INTRODUCTION
Wild birds represent the reservoir of all type A influenza viruses that infect mammals [Reference Hinshaw, Webster and Beare1]. However, the transmission pathways and specific adaptations required for avian viruses to become established in mammalian populations, especially human populations, are poorly defined due to the complexity and diversity of potential interfaces connecting wildlife and human populations. Infection of humans with avian strains through direct contact with infected domestic bird populations has been reported [Reference Beigel2], and highly pathogenic avian influenza (HPAI) H5N1 has been directly transmitted from wild swans to humans in Azerbaijan [Reference Gilsdorf3]. It has been suggested that direct transmission of type A influenza viruses can occur between ducks, a major wildlife reservoir for these viruses, and humans [4]. Thus, human contact with wild ducks during hunting or bird-banding may be a potential exposure pathway to the virus, and this possibility is supported by the detection of antibodies in humans to influenza virus subtypes (H11) found only in wild birds [Reference Gill5].
Direct transmission of a type A influenza from a wild bird to a waterfowl hunter or biologist might have at least two significant outcomes; a direct introduction of a novel virus that could be sustained by human-to-human transmission, or a possible reassortment event where avian genes could be incorporated into an existing seasonal human influenza strain. Such reassortments previously have been demonstrated, but it is unknown if they resulted from human–domestic animal contact or direct contact with wild birds [Reference Claas6]. As contact is the initial prerequisite for any transmission event, it is important to understand that the risk of contact between a reservoir and a potential new host species (in this case wild ducks and humans) is influenced by seasonal, temporal, and population- or species-based variation in virus prevalence within the reservoir as well as variation in contact rates associated with human behaviour. Human behaviour can be characterized by hunting-specific behaviours brought about by regulatory constraints and selective individual behaviours that may influence the probability of virus exposure [Reference Dishman, Stallknecht and Cole7]. Similarly, the probability of a reassortment event is dependent on the co-infection with a human strain which also is seasonally dependent.
This study quantitatively estimated the risk of human exposure to avian influenza viruses associated with waterfowl hunting activities, particularly hunting of ducks and geese. The effects of temporal and spatial variation were taken into account by modelling the exposure risk associated with hunting activities from September to January (based on the dates for the hunting season of 2010–2011) in three different states: Georgia (GA), Louisiana (LA) and Minnesota (MN). Differences in hunting practices and intensity among these states, as well as the difference in avian influenza virus (AIV) prevalence between juvenile and adult birds were accounted for. Exposure risk (per hunter-day) is compared among states to evaluate the potential for regional variation, which has great application to risk management. Finally, the monthly variation in exposure risk is compared to the seasonal incidence of human influenza A viruses in order to discuss the potential for co-infection.
MATERIALS AND METHODS
Hunting season data
Potential hunter exposure to avian type A influenza viruses during hunting activities were evaluated for GA, LA, and MN. MN and LA are both located in the Mississippi flyway, but their hunting season months are different. In MN, duck hunting occurs in October and November, and geese are hunted from September to December [8]. In LA there is an early teal season in September as well as the regular duck hunting season that runs from November to January; geese are hunted from November to January [9]. In 2010, 69 600 duck hunters and 51 600 goose hunters harvested 524 000 ducks and 190 400 geese, respectively, in MN, while in LA 89 300 duck hunters and 10 700 goose hunters harvested more than 2·7 million ducks and 65 100 geese – the largest harvest in the USA [Reference Raftovich10]. GA is located in the Atlantic flyway, and the duck season coincides with that of LA [11], except that geese are hunted in September, as in MN. However, the numbers of hunters and harvested waterfowl are considerably lower than in LA – 21 900 and 8800 hunters harvested 218 600 ducks and 23 700 geese in GA, respectively, in 2010 [Reference Raftovich10].
A unit representing one individual hunter's harvest activity during a single day (hunter-day) was used to calculate exposure risk, since multiple days of hunting by the same hunter represent independent exposure events. Assuming that most hunting activities occur on weekends, the total number of hunter-days in a state's hunting season were distributed across the study months by assigning each month a weight based on the number of weekends of open season occurring during that month [8, 9, 11]. For instance, in GA the months of September, November, December and January had, respectively, 3, 2, 3 and 5 weekends of duck hunting activities in the season 2010–2011 (Table 1), for 13 hunting weekends in total. The total number of duck hunter-days in this state was multiplied by 3/13, 2/13, 3/13 and 5/13 to calculate the number of hunter-days in each month, and rounded to the nearest integer. Special season dates for specific regions within a state were not individually specified.
