of CRP (>10 mg/L). These studies, comprising 3868 patients and 63 212 controls, showed that the prevalence of inflammation in controls was 10% (95% CI 3–26%) and that in patients it was 13% (95% CI 4–36%); see Supplementary Figs S8 and S9. Based on these studies, the meta-analytic OR for inflammation in depressed patients compared with matched controls was 1.44 (95% CI 0.80–2.61; p = 0.23); see Supplementary Fig. S10.
A sensitivity analysis excluding poor quality studies, comprising 5045 patients and 105 372 controls, showed that the meta-analytic OR for inflammation in depressed patients compared with matched controls was 1.56 (95% CI 1.29–1.88; p < 0.0001); see Supplementary Table S1 and Fig. S11. A further sensitivity analysis only including studies that matched patients and controls by BMI, comprising 2624 patients and 79 887 controls, showed that the meta-analytic OR for inflammation in depressed patients compared with matched controls was 1.59 (95% CI 1.08–2.34; p = 0.02); see Supplementary Fig. S12. Finally, a sensitivity analysis excluding studies where depression was not active in all patients showed that the meta-analytic OR for inflammation in depressed patients compared with matched controls was 1.47 (95% CI 1.22–1.75; p = 0.02); see Supplementary Fig. S13.
Prevalence of elevated CRP levels (>1 mg/L) in depressed patients
Results based on all available studies
Twenty-five studies comprising 8887 patients with depression were used for this analysis. The meta-analytic pooled prevalence of elevated CRP >1 mg/L in depressed patients was 58% (95% CI 47–69%); see Fig. 3. There was evidence of heterogeneity (I 2 = 98.7%; 95% CI 98.5–98.9%; Cochrane's Q = 1862; p = <0.01). Further analyses after grouping studies by setting showed that the prevalence of elevated CRP in inpatient samples (N = 1023) was 56% (95% CI 46–66%; I 2 = 81.8%; Cochrane's Q = 22); in outpatient samples (N = 697) was 59% (95% CI 50–67%; I 2 = 74.9%; Cochrane's Q = 40); and in population-based samples (N = 7167) was 57% (95% CI 34–77%; I 2 = 99.5%; Cochrane's Q = 1774).
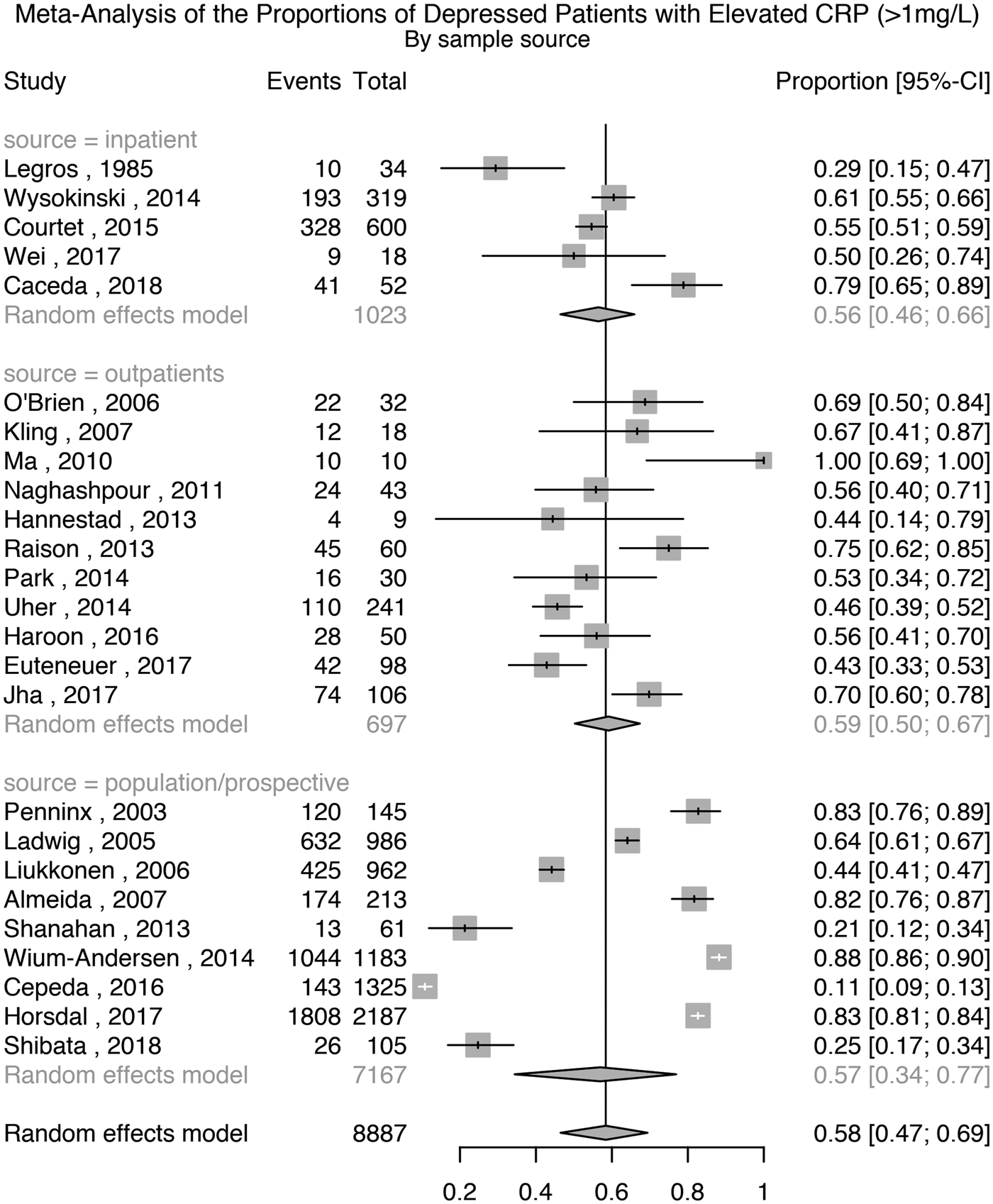
Fig. 3. Prevalence of elevated CRP (>1 mg/L) in depressed patients.
Analyses excluding poor quality studies or cases with past depression