Table 1. Parameters used in the model to assess the risk of exposure of duck and goose hunters to avian influenza viruses in three US states

Juv, Juvenile (hatching year) birds; Adt, adult birds; EID, egg infective dose.
* Based on the proportion juveniles/adults reported for mallard ducks.
† Prevalence in December and January was adjusted according to expert opinion (see text for details).
Exposure assessment
Data used to calculate hunter exposure probabilities are summarized by month in Table 1. The model was set up in R 2.14.2 (free software environment available at http://www.r-project.org/). Values drawn from probability distributions were sampled through 1 000 000 iterations. Exposure was assessed independently for duck and goose hunters. Risk was assumed to depend on the species and number of birds harvested per hunter-day in each state [Reference Wagner12]. Although a hunter may hunt both ducks and geese throughout the season, the risks were calculated independently for hunter-days where geese were hunted and hunter-days where ducks were hunted.
Exposure per hunter-day was estimated as the product of the number of harvested birds shedding virus, the viral load per gram faecal dropping shed by an infected bird, and the number of grams per fecal dropping. The mathematical expression for the calculation is as follows:
where exposure (E) is assumed to be a random variable dependent upon the expected number of infected birds (B) of species i (duck or goose) harvested in a given day by a single hunter in month t and state s and the viral load shed by infected birds. The average weight of each duck dropping was assumed to be 4·4 g [Reference Murphy13]. Due to the absence of data, we assumed that viral shedding in infected geese is similar to shedding in ducks, which is likely to be an overestimation.
As the probability of a duck being infected depends on age, the total number of harvested birds per hunter-day was stratified into juveniles and adults, based on available data from mallard populations [9]. Thus, the final estimation of the number of a given species of harvested birds shedding virus is described by the following binomial probability distribution:
where λ is the expected number of birds of species i (duck or goose) of age j (juvenile or adult) harvested in a given day by a single hunter in state s, and P ijt is the probability that a harvested bird of species i of age j is shedding AIV in month t (Table 1). All iterations in which the number of infected harvested birds was zero were counted as hunter-days with no virus exposure.
The value λijs was defined by a Poisson distribution as outlined below, with the mean equal to the total number of ducks harvested in each state divided by the total number of hunter-days in that state for the same year [Reference Raftovich10] (Table 1):
The choice of a Poisson distribution was based on the fact that the states impose a maximum number of harvested birds per hunter, which is assumed to limit overdispersion in the distribution.
The probability of infection in ducks each month (P ijt) was estimated based on published prevalence data (Table 1) where sampling month and bird age were included [Reference Stallknecht14–Reference Hanson16]. Only data from LA were used to calculate virus prevalence in ducks in September; prevalence in early migrating teal that would be encountered by hunters in LA is lower than that described for birds on northern staging areas [Reference Stallknecht14]. We assumed that this also would be the case in GA. Similarly, only data from the surveys in MN, New York and Alberta were used to calculate the prevalence in October for MN. Based on the literature, the prevalence for December and January is 0·3% in juveniles and 0% in adults. However, adjustments based on expert opinion were made to avoid 0% prevalence, as the finding is believed to be due to a low sensitivity of the method, not to a true zero prevalence. Prevalence data for geese were not readily available; however, compared to studies on duck populations, AIV prevalence estimates for geese are generally low throughout the year. For the analysis we used estimates of infection ranging from 1% to 2% for juveniles and 0·1% to 0·5% for adults [Reference Stallknecht17] (Table 1).
The expected viral load, measured in egg infectious dose (EID), in 1 g of duck faeces was calculated based on unpublished data (J. Brown) that provided virus titres for mallard faeces from days 1–15 post-infection with H3N8 AIV. The data comes from a study that followed eight birds, and the total number of observations was 55. These data were used to fit a probability distribution using the distribution fitting tool (parameter estimation method) of @Risk 5.5 (Palisade Corporation, USA) for continuous data, with no filtering, and a lower bound of zero. The best fit, as determined by χ2 ranking, was a normal distribution of the log10 EID with mean 5·6 and standard deviation 2 log10. This distribution is also consistent with the estimates of Schijven et al. [Reference Schijven, Teunis and de Roda Husman18] in their quantitative risk assessment of influenza virus infection via water.
It was expected that a large number of hunter-days would result in no viral exposure, considering the low prevalence of AIV among birds. No viral exposure resulted if no infected birds were harvested in a given model iteration. The percentage of iterations resulting non-zero exposure (probability that at least one infected bird is harvested) was recorded as Enon-zeroist. In cases when viral exposure was estimated to occur, the non-zero value calculated for the random variable E ist was recorded in a variable estimating the expected viral load in case of exposure, Eviral-loadist.
Population risk
The total number of hunter-days per month (Table 1) was calculated from the total reported for 2010 [Reference Raftovich10], the most recent year available at the time of the study. Assuming that the risk of infection per hunter-day is directly proportional to the AIV exposure risk (probability of inoculation and dose-response having the same expected values in all states and months), we applied the exposure risk to the population of hunters per state and month to give a graphical representation of when and where the population risk would be higher. Since no assumption was made regarding the probability of infection, the population risk calculated is intended to reflect only the states and months associated with higher risk of exposure based on the percentage of hunter-days exposed and the viral load per exposed hunter-day.
The proportion of hunter-days resulting in viral exposure (Enon-zeroist) was multiplied by the number of hunter-days for species i, in state s and month t, in order to determine the number of exposed hunter-days. When exposure was estimated to occur, the expected viral load was given by the distribution of the non-zero values of the random variable, Eviral-loadist.
The total population risk was therefore calculated as follows:
 $$\eqalign{ \tab{\rm Population\ risk}_{st}\cr \tab \quad \equals \sum _{i} \lpar {\rm hunter} \hbox {-} {\rm days}_{ist} \lowast E{\rm non} \hbox {-} {\rm zero}_{ist} \lowast E{\rm viral} \hbox {-} {\rm load}_{ist} \rpar . \cr} $$
$$\eqalign{ \tab{\rm Population\ risk}_{st}\cr \tab \quad \equals \sum _{i} \lpar {\rm hunter} \hbox {-} {\rm days}_{ist} \lowast E{\rm non} \hbox {-} {\rm zero}_{ist} \lowast E{\rm viral} \hbox {-} {\rm load}_{ist} \rpar . \cr} $$Although this does not represent a true quantitative estimate of the population risk of AIV infection it does provide an estimation of the variation in expected relative risk among study states and months. This graphical result was overlaid with the estimated prevalence of human type A influenza virus infections during the same period to evaluate the likelihood of co-infection with avian and human viruses. Information on the number of laboratory-confirmed cases of influenza A infection by month for 2005–2010 was obtained by summing weekly information published by the Centers for Disease Control for each study state individually [19]. The incidence of human influenza infection cannot be determined directly from surveillance data, but it is assumed that the true incidence is proportional to the number of laboratory-confirmed cases in surveillance sites [Reference Reed20]. The graphical representation of both the population risk of AIV exposure and the risk of infection with human type A influenza virus does not contain any mathematical scale, but visually compares proportional risk along time.
RESULTS
The majority of exposure simulations resulted in no hunter exposure to influenza A viruses from hunted waterfowl during a hunter-day. Of the iterations for each of the state/month scenarios, 91–99% resulted in no viral exposure of duck hunters (none of the harvested birds were infected), and 98·6–99·7% of the iterations for goose hunters also resulted in no exposure. Figure 1 provides the proportion of hunter-days resulting in exposure to AIV by state and hunting month, and the distribution of the viral load when exposure occurs. While the probability of exposure varied among states and across months, the median viral load of exposure showed little variation.

Fig. 1. Summary measures for the random variable Eviral-loadist, which represents the estimated viral load a hunter is exposed to when a hunter-day results in exposure. The right axis indicates the percentage of iterations which resulted in non-zero exposure (Enon-zeroist) (![]() ). GA, Georgia; LA, Louisiana; MN, Minnesota.
). GA, Georgia; LA, Louisiana; MN, Minnesota.
The probability of exposure (Enon-zeroist) per hunter-day within each state reflects the AIV prevalence in wild waterfowl, with a higher probability of AIV exposure in the beginning of the hunting season in each state (September in LA and GA, October in MN), and declining in December and January. In addition, relative exposure risks among different states vary according to the expected harvest per hunter-day. For example, in November, the first month in which ducks are hunted in all three states, the probability of exposure is highest in LA, where hunters harvest an average 3·3 ducks per day, and lowest in MN where the average harvest is lower than 1·3 ducks per day (Table 1).