A sensitivity analysis excluding four poor quality studies showed that the prevalence of elevated CRP >1 mg/L in depressed patients was 57% (95% CI 43–69%); Supplementary Table S1 and Fig. S14. There was evidence of heterogeneity (I 2 = 98.9%; 95% CI 98.7–99.1%; Cochrane's Q = 1858; p = <0.01). A further sensitivity analysis excluding studies where depression was not active in all patients showed that the prevalence of elevated CRP in depressed patients was 58% (95% CI 45–69%); Supplementary Fig. S15. There was evidence of heterogeneity (I 2 = 98.8%; 95% CI 98.6–99.0%; Cochrane's Q = 1861; p = <0.01).
Analysis after excluding cases of suspected infection (CRP>10 mg/L)
Eight studies also reported the prevalence of elevated CRP after excluding participants with suspected infection, defined as CRP >10 mg/L. A separate meta-analysis based on these studies, comprising 4456 patients that excluded patients with CRP levels >10 mg/L showed that the prevalence of elevated CRP >1 mg/L was 50% (95% CI 29–72%); see Supplementary Fig. S16 There was evidence of heterogeneity (I 2 = 99.1%; 95% CI 98.9–99.3%; Cochrane's Q = 816; p = <0.01).
Association between prevalence of elevated CRP levels (>1 mg/L) and characteristics of depressed patients
Meta-regression analyses did not find any significant association of elevated CRP with sex, age, BMI, non-White ethnicity, being antidepressant-free or sample source (see Supplementary Results).
Assessment of publication bias
A funnel plot of the 25 studies assessing the prevalence of elevated CRP in depression appeared visually symmetrical. Egger's regression test for funnel plot asymmetry was non-significant (t = −0.43; df = 23; p = 0.67), suggesting there was no evidence of publication bias (Supplementary Fig. S17).
Odds ratio for elevated CRP levels (>1 mg/L) in depressed patients
Fifteen studies reported the prevalence of elevated CRP >1 mg/L in 5177 depressed patients and 106 682 matched non-depressed controls (see Supplementary Table S2 for matching details). The meta-analytic OR for elevated CRP in depressed patients compared with matched controls was 1.47 (95% CI 1.18–1.82; p = 0.0005); see Fig. 4. There was evidence of heterogeneity (I 2 = 75.6%; 95% CI 59.8–85.2%; Cochrane's Q = 57; p = <0.01).
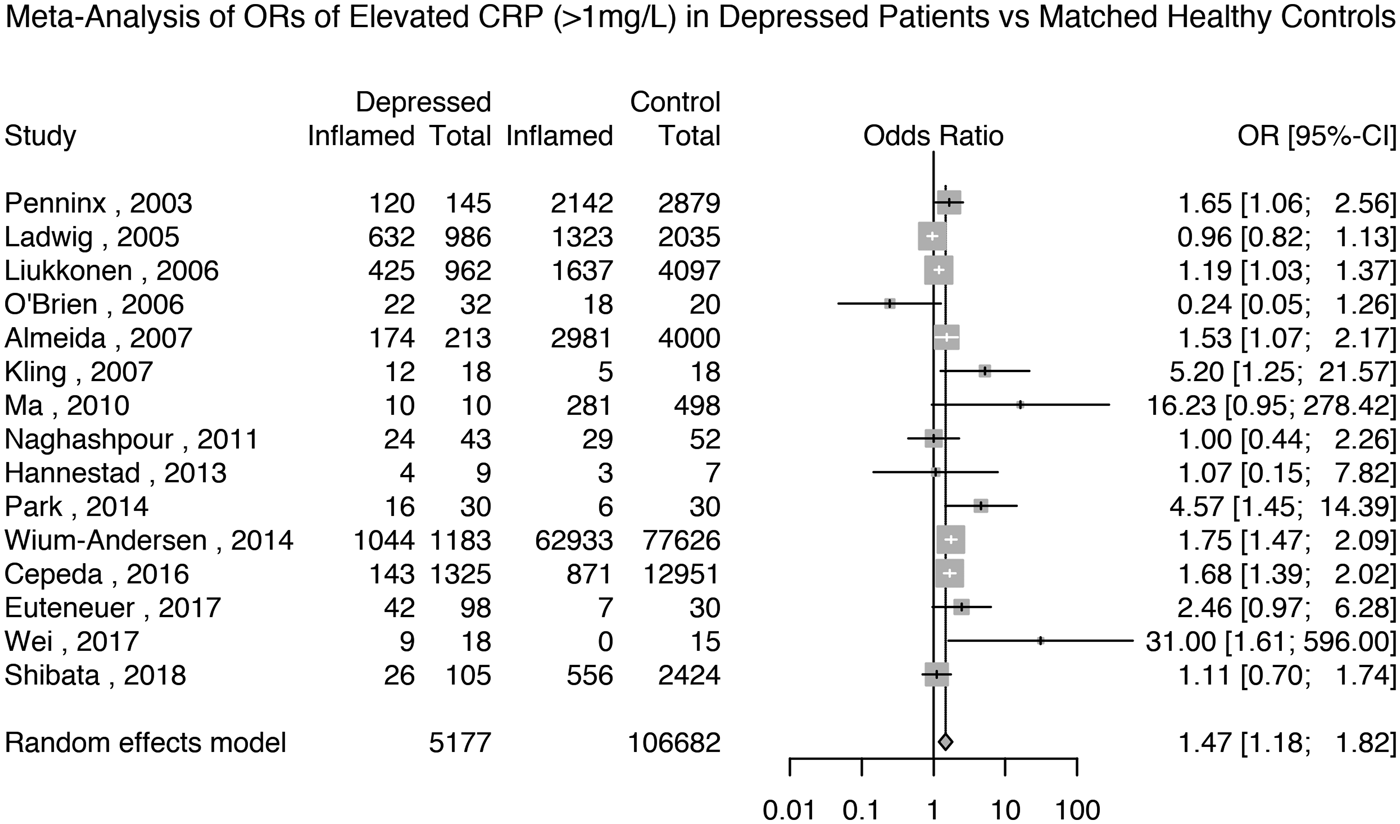
Fig. 4. Odds ratio for elevated CRP (>1 mg/L) in depressed patients compared with matched controls.
Based on the same studies, we meta-analysed the prevalence of elevated CRP in depressed patients and matched non-depressed controls separately. The prevalence of elevated CRP >1 mg/L in controls was 44% (95% CI 26–65%) and that in depressed patients was 59% (95% CI 41–75%); see Supplementary Figs S18 and S19.