The distribution of Eviral-loadijs was highly skewed to low viral loads as illustrated in Figure 2 for LA in December. The minimum estimated viral load shed by an infected, harvested bird in a hunter-day was 4·38 EID50 (0·64 log10 EID50).

Fig. 2. Distribution of estimated values for the random variable Eviral-loadist, for Louisiana, in December, for iterations with non-zero exposure (2·1%).
Figure 3 provides the probability of viral exposure (Enon-zeroist) in a hunter-day, as well as the expected number of hunter-days per state per month. Although the probability of exposure was highest in LA in September, the number of hunter-days was not as high as in MN the following month when the probability of exposure was only slightly lower. The number of hunter-days was highest in LA during each study month, but the probability of exposure decayed rapidly along the hunting season.
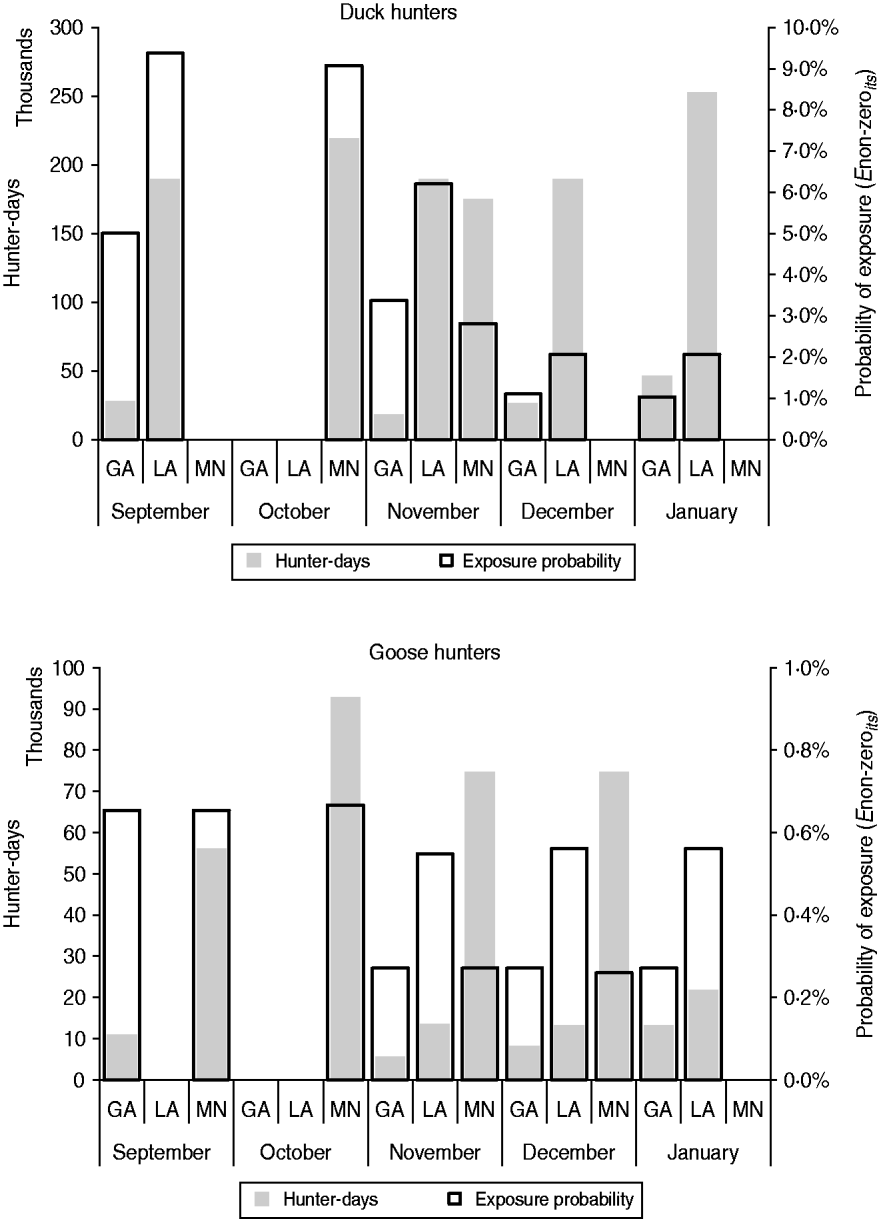
Fig. 3. Expected size of the hunter population in each state and month, according to the number of weekends of open hunting per month (left axis) and probability of hunter exposure to any load of avian influenza viruses (Enon-zeroist) (right axis). GA, Georgia; LA, Louisiana; MN, Minnesota.