A sensitivity analysis based on 12 studies after excluding poor quality studies showed that the meta-analytic OR for elevated CRP in depressed patients compared with matched controls was 1.51 (95% CI 1.22–1.88; p = 0.0002); see Supplementary Table S1 and Fig. S20. A sensitivity analysis only including the nine studies that matched the patients and controls by BMI showed that the meta-analytic OR for elevated CRP >1 mg/L in depressed patients compared with matched controls was 1.52 (95% CI 1.12–2.07; p = 0.01); see Supplementary Fig. S21. A further sensitivity analysis excluding studies where depression was not active in all patients showed that the meta-analytic OR for elevated CRP in depressed patients compared with healthy controls was 1.47 (95% CI 1.19–1.81; p = 0.0003); see Supplementary Fig. S22. Finally, a sensitivity analysis of the four studies excluding subjects with very high levels of CRP (>10 mg/L) showed that the meta-analytic OR for elevated CRP in depressed patients compared with healthy controls was 1.29 (95% CI 0.38–4.30; p = 0.68); see Supplementary Fig. S23.
Very high CRP levels (>10 mg/L) in depressed patients and healthy controls
We used data from four available studies comprising 3926 patients and 62 748 matched healthy controls. The meta-analytic pooled prevalence of very high CRP in depressed patients matched to healthy controls was 3% (95% CI 1–11%); in the same studies, prevalence of very high CRP in healthy controls matched to depressed patients was 1% (95% CI 0–4%); the meta-analytic OR for very high CRP in depressed patients compared with matched controls was 1.52 (95% CI 1.13–2.05; p = 0.006); see Supplementary Figs S24–26.
Discussion
To our knowledge, this is one of the first systematic reviews and meta-analyses of the prevalence of low-grade inflammation in patients with depression. We report that a notable proportion of depressed patients show evidence of inflammation. Approximately one in four patients with depression show CRP levels >3 mg/L, a widely used threshold to define low-grade inflammation in the literature. The prevalence is unaltered after excluding poor quality studies, or after excluding studies where depression was not active. After excluding patients with suspected infection, the prevalence of low-grade inflammation is about one in six. We also report that approximately three patients out of five have mildly elevated CRP (>1 mg/L). The prevalence is unaltered after excluding poor quality studies, or after excluding studies where depression was not active. After excluding patients with suspected infection, the prevalence of elevated CRP is one in two. Meta-regression analyses show that the prevalence of inflammation in depression is not associated with sex, age, BMI, ethnicity or sample source.
Using matched non-depressed controls, we quantified the odds ratios of low-grade inflammation and of elevated CRP in depressed patients. We report that the proportion of patients with depression showing elevated inflammatory markers as compared to matched healthy controls is remarkably stable: the ORs were 1.46 for CRP levels >3 mg/L, 1.47 for CRP levels >1 mg/L and 1.52 for CRP levels >10 mg/L. There was no evidence of publication bias within the included studies, but there was evidence of heterogeneity in all analyses.
Knowing inflammation levels in patients with depression could be important for several reasons, particularly for predicting the risk of physical illness and for predicting response to psychiatric treatment. Inflammation is a potentially causal risk factor for CVD (Pearson et al., Reference Pearson, Mensah, Alexander, Anderson, Cannon III, Criqui, Fadl, Fortmann, Hong and Myers2003), because CVD is associated with circulating IL-6 and CRP levels (Pradhan et al., Reference Pradhan, Manson, Rossouw, Siscovick, Mouton, Rifai, Wallace, Jackson, Pettinger and Ridker2002; Danesh et al., Reference Danesh, Wheeler, Hirschfield, Eda, Eiriksdottir, Rumley, Lowe, Pepys and Gudnason2004; Danesh et al., Reference Danesh, Kaptoge, Mann, Sarwar, Wood, Angleman, Wensley, Higgins, Lennon and Eiriksdottir2008) and with genetic variants regulating levels/activity of IL-6 (IL6R Genetics Consortium Emerging Risk Factors Collaboration, 2012; Interleukin-6 Receptor Mendelian Randomisation Analysis Consortium, 2012). Depression is co-morbid with CVD (Hare et al., Reference Hare, Toukhsati, Johansson and Jaarsma2013). Depression increases the risk of incident CVD, and is a marker of poor prognosis after myocardial infarction (Nicholson et al., Reference Nicholson, Kuper and Hemingway2006). Inflammation could be a shared mechanism for these conditions. Using Mendelian randomisation analysis of the UK Biobank sample, we previously found that out of all cardiovascular risk factors, IL-6, CRP and triglycerides are likely to be causally linked with depression (Khandaker et al., Reference Khandaker, Zuber, Rees, Carvalho, Mason, Foley, Gkatzionis, Jones and Burgess2019). Therefore, cardiovascular risk screening in depressed patients who show evidence of inflammation could be useful. Our work suggests that such screening will be relevant for about a quarter of patients with depression.
We focussed on CRP levels as our preferred measure of inflammation because it has been widely used in different fields of medicine to measure inflammation, and standardised cut-offs for CRP exist in the literature. The American Heart Association and Center for Disease Control and Prevention have proposed clear CRP thresholds as indicators of inflammation levels (<1 = ‘low’, 1–3 = ‘medium’, >3 mg/L = ‘high’) (Pearson et al., Reference Pearson, Mensah, Alexander, Anderson, Cannon III, Criqui, Fadl, Fortmann, Hong and Myers2003). Our findings are consistent with previous meta-analyses reporting higher mean concentrations of CRP, IL-6 and other inflammatory markers in depressed patients compared with controls (Howren et al., Reference Howren, Lamkin and Suls2009; Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctôt2010; Haapakoski et al., Reference Haapakoski, Mathieu, Ebmeier, Alenius and Kivimäki2015; Goldsmith et al., Reference Goldsmith, Rapaport and Miller2016). Our study adds to the literature by providing information on the proportion of depressed patients who have evidence of inflammation.
In addition to depression and CVD, inflammation is associated with other physical and psychiatric disorders including diabetes mellitus (Pradhan et al., Reference Pradhan, Manson, Rifai, Buring and Ridker2001), schizophrenia (Miller et al., Reference Miller, Buckley, Seabolt, Mellor and Kirkpatrick2011; Khandaker et al., Reference Khandaker, Cousins, Deakin, Lennox, Yolken and Jones2015) and dementias (Schmidt et al., Reference Schmidt, Schmidt, Curb, Masaki, White and Launer2002). Inflammation is also an important predictor of increased all-cause mortality (Zacho et al., Reference Zacho, Tybjærg-Hansen and Nordestgaard2010; Sung et al., Reference Sung, Ryu, Chang, Byrne and Kim2014; Li et al., Reference Li, Zhong, Cheng, Zhao, Zhang, Hong, Wan, He and Wang2017). Therefore, routine CRP screening in patients with depression, and identification and treatment of the cause of inflammation could improve overall health-related mortality and morbidity. Public health interventions aimed at reducing inflammation could improve mortality and morbidity associated with a number of conditions.
It is unlikely that anti-inflammatory drugs will be useful for all patients with depression (Khandaker et al., Reference Khandaker, Dantzer and Jones2017). Measurement of CRP levels could inform patient selection in RCTs of anti-inflammatory drugs for depression. We are aware of two studies that are testing novel anti-inflammatory drugs such as monoclonal antibodies (mAb) against the IL-6/IL-6R pathway. One study testing the efficacy and safety of sirukumab (anti-IL-6 mAb) for depression has completed recruitment (NCT02473289). We are conducting an RCT of tocilizumab (anti-IL-6R mAb) for patients with depression (Khandaker et al., Reference Khandaker, Oltean, Kaser, Dibben, Ramana, Jadon, Dantzer, Coles, Lewis and Jones2018). Both of these studies are based on patients with CRP levels ⩾3 mg/L. Secondary analysis of existing RCTs suggests mAb against specific inflammatory cytokines, such as IL-6/IL-6R, could be helpful for depression (Sun et al., Reference Sun, Wang, Salvadore, Hsu, Curran, Casper, Vermeulen, Kent, Singh and Drevets2017; Kappelmann et al., Reference Kappelmann, Lewis, Dantzer, Jones and Khandaker2018). However, definitive efficacy trials need to be completed before anti-inflammatory drugs can be considered in psychiatric clinical practice. Our findings suggest that up to a quarter of depressed patients show signs of low-grade inflammation. Future studies should explore the potential causes for this, and also whether depressed patients with higher CRP levels may benefit from anti-inflammatory treatments.
Studies included in this review varied on setting, country and analytic methods, and the proportion of depressed patients with elevated CRP (>3 mg/L) in these studies ranged between 0% and 60%. In our analyses, the prevalence of inflammation was not associated with participant age and sex, antidepressant treatment, ethnicity or source of sample. This is the case despite the samples spanning all age groups [median age: 42.2 years; inter-quartile range (IQR): 37–59]. Both sexes were well represented (median proportion of males: 36%, IQR: 17–41%). The samples comprised both antidepressant-free and treated populations (antidepressant-free = 6 studies; 100% treated = 3 studies; mixed populations = 10 studies). Included studies covered samples collected from inpatient (N = 6), outpatient (N = 15) and general population (N = 9). One reason for not detecting an association between inflammation and sociodemographic factors could be that a number of studies matched patients and controls on these factors.