Overall, the probability of exposure associated with goose hunting activities was about ten times lower than with duck hunting. Because the prevalence of AIV in geese, already assumed to be low, was varied only by month and not by state, the effect of bird harvest size on the estimated probability of exposure per hunter-day is even more evident (Fig. 3). With equal harvest per hunter-day, the probability of exposure is the same in GA and MN. However, as the number of goose hunter-days is nearly five times greater in MN, the population risk (as estimated by the expected number of exposures among geese hunters in the state) can be expected to be highest in MN for all hunting months, compared to GA.
The population-level exposure risk, summed for the populations of duck and goose hunters, and prevalence of human influenza infection for surveillance years 2005–2010 are shown in Figure 4. In most seasons when the risk of AIV infection among hunters is highest (e.g. LA in September) circulating influenza A infection in humans is very low. Conversely, during months of relatively high levels of human influenza A activity in the population, hunter exposure risk to AIV is minimal. The distribution of human influenza A activity during the atypical flu season of 2009– 2010, however, did not follow this pattern. In that year, the peak of influenza infection in humans coincided with the months of higher prevalence of infection in birds, and therefore the months of higher population risk.

Fig. 4. Comparison between the relative population risk of avian influenza infection in hunters (combining exposure risk and active hunter population per month) and the prevalence of human influenza A (lines, right axis) per month, from September to January. Different lines represent different influenza seasons. GA, Georgia; LA, Louisiana; MN, Minnesota.
DISCUSSION
The risk of AIV infection associated with hunting activities is expected to be very low. Gill et al. [Reference Gill5] reported influenza antibodies in 1/39 duck hunters and 2/68 employees of the Department of Natural Resources. All those with detectable antibody had substantial lifetime exposures to wildlife and there was no indication of disease associated with these exposures. The potential transmission mechanisms of AIVs from an infected, harvested bird to a hunter are speculative. Handling of faecally contaminated materials (e.g. feathers, viscera, fomites, etc.) during and immediately following the harvest of the bird(s) may result in airborne inhalation or direct contact via self-inoculation of conjunctival mucosa [Reference Hayden and Croisier21]. It is likely that only a small amount of infected faeces would be involved in the transmission event, but estimates of the probability of inoculation as well as the likely inoculant volume are not available.
Previous risk assessments have reviewed a large number of publications assessing the infectious dose of different strains of AIVs in humans and mice via the intranasal route. The United States Food Safety and Inspection Service [22] used an ID50 (viral dose that will infect 50% of the experimental group) ranging from 7·8 to 9·5 log10 EID50, based on studies by Beare & Webster [Reference Beare and Webster23]. But other human studies reported ID50 as low as 4·9 log10 EID50. In an assessment of the risk of AIV infection to humans via water, Schijven et al. [Reference Stallknecht17] simulated an exponential dose response with the parameter varying up to fiveold (from 1 to 0·00 001). The resulting exponential dose response would give a 50% probability of infection for doses from −0·16 to 4·84 log10 EID50. It is important to note that the viral loads estimated and presented in this study represent the total load potentially shed by an infected, harvested bird and do not represent the potential dose associated with hunter exposure.
Due to the great uncertainty associated with the probability of transmission of AIV from an infected bird to a hunter, as well as the lack of precise estimates for the dose response in humans, the construction of a human infection model was not considered to improve the risk assessment in this study. Consequently, the actual risk of AIV infection among duck and goose hunters was not calculated because of the lack of reliable estimates of inoculation rate and dose response in humans. Instead, we focused on modelling the determinants of exposure risk, assessing variation among locations and hunting months, and presented these risks in relation to the levels of circulating seasonal influenza A infections in humans.