The meta-analytic prevalence of low-grade inflammation (CRP >3 mg/L) in non-depressed controls seen in our analysis is 16%, which is lower than the prevalence of inflammation reported in some general population studies. For instance, Ford et al. (Reference Ford, Giles, Mokdad and Myers2004) reported the prevalence of low-grade inflammation to be about 25% in a sample of adult US women. Khera et al. (Reference Khera, McGuire, Murphy, Stanek, Das, Vongpatanasin, Wians, Grundy and de Lemos2005) reported the prevalence of CRP >3 mg/L to be >30% in US adult males and females. One reason for these high prevalence reports in general population samples could be that these studies include both healthy and diseased individuals including those with chronic inflammatory physical illness. Therefore, for a more accurate comparison of the prevalence of inflammation between depressed cases and healthy controls, we have used studies that included cases matched to healthy controls for the calculation of odds ratios. In our results, the stability of the odds ratios for elevated CRP in depressed patients compared with healthy controls across different CRP thresholds (ORs = 1.46 for CRP levels >3 mg/L; OR = 1.47 for CRP levels >1 mg/L; and OR = 1.52 for CRP levels >10 mg/L) provides confidence that patients are more likely to have evidence of inflammation than healthy controls. Furthermore, excluding patients with very high levels of inflammation did not significantly affect the odds ratio for low-grade inflammation (>3 mg/L) in depressed subjects (OR = 1.44).
Strengths of this work include the systematic nature of the literature search, which identified a large number of relevant studies comprising 13 541 patients and 155 728 controls from different countries and settings, and spanning diverse ethnic and age groups. The methods were laid out prospectively and published on PROSPERO (Osimo et al., Reference Osimo, Baxter, Jones and Khandaker2018a). We assessed the studies for quality using the validated Newcastle–Ottawa Scale (Stang, Reference Stang2010). We conducted multiple sensitivity analyses to examine the robustness of the findings. There was no evidence of publication bias, suggesting that we covered a range of results spanning the whole expected distribution of means.
Limitations of this work include sample heterogeneity: the studies we included used different methods to assess depression (albeit a valid method was required for inclusion), and samples were recruited from different sources making it difficult to test the association between the prevalence of inflammation and depression severity. However, we have reported meta-analytic results separately by sample source (community, inpatient, etc.), which could be taken as an indirect indicator of depression severity. Inflammation prevalence did not differ much by sample source. However, due to the lack of comparable data on depression severity, we could not assess this directly. Study setting and sample characteristics could account for some of the observed heterogeneity. We used random-effects meta-analyses in order to take care of inter-study variability. Another limitation is that we were not able to account for comorbidities, partly because for some studies this information was not reported. By design we have focused on dichotomous measure of inflammation, so we cannot comment on the distributions of continuous CRP values in patients/controls; these have been subject to previous meta-analyses reporting higher mean levels of CRP in depression compared with controls (Howren et al., Reference Howren, Lamkin and Suls2009; Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctôt2010; Haapakoski et al., Reference Haapakoski, Mathieu, Ebmeier, Alenius and Kivimäki2015; Goldsmith et al., Reference Goldsmith, Rapaport and Miller2016).
In summary, this systematic review and meta-analysis provides a robust estimate of the prevalence of low-grade inflammation in depressed patients, which is about one in four. We also report that depressed patients are about 50% more likely to have evidence of inflammation as compared to matched non-depressed controls. These findings are relevant for future treatment studies of anti-inflammatory drugs and for clinical practice, particularly for predicting response to antidepressants and for predicting co-morbid, immune-related physical illness, such as CVD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719001454
Author ORCIDs
Emanuele Felice Osimo, 0000-0001-6239-5691
Acknowledgements
Dr Khandaker acknowledges grant support from the Wellcome Trust (201486/Z/16/Z), UK Medical Research Council (MC_PC_17213), and MQ: Transforming Mental Health (MQDS17/40). PBJ acknowledges grant support from the Wellcome Trust (095844/Z/11/Z & 088869/Z/09/Z) and NIHR [RP-PG-0616-20003 and the Collaboration for Leadership in Applied Health Research & Care (CLAHRC) East of England]. The funding bodies had no role in design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Financial disclosures
The authors have no conflict of interests or financial disclosures to declare.