Risk was modelled per hunter-day as this was the unit of exposure. This does not account for the fact that a single hunter often has multiple hunter-days in a season. As the majority of hunter-day exposure scenarios (>90%) did not result in exposure to influenza A viruses, and the risk of virus transmission per hunter-day is assumed to be independent of whether exposure previously occurred, it is highly unlikely that two effective transmission events would occur in the same person; thus, we believe that the use of hunter-days did not compromise the assessment. For the same reason, we did not consider overlap in the populations of duck and goose hunters, although individual hunters could be included in both populations on the same day. In this case, the exposure per hunter-day would be different; but again, the low exposure risk, especially associated with goose hunting, provides confidence that the results of the model were not compromised by this assumption.
Except for the estimated population-based differences in AIV prevalence between ducks and geese, individual species variation in virus prevalence among hunted birds was not considered [Reference Deibel24]. However, our prevalence assumptions were broadly based on mallards; and this very abundant North American species has a relatively high prevalence of infection compared to other hunted duck species [Reference Olsen25]. Species-related differences in AIV infection may be important to consider in future assessments, as species harvested in the states represented in this study vary considerably, and this may reduce hunter exposure risk estimates.
This assessment highlighted key factors responsible for the variation in risk. Differences in risk per hunter-day depend on the prevalence of AIV in birds, and the harvest per hunter-day. Therefore, risk cannot be generalized within the hunter population and varies with specific activities (duck vs. goose hunting), season (in relation to declining prevalence in the waterfowl population), and geographical areas (in relation to prevalence in the waterfowl population, the timing of the hunting season, and harvest rates). But hunter exposure risk variation due to different prevalence of AIV in birds seems to have a significant effect in the final estimated population risk, as illustrated by a higher exposure risk observed in early season months, especially in MN.
We assumed a homogenous distribution of hunter-days throughout the weekends of open season. If hunting activities are concentrated during the holiday season, the overall population risk would be smaller, because the holiday months of December and January represent lower AIV exposure risk per hunter-day. However, December and January are the months with higher prevalence of human influenza virus infections. Nonetheless, our results indicate that the risk of AIV infection among hunters does not coincide with the peak season of influenza A infection in humans in most years. This temporal separation was apparent in the patterns observed during all human influenza seasons except 2009–2010 when the novel H1N1 human pandemic occurred [19]. Prior to the human influenza pandemic, human cases did not increase until February; and this is far removed from waterfowl hunting activities. Consequently, the risk of human exposure to an AIV during peak circulation of human influenza may be restricted to years when novel human strains have already emerged. Nonetheless, even during human pandemic years, the risk of hunter-exposure to AIV is very small.
In this study we used estimates of AIV prevalence from the literature on low pathogenic avian influenza (LPAI) viruses in wild waterfowl. Extrapolating these results to the risk of HPAI H5N1 transmission to humans requires the assumptions that (a) birds could be infected with highly pathogenic strains with no clinical signs, allowing people to be exposed to them during regular hunting activities; and (b) the prevalence of HPAI H5N1 would be comparable to LPAI virus estimates. With regard to hunting-related contact, two important observations from experimental studies should be considered in relation to HPAI H5N1. First, geese and some species of ducks (wood ducks) would probably show clinical signs [Reference Brown, Stallknecht and Swayne26, Reference Tuckwell, Toubiana and Vibert27] and significant mortality, decreasing the chance of harvest by hunters. Second, in mallards, which represent the most common species harvested by hunters in North America, viral shedding rates for HPAI H5N1 are less than normally observed with LPAI virus [Reference Brown28]. Finally, it is highly likely based on the few HPAI H5N1 isolations reported from healthy waterfowl despite extensive surveillance activities in Europe and Asia, that prevalence of HPAI H5N1 would be extremely low in wild waterfowl even if established in these populations.
We have demonstrated in this study that variation in hunting intensity among states can result in variation in the population risk of human exposure to AIV during waterfowl hunting activities, but the main determinants of exposure risk per hunter-day are spatial and temporal variations in the prevalence of AIV in birds. Moreover, the risk of human exposure to AIV is temporally distinct from the risk of human influenza A infection, making recombination events due to co-infection in the same individual highly unlikely. The parameters used in this risk model were focused on North America, but the approaches can have global application in understating potential human exposure associated with hunting activities, and managing risks.
ACKNOWLEDGEMENTS
This work was funded through Cooperative Agreement 1U19Cl0004501 with the Center of Disease Control. The CDC did not have any involvement in the study design, implementation, or publishing of this study and the research presented herein represents the opinions of the authors, but not necessarily the opinions of the funding agencies.
DECLARATION OF INTEREST
None.







